Innovations and Patent Trends in the Development of USFDA Approved Protein Kinase Inhibitors in the Last Two Decades
Abstract
1. Introduction
| S. No. | Kinase | Families | Subfamilies | Functions |
|---|---|---|---|---|
| Serine/Threonine-Specific Protein Kinases | ||||
| 1 | AGC | PKA, PKG, PKC, DMPK, NDR, AKT, SGK, RSK, PKN, GRK, PDK1, RSKR, RSKL, MAST | DMPK: GEK, ROCK, CRIK PKC: Alpha, Delta, Gamma, Epsilon RSK: MSK, P70 RSKL: RSKL1, RSKL2 MAST: MAST1, MAST 2, MAST3, MAST4, MASTL | They are implicated in various cellular activities and are prospective targets to treat cancer, inflammation, viral infections, obesity, diabetes, and neurological disorders [8] |
| 2 | CAMK | Calcium/calmodulin-dependent protein kinase-CAMK1, Unique VACAMKL, PSK, DAPK, MLCK, TRIO, CASK, CAMK2, PHK, DCAMKL, MAPKAPK, CAMKL, TSK, PIM, TRB1, Unique STK33, PKD, RAD53 | MAPKAPK: MNK, MAPKAPK1, MAPKAPK2, MAPKAPK3, JNK CAMKL: AMPK, BRSK, MELK, MARK, QIK, NUAK, NIMI, SNRK, PASK, CHK1, LKB1, HUNK | They are implicated in the phosphorylation of transcription factors and the control of gene expression. They also control the life cycle of the cell [9] |
| 3 | CK1 | Casein kinase 1, TTBK, VRK | - | They are involved in the phosphorylation of significant governing molecules in cellular translation/transcription, cell–cell adhesion, and receptor coupled signal transduction. They control main signaling trails, particularly in cancer evolution [10] |
| 4 | CMGC | CDK, MAPK, GSK3, CLK families, CDKL, CLK, RCK, DYRK | - | Critical role in cell cycle regulation and intracellular signal transduction [11] |
| 5 | STE | Homologs of yeast Sterile 7/MAP3K, Sterile 11/MAP2K, Sterile 20/MAP4K | MAP4K: FRAY, STLK, PAKA, PAKB, MST, YSK, TAO, MSN, NINAC, KHS, SLK | Crucial role in MAP kinase pathways, which require a sequential PK reaction to activate the next kinase in the pathway, especially in cascade process [12] |
| Tyrosine-Specific Protein Kinases | ||||
| 6 | TK | Tyrosine kinase | Receptor Tyrosine Kinases (RTKs): EGFR, EPH, SEV, ALK, TRK, INSR, CCK4, AXL, VEGFR, FGFR, MUSK, LMR, DDR, ROR, TIE, SEF, PDGFR, RET, MET, RYK | They play a vital role in controlling cellular differentiation, cell division, and morphogenesis. They primarily act as growth factor receptors and in downstream signaling [13] |
| Non-Receptor Tyrosine Kinases (nRTKs): CSK, JAK, SRC (SFKs, BCR), BTK, ACK, SYK, FER, TEC, ABL, FAK | They are involved in signaling cascades, particularly those implicated in growth hormone and cytokine signaling. Some of them are involved in synaptic transmission, myelination, axon guidance, and oligodendrocyte formation [13] | |||
| 7 | TKL | Tyrosine kinase-like | IRAK, MLKL, LIMK, TESK, LRRK, ALK, ACTR, TGFR, MISR, BMPR, RAF, KSR, TAK, ILK, DLK, LZK, MLK, ZAK, RIPK, ANKRP, SGK, RIPK | They control apoptosis, cell differentiation/growth, angiogenesis, vascular development, and the protective response against pathogens [5,14] |
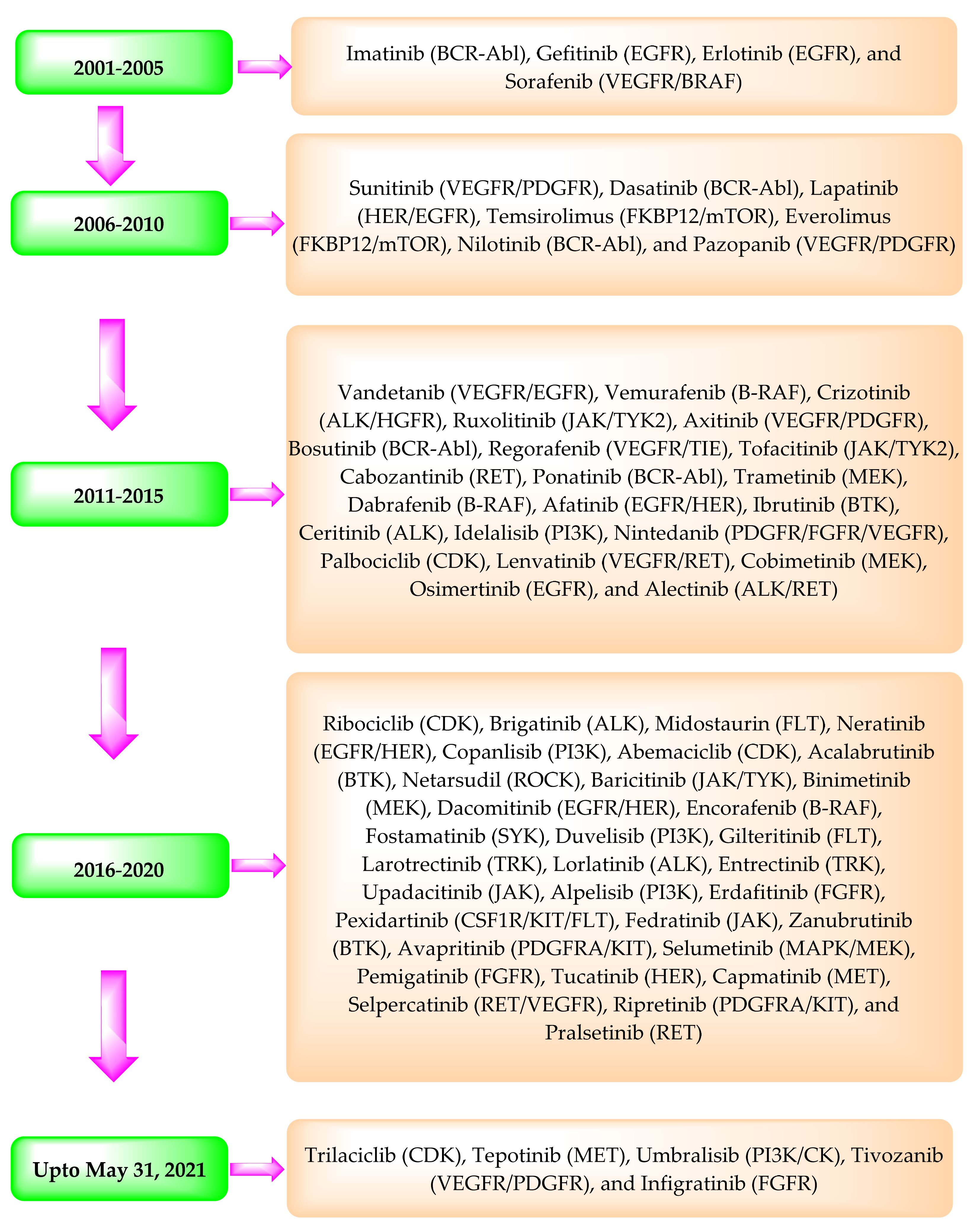
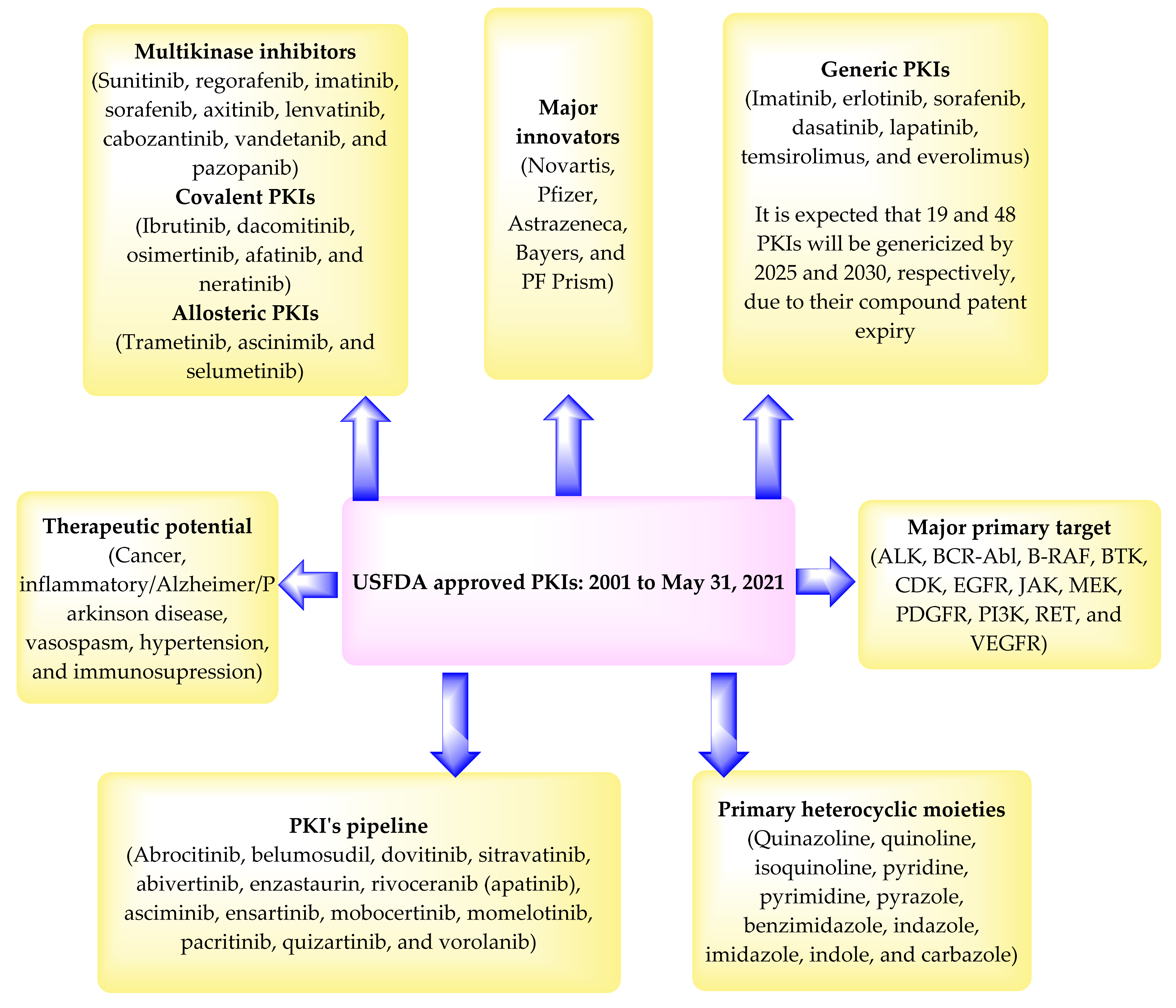
2. USFDA Approved Protein Kinase Inhibitors
3. Patent Searching
4. Summary of the Patents
4.1. Imatinib Mesylate
4.2. Gefitinib
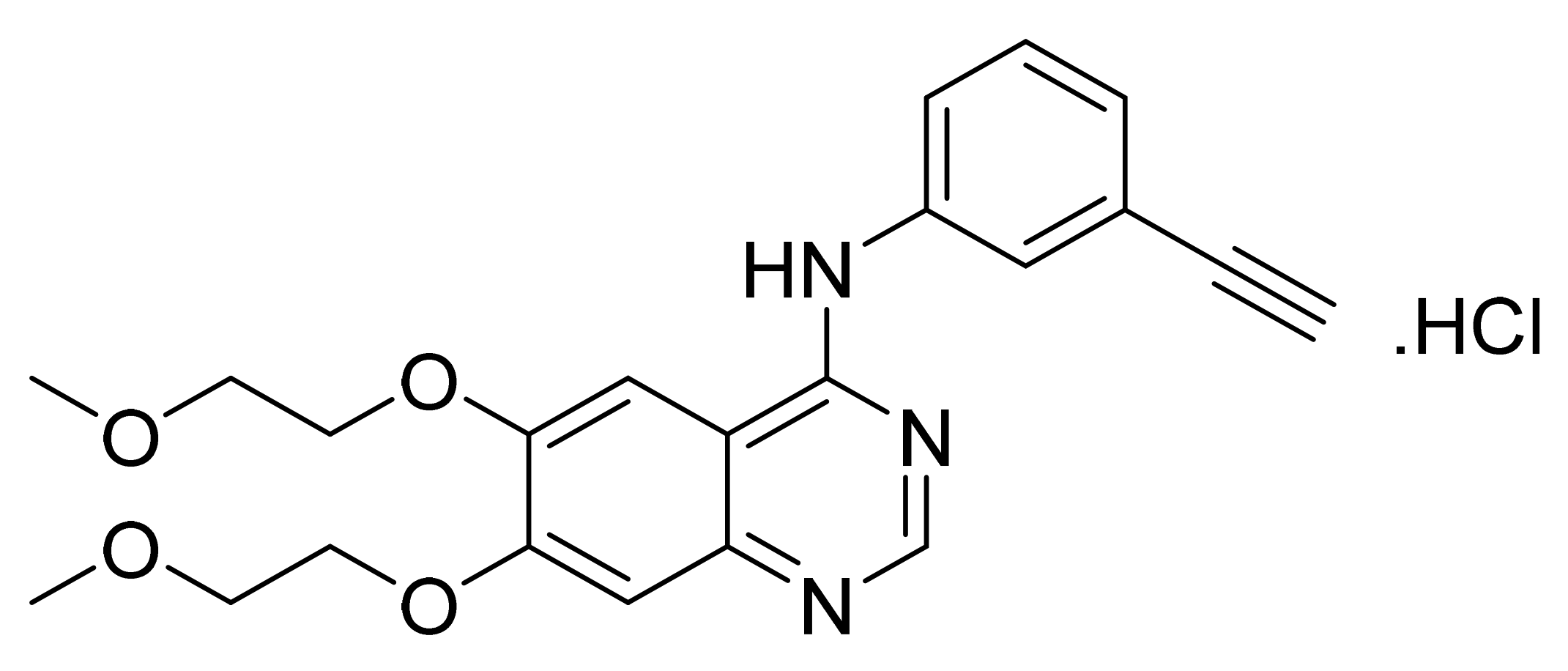
4.3. Erlotinib Hydrochloride
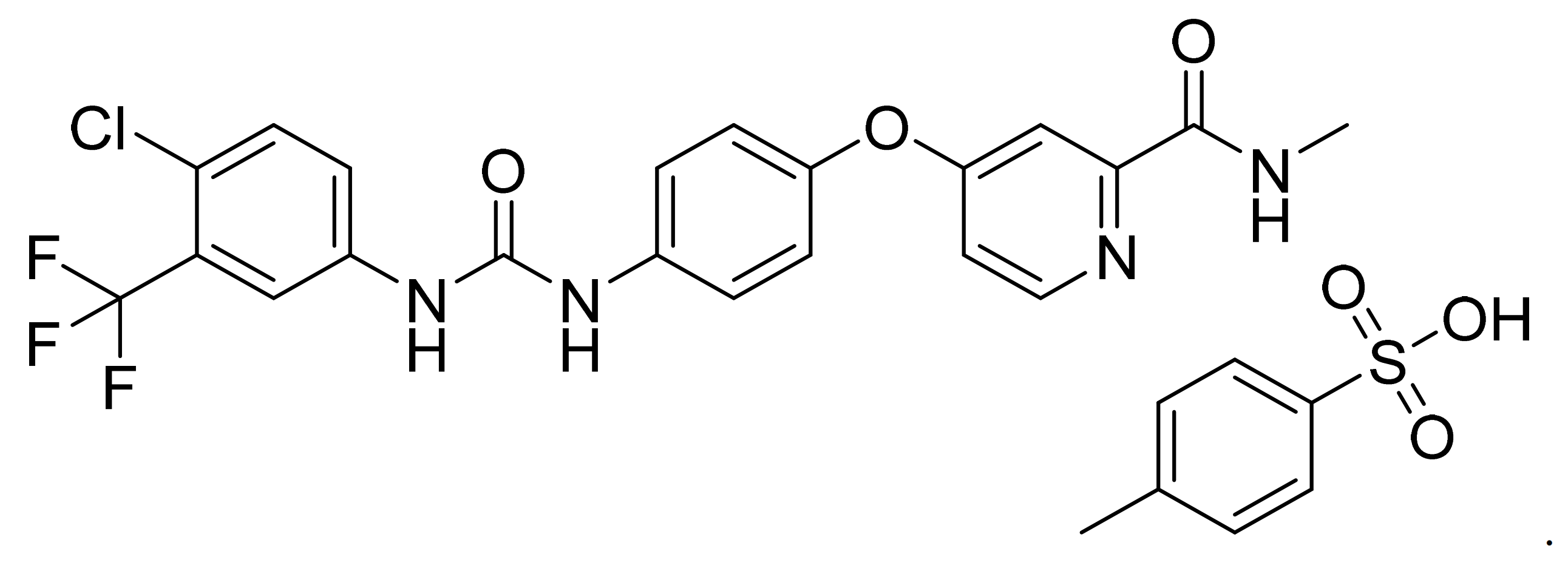
4.4. Sorafenib Tosylate
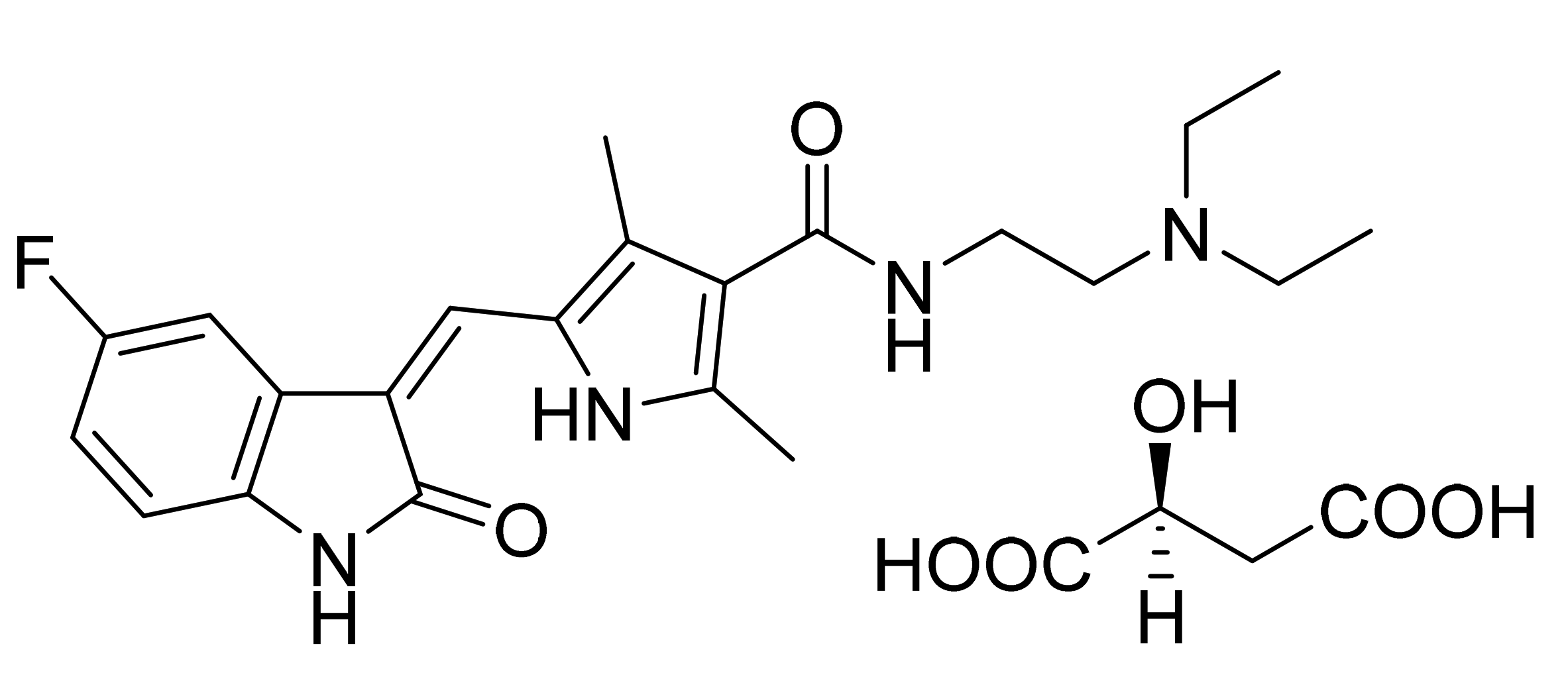
4.5. Sunitinib Malate
4.6. Dasatinib Monohydrate
4.7. Lapatinib Ditosylate Monohydrate
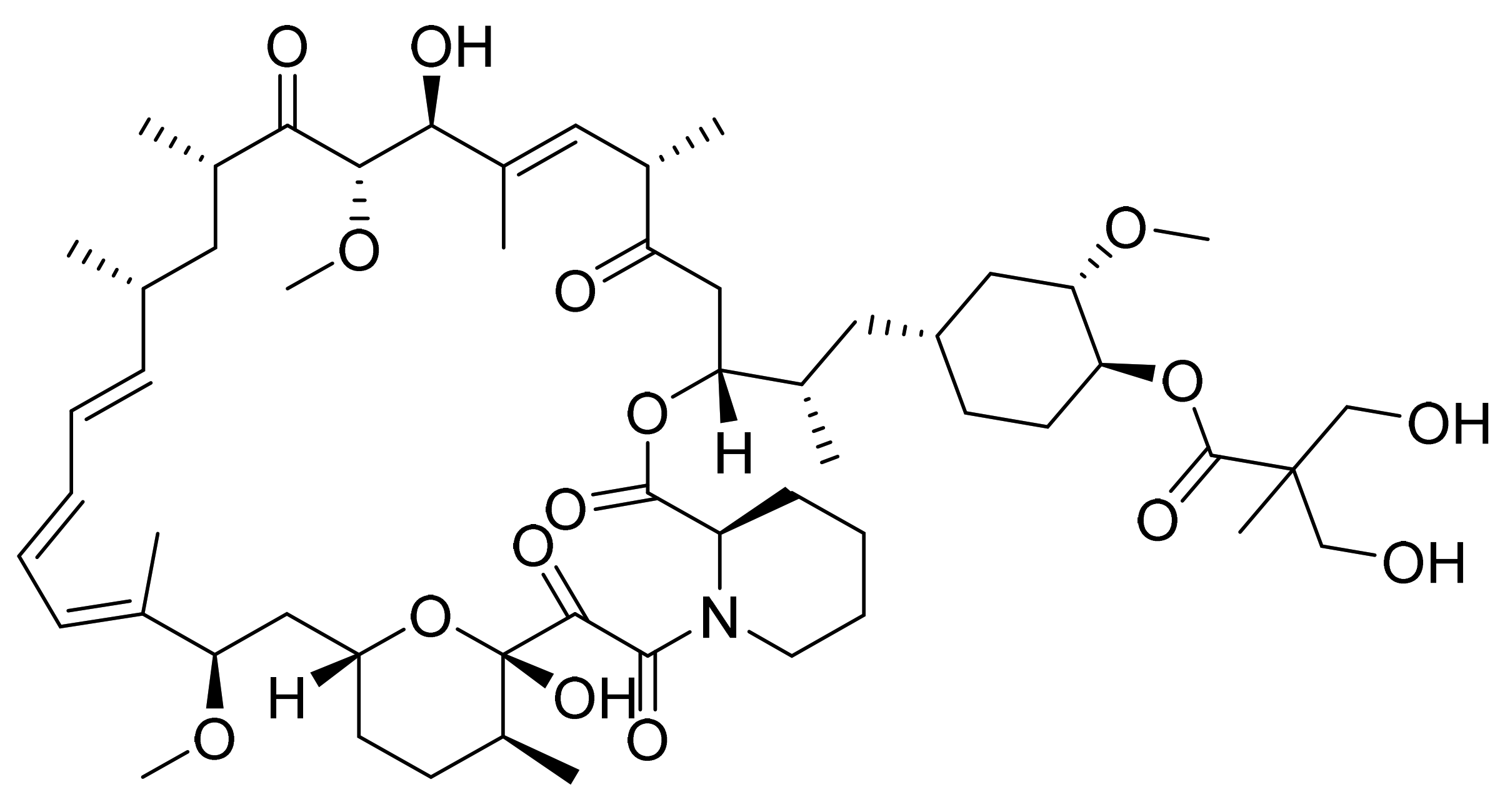
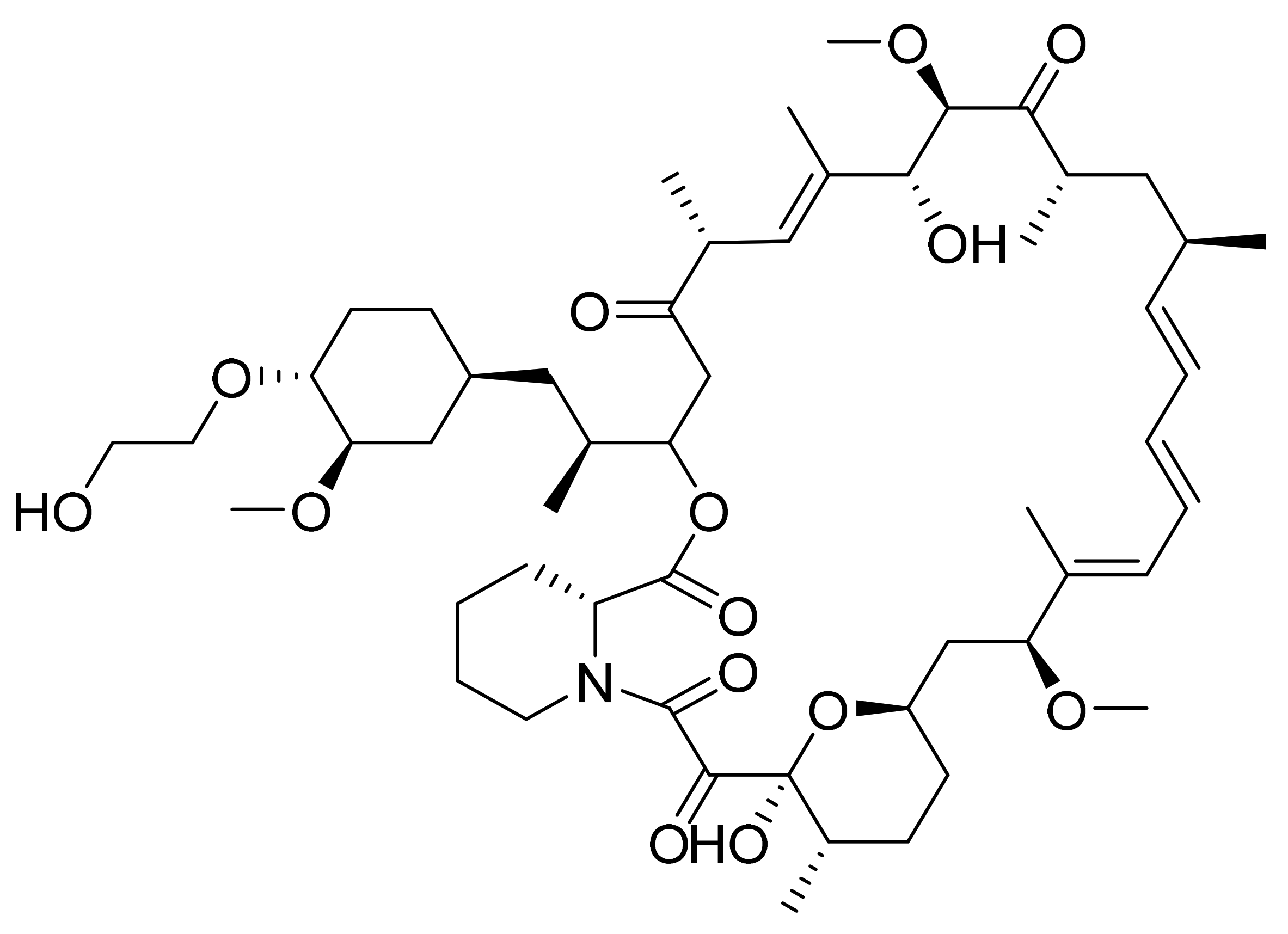
4.8. Temsirolimus
4.9. Everolimus
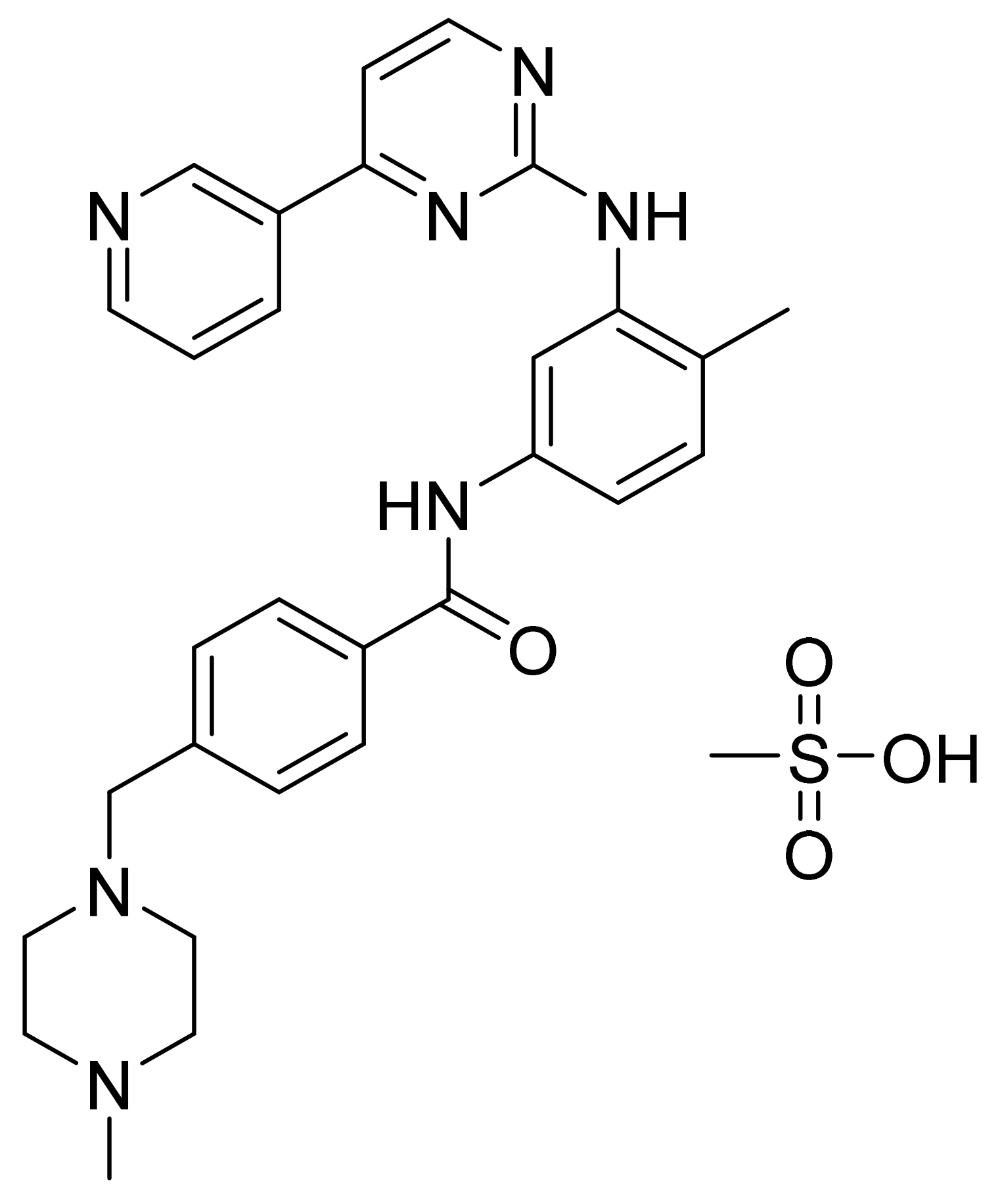
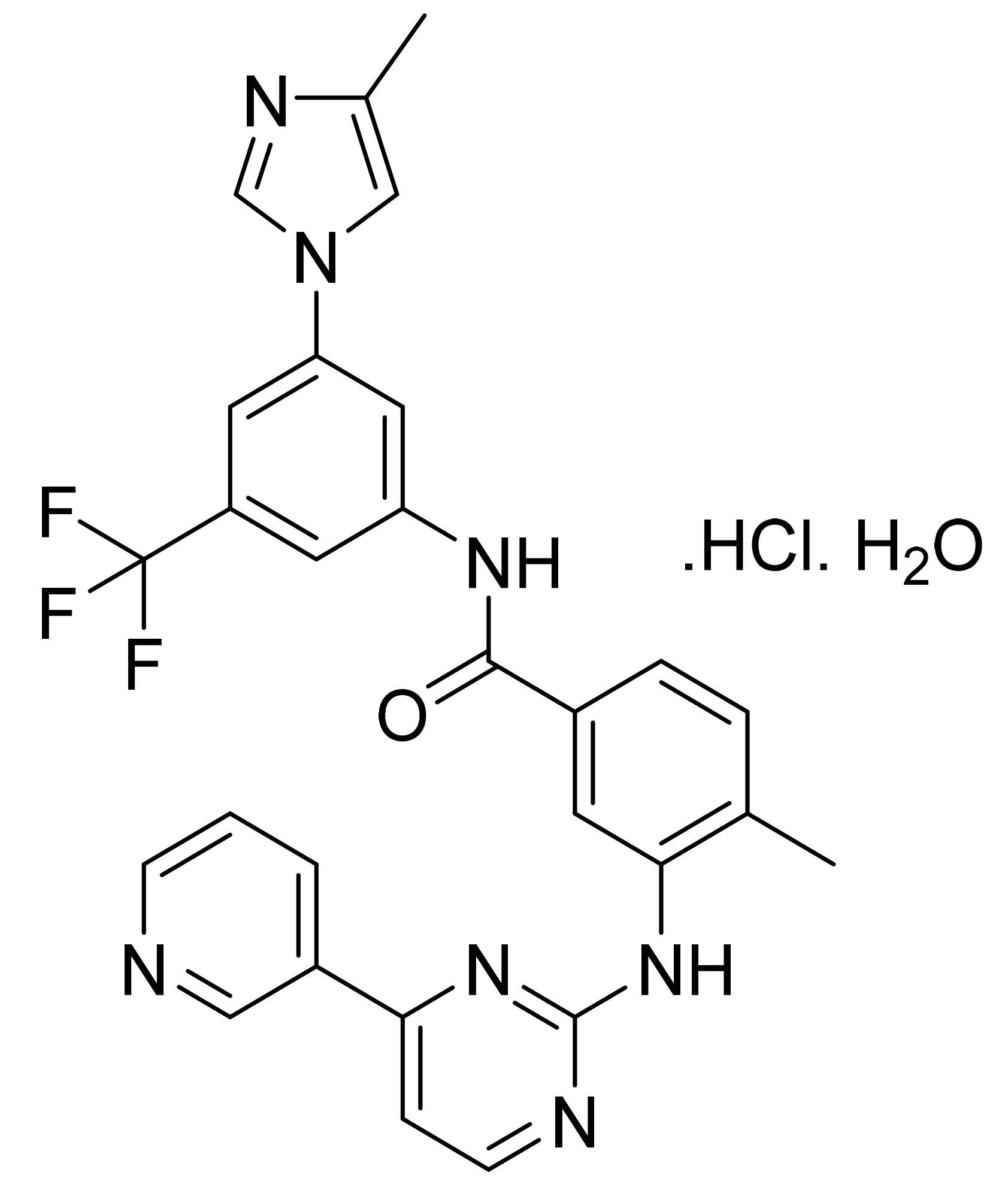
4.10. Nilotinib Hydrochloride Monohydrate
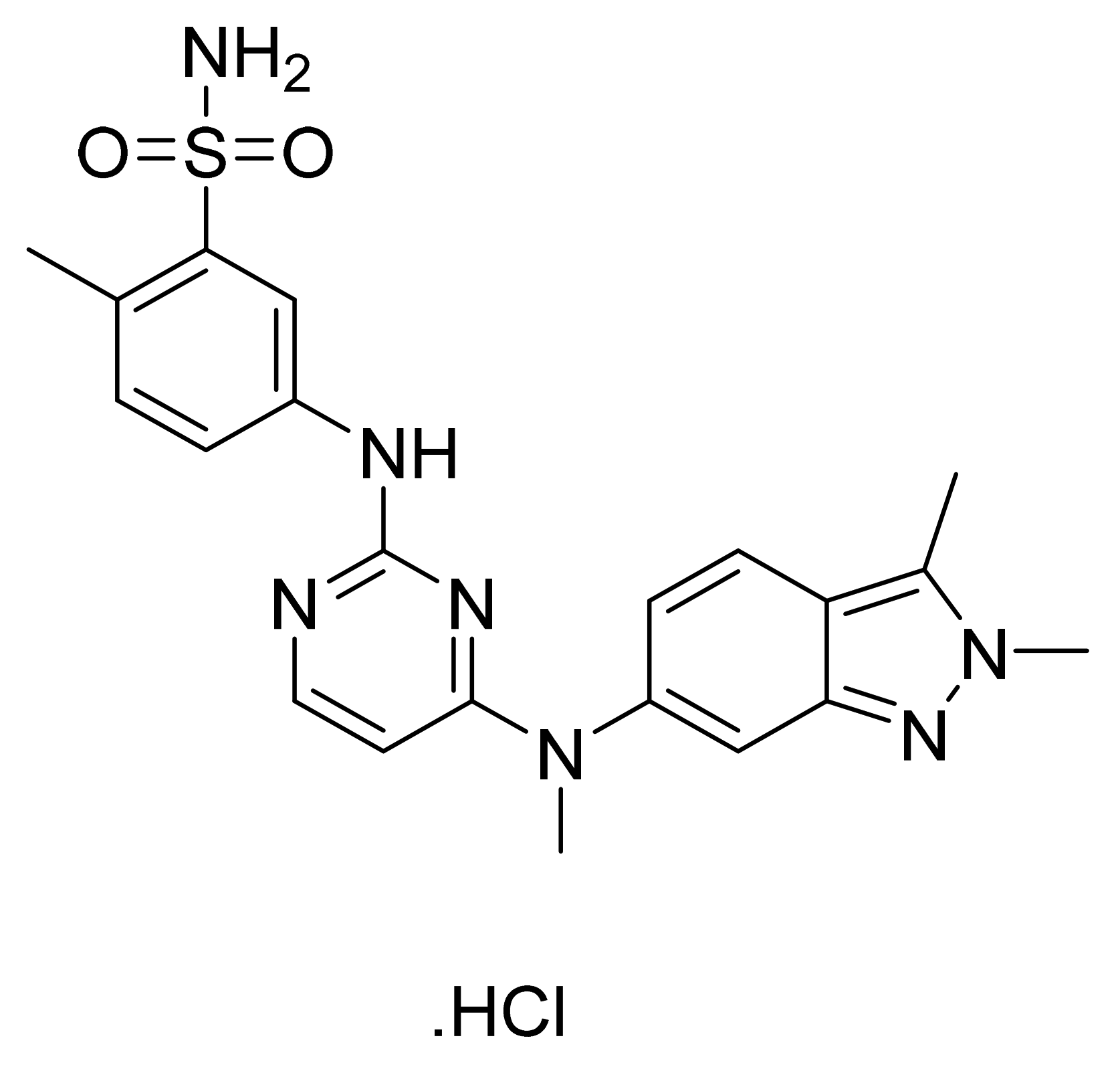
4.11. Pazopanib Hydrochloride
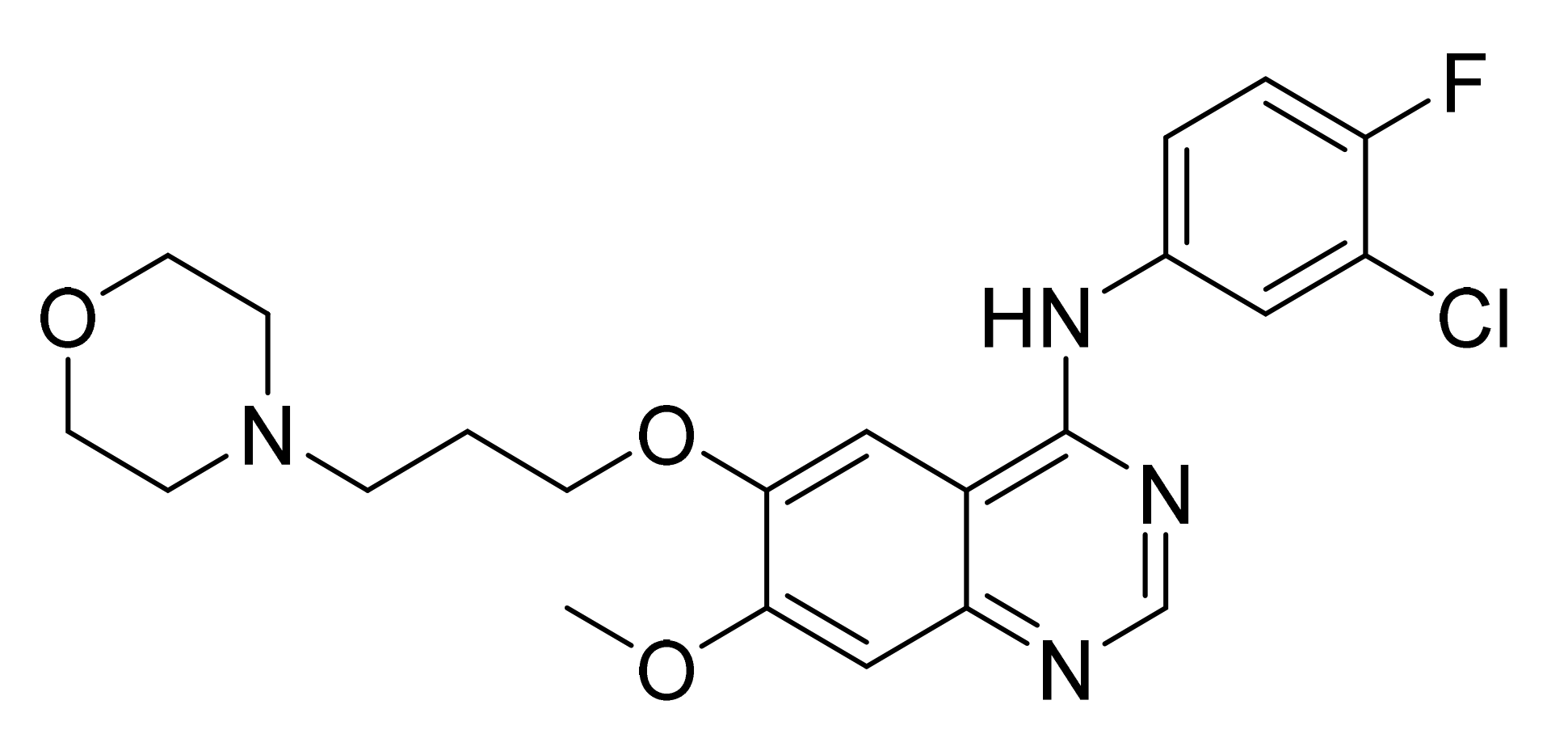
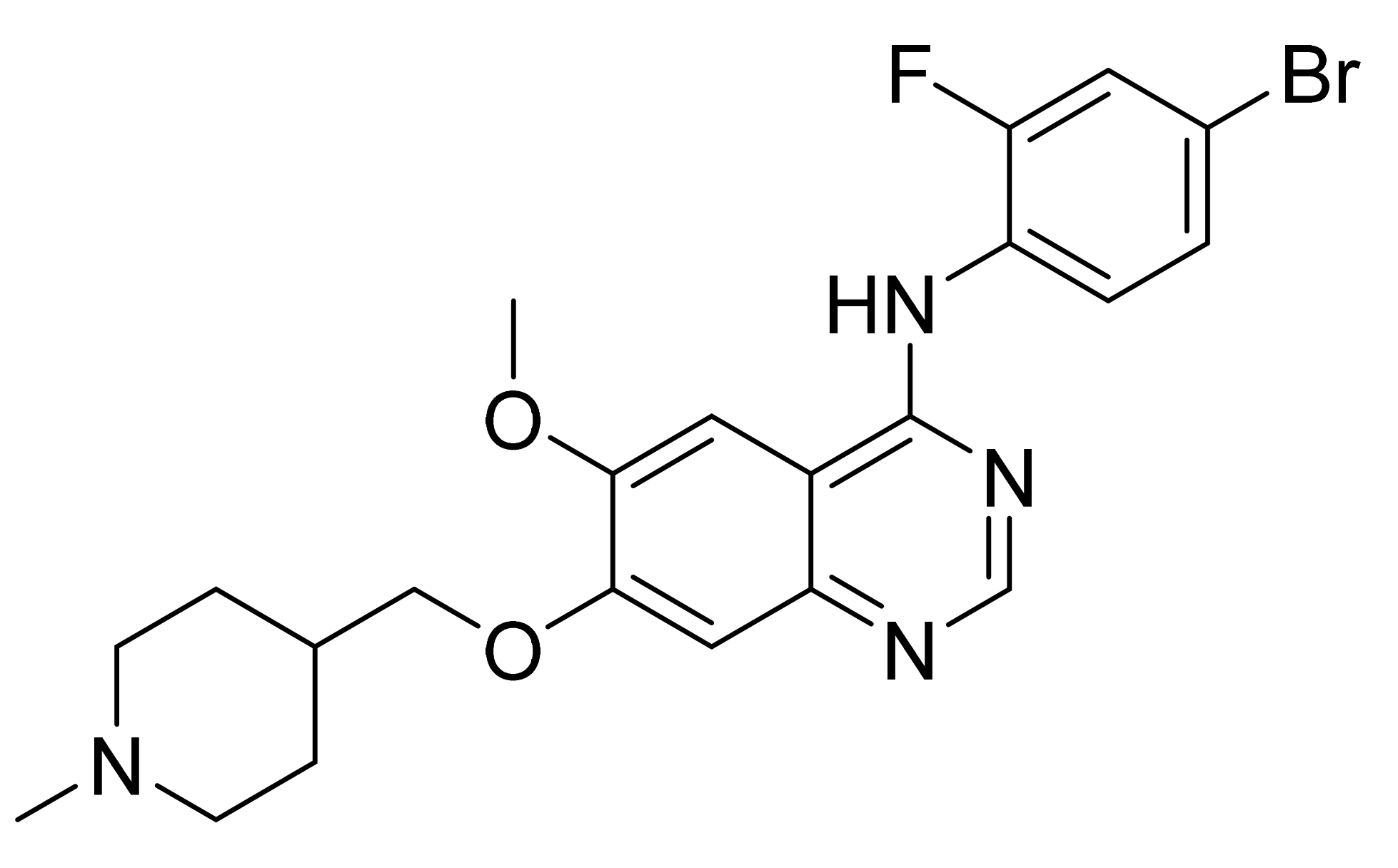
4.12. Vandetanib
4.13. Vemurafenib
4.14. Crizotinib
4.15. Ruxolitinib Phosphate
4.16. Axitinib
4.17. Bosutinib Monohydrate
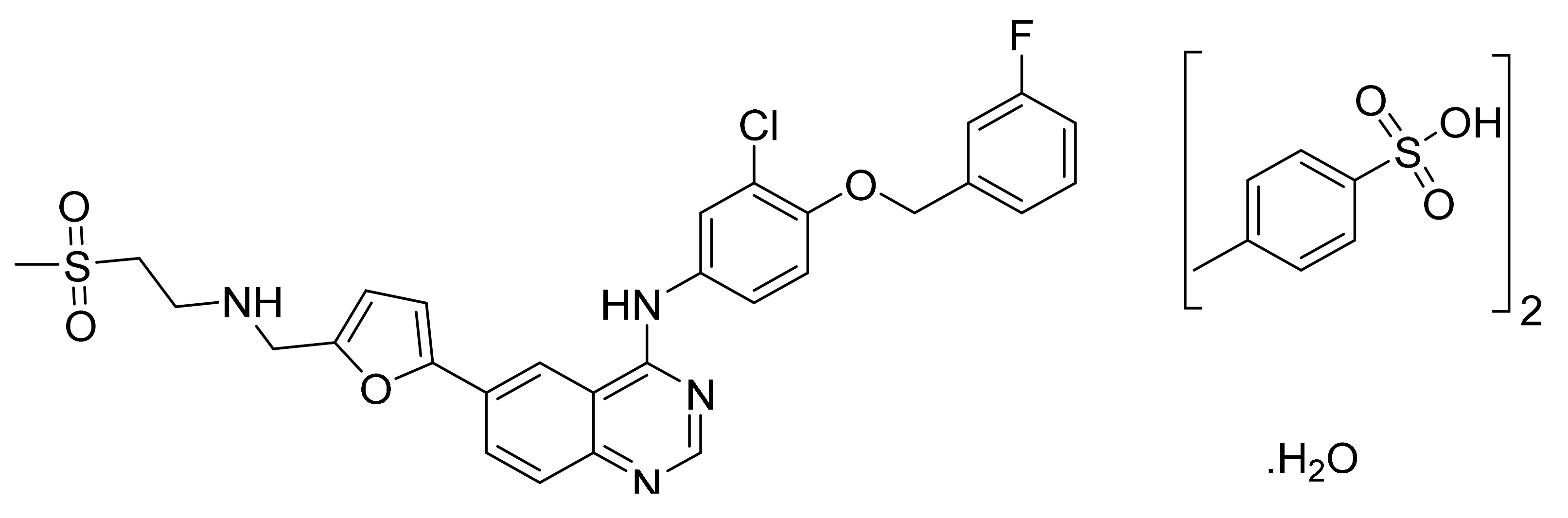
4.18. Regorafenib Monohydrate
4.19. Tofacitinib Citrate
4.20. Cabozantinib S-Malate
4.21. Ponatinib Hydrochloride
4.22. Trametinib Dimethyl Sulfoxide
4.23. Dabrafenib Mesylate
4.24. Afatinib Dimaleate
4.25. Ibrutinib
4.26. Ceritinib
4.27. Idelalisib
4.28. Nintedanib Esylate
4.29. Palbociclib
4.30. Lenvatinib Mesylate
4.31. Cobimetinib Fumarate
4.32. Osimertinib Mesylate
4.33. Alectinib Hydrochloride
4.34. Ribociclib Succinate
4.35. Brigatinib
4.36. Midostaurin
4.37. Neratinib Maleate
4.38. Copanlisib Dihydrochloride
4.39. Abemaciclib
4.40. Acalabrutinib
4.41. Netarsudil Dimesylate
4.42. Baricitinib
4.43. Binimetinib
4.44. Dacomitinib Monohydrate
4.45. Encorafenib
4.46. Fostamatinib Disodium Hexahydrate
4.47. Duvelisib Hydrate
4.48. Gilteritinib Fumarate
4.49. Larotrectinib Sulfate
4.50. Lorlatinib
4.51. Entrectinib
4.52. Upadacitinib Hemihydrate
4.53. Alpelisib
4.54. Erdafitinib
4.55. Pexidartinib Hydrochloride
4.56. Fedratinib Dihydrochloride Monohydrate
4.57. Zanubrutinib
4.58. Avapritinib
4.59. Selumetinib Sulfate
4.60. Pemigatinib
4.61. Tucatinib
4.62. Capmatinib Dihydrochloride Monohydrate
4.63. Selpercatinib
4.64. Ripretinib
4.65. Pralsetinib
4.66. Trilaciclib Dihydrochloride
4.67. Tepotinib Hydrochloride Monohydrate
4.68. Umbralisib Tosylate
4.69. Tivozanib Hydrochloride Monohydrate
4.70. Infigratinib Phosphate
5. Expert Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Keohane, E.M.; Otto, C.N.; Walenga, J.M. Rodak’s Hematology-E-Book: Clinical Principles and Applications; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Prescott, J.C.; Braisted, A. Identification of Kinase Inhibitors. PCT Patent Application Publication Number WO2005034840A2, 21 April 2005. [Google Scholar]
- Charrier, J.D.; Durrant, S. Protein Kinase Inhibitors. U.S. Patent Application Publication Number US20120028966A1, 2 February 2012. [Google Scholar]
- Liu, C.; Ke, P.; Zhang, J.; Zhang, X.; Chen, X. Protein kinase inhibitor peptide as a tool to specifically inhibit protein kinase A. Front. Physiol. 2020, 11, 574030. [Google Scholar] [CrossRef] [PubMed]
- Plowman, G.; Whyte, D.; Manning, G.; Sudarsanam, S.; Martinez, R. Novel human protein kinases and protein kinase-like enzymes. U.S. Patent Application Publication Number US20040048310A1, 11 March 2004. [Google Scholar]
- Buljan, M.; Ciuffa, R.; van Drogen, A.; Vichalkovski, A.; Mehnert, M.; Rosenberger, G.; Lee, S.; Varjosalo, M.; Pernas, L.E.; Spegg, V.; et al. Kinase interaction network expands functional and disease roles of human kinases. Mol. Cell 2020, 79, 504–520.e9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.J.; Linley, A.; Hammond, D.E.; Hood, F.E.; Coulson, J.M.; MacEwan, D.J.; Ross, S.J.; Slupsky, J.R.; Smith, P.D.; Eyers, P.A.; et al. New perspectives, opportunities, and challenges in exploring the human protein kinome. Cancer Res. 2018, 78, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, J.M.; Pastor-Flores, D.; Bauer, A.F.; Schulze, J.O.; Biondi, R.M. AGC protein kinases: From structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim. Biophys. Acta 2013, 1834, 1302–1321. [Google Scholar] [CrossRef]
- Junho, C.V.C.; Caio-Silva, W.; Trentin-Sonoda, M.; Carneiro-Ramos, M.S. An overview of the role of calcium/calmodulin-dependent protein kinase in cardiorenal syndrome. Front. Physiol. 2020, 11, 735. [Google Scholar] [CrossRef]
- Schittek, B.; Sinnberg, T. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol. Cancer 2014, 13, 231. [Google Scholar] [CrossRef]
- Strang, B.L. RO0504985 is an inhibitor of CMGC kinase proteins and has anti-human cytomegalovirus activity. Antivir. Res. 2017, 144, 21–26. [Google Scholar] [CrossRef]
- Sawa, M.; Masai, H. Drug design with Cdc7 kinase: A potential novel cancer therapy target. Drug Des. Devel. Ther. 2009, 2, 255–264. [Google Scholar] [CrossRef]
- Matrone, C.; Petrillo, F.; Nasso, R.; Ferretti, G. Fyn tyrosine kinase as harmonizing factor in neuronal functions and dysfunctions. Int. J. Mol. Sci. 2020, 21, 4444. [Google Scholar] [CrossRef]
- Petrie, E.J.; Hildebrand, J.M.; Murphy, J.M. Insane in the membrane: A structural perspective of MLKL function in necroptosis. Immunol. Cell Biol. 2017, 95, 152–159. [Google Scholar] [CrossRef]
- Rauch, J.; Volinsky, N.; Romano, D.; Kolch, W. The secret life of kinases: Functions beyond catalysis. Cell Commun. Signal. 2011, 9, 23. [Google Scholar] [CrossRef]
- Ochoa, D.; Bradley, D.; Beltrao, P. Evolution, dynamics and dysregulation of kinase signalling. Curr. Opin. Struct. Biol. 2018, 48, 133–140. [Google Scholar] [CrossRef]
- Brognard, J.; Hunter, T. Protein kinase signaling networks in cancer. Curr. Opin. Genet. Dev. 2011, 21, 4–11. [Google Scholar] [CrossRef]
- Oprea, T.I.; Bologa, C.G.; Brunak, S.; Campbell, A.; Gan, G.N.; Gaulton, A.; Gomez, S.M.; Guha, R.; Hersey, A.; Holmes, J.; et al. Unexplored therapeutic opportunities in the human genome. Nat. Rev. Drug Discov. 2018, 17, 317–332. [Google Scholar] [CrossRef]
- Essegian, D.; Khurana, R.; Stathias, V.; Schürer, S.C. The clinical kinase index A method to prioritize understudied kinases as drug targets for the treatment of cancer. Cell Rep. Med. 2020, 1, 100128. [Google Scholar] [CrossRef]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353–377. [Google Scholar] [CrossRef]
- Klaeger, S.; Heinzlmeir, S.; Wilhelm, M.; Polzer, H.; Vick, B.; Koenig, P.A.; Reinecke, M.; Ruprecht, B.; Petzoldt, S.; Meng, C.; et al. The target landscape of clinical kinase drugs. Science 2017, 358, eaan4368. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol. Res. 2021, 165, 105463. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef]
- Zimmermann, J. Pyrimidine Derivatives and Processes for the Preparation Thereof. U.S. Patent Number US5521184A, 28 May 1996. [Google Scholar]
- Zimmermann, J.; Sutter, B.; Buerger, H.M. Crystal Modification of a N-Phenyl-2-Pyrimidineamine Derivative, Processes for its Manufacture and Its Use. U.S. Patent Number USRE43932E1, 15 January 2013. [Google Scholar]
- Sim, E.H.; Yang, I.A.; Wood-Baker, R.; Bowman, R.V.; Fong, K.M. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2018, 1, CD006847. [Google Scholar] [CrossRef]
- Barker, A.J. Quinazoline Derivatives Useful for Treatment of Neoplastic Disease. U.S. Patent Number US5457105A, 10 October 1995. [Google Scholar]
- Gibson, K.H. Quinazoline Derivatives. U.S. Patent Number US5770599A, 23 June 1998. [Google Scholar]
- Steins, M.; Thomas, M.; Geibler, M. Erlotinib. Recent Results Cancer Res. 2018, 211, 1–17. [Google Scholar] [CrossRef]
- Schnur, R.C.; Arnold, L.D. Alkynl and Azido-Substituted 4-Anilinoquinazolines. U.S. Patent Number USRE41065E1, 29 December 2009. [Google Scholar]
- Norris, T.; Raggon, J.W.; Connell, R.D.; Moyer, J.D.; Morin, M.J.; Kajiji, S.M.; Foster, B.A.; Ferrante, K.J.; Silberman, S.L. Stable Polymorph on N-(3-ethynylphenyl)-6,7-Bis (2methoxyethoxy)-4-Quinazolinamine Hydrochloride, Methods of Production, and Pharmaceutical Uses Thereof. U.S. Patent Number US6900221B1, 31 May 2005. [Google Scholar]
- Chen, F.; Fang, Y.; Zhao, R.; Le, J.; Zhang, B.; Huang, R.; Chen, Z.; Shao, J. Evolution in medicinal chemistry of sorafenib derivatives for hepatocellular carcinoma. Eur. J. Med. Chem. 2019, 179, 916–935. [Google Scholar] [CrossRef]
- Riedl, B.; Dumas, J.; Khire, U.; Lowinger, T.B.; Scott, W.J.; Smith, R.A.; Wood, J.E.; Monahan, M.K.; Natero, R.; Renick, J.; et al. Omega-Carboxyaryl Substituted Diphenyl Ureas as Raf Kinase Inhibitors. U.S. Patent Number US7235576B1, 26 June 2007. [Google Scholar]
- Alfons, T.G.; Jana, L. Hermodynamically Stable Form of a Tosylate Salt. U.S. Patent Number US8877933B2, 27 August 2009. [Google Scholar]
- Carlisle, B.; Demko, N.; Freeman, G.; Hakala, A.; MacKinnon, N.; Ramsay, T.; Hey, S.; London, A.J.; Kimmelman, J. Benefit, Risk, and Outcomes in Drug Development: A Systematic Review of Sunitinib. J. Natl. Cancer Inst. 2015, 108, djv292. [Google Scholar] [CrossRef]
- Tang, P.C.; Miller, T.A.; Li, X.; Sun, L.; Wei, C.C.; Shirazian, S.; Liang, C.; Vojkovsky, T.; Nematalla, A.S.; Hawley, M. Pyrrole Substituted 2-Indolinone Protein Kinase Inhibitors. U.S. Patent Number US7125905B2, 11 August 2005. [Google Scholar]
- Tang, P.C.; Miller, T.A.; Li, X.; Sun, L.; Wei, C.C.; Shirazian, S.; Liang, C.; Vojkovsky, T.; Nematalla, A.S.; Hawley, M. Pyrrole Substituted 2-Indolinone Protein Kinase Inhibitors. U.S. Patent Number US6573293B2, 24 October 2002. [Google Scholar]
- Korashy, H.M.; Rahman, A.F.; Kassem, M.G. Dasatinib. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 205–237. [Google Scholar] [CrossRef]
- Das, J.; Padmanabha, R.; Chen, P.; Norris, D.J.; Doweyko, A.M.P.; Barrish, J.C.; Wityak, J. Cyclic Protein Tyrosine Kinase Inhibitors. U.S. Patent Number US6596746B1, 22 July 2003. [Google Scholar]
- Lajeunesse, J.; Dimarco, J.D.; Galella, M.; Chidambaram, R. Process for Preparing 2-Aminothiazole-5-Aromatic Carboxamides as Kinase Inhibitors. U.S. Patent Number US7491725B2, 17 February 2009. [Google Scholar]
- Gross-Goupil, M.; Bernhard, J.C.; Ravaud, A. Lapatinib and renal cell carcinoma. Expert Opin. Investig. Drugs 2012, 21, 1727–1732. [Google Scholar] [CrossRef]
- Carter, M.C.; Cockerill, G.S.; Lackey, K.E. Bicyclic Heteroaromatic Compounds as Protein Tyrosine Kinase Inhibitors. U.S. Patent Number US8513262B2, 20 September 2013. [Google Scholar]
- Mcclure, M.S.; Osterhout, M.H.; Roschangar, F.; Sacchetti, M.J. Quinazoline Ditosylate Salt Compounds. U.S. Patent Number US7157466B2, 2 January 2007. [Google Scholar]
- Bukowski, R.M. Temsirolimus: A safety and efficacy review. Expert Opin. Drug Saf. 2012, 11, 861–879. [Google Scholar] [CrossRef]
- Skotnicki, J.S.; Leone, C.L.; Schiehser, G.A. Rapamycin Hydroxyesters. U.S. Patent Number USRE44768E1, 18 February 2014. [Google Scholar]
- Guarini, A.; Minoia, C.; Giannoccaro, M.; Rana, A.; Iacobazzi, A.; Lapietra, A.; Raimondi, A.; Silvestris, N.; Gadaleta, C.D.; Ranieri, G. mTOR as a target of everolimus in refractory/relapsed Hodgkin lymphoma. Curr. Med. Chem. 2012, 19, 945–954. [Google Scholar] [CrossRef]
- Cottens, S.; Sedrani, R. O-alkylated Rapamycin Derivatives and Their Use, Particularly as Immunosuppressants. U.S. Patent Number US5665772A, 9 September 1997. [Google Scholar]
- Vaid, A. Nilotinib as first-line therapy for chronic myeloid leukemia. Indian J. Cancer 2011, 48, 438–445. [Google Scholar] [CrossRef]
- Breitenstein, W.; Furet, P.; Jacob, S.; Manley, P.W. Inhibitors of Tyrosine Kinases. U.S. Patent Number US7169791B2, 30 January 2007. [Google Scholar]
- Manley, P.W.; Shieh, W.C.; Sutton, P.A.; Karpinski, P.P.H.; Wu, R.R.; Monnier, S.M.; Brozio, J. Salts of 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-benzamide. U.S. Patent Number US8163904B2, 24 April 2012. [Google Scholar]
- Manley, P.W.; Shieh, W.C.; Sutton, P.A.; Karpinski, P.P.H.; Wu, R.R.; Monnier, S.M.; Brozio, J. Crystalline Forms of 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-benzamide. U.S. Patent Number US8415363B2, 9 April 2013. [Google Scholar]
- Schutz, F.A.; Choueiri, T.K.; Sternberg, C.N. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit. Rev. Oncol. Hematol. 2011, 77, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Boloor, A.; Cheung, M.; Davis, R.; Harris, P.A.; Hinkle, K.; Mook, R.A., Jr.; Stafford, J.A.; Veal, J.M. Pyrimidineamines as Angiogenesis Modulators. U.S. Patent Number US7105530B2, 12 September 2006. [Google Scholar]
- Boloor, A.; Cheung, M.; Hinkle, K.; Hinkle, K.; Veal, J.M.; Harris, P.A.; Mook, R.A., Jr.; Stafford, J.A. Chemical Compounds. U.S. Patent Number US8114885B2, 12 September 2012. [Google Scholar]
- Sim, M.W.; Cohen, M.S. The discovery and development of vandetanib for the treatment of thyroid cancer. Expert Opin. Drug Discov. 2014, 9, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Johnstone, C.; Clayton, E.; Stokes, E.S.E.; Lohmann, J.J.M.; Hennequin, L.F.A. Quinazoline Derivatives and Pharmaceutical Compositions Containing Them. U.S. Patent Number USRE42353E1, 10 May 2011. [Google Scholar]
- Kim, A.; Cohen, M.S. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert Opin. Drug Discov. 2016, 11, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.R.; Bollag, G.; Bremer, R.; Cho, H.; Hirth, K.P.; Ibrahim, P.N.; Tsai, J.; Zhang, C.; Zhang, J. Compounds and Methods for Kinase Modulation, and Indications Therefor. U.S. Patent Number US8143271B2, 27 March 2012. [Google Scholar]
- Roskoski, R., Jr. The preclinical profile of crizotinib for the treatment of non-small-cell lung cancer and other neoplastic disorders. Expert Opin. Drug Discov. 2013, 8, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Ia, L.; Kung, P.P.; Shen, H.; Tran, D.M.; Cui, J.J.; Funk, L.A.; Meng, J.J.; Nambu, M.D.; Pairish, M.A. Enantiomerically Pure Aminoheteroaryl Compounds as Protein Kinase Inhibitors. U.S. Patent Number US7858643B2, 28 December 2010. [Google Scholar]
- Cui, J.J.; Tran, D.M.B. Polymorphs of a c-MET/HGFR Inhibitor. U.S. Patent Number US8217057B2, 10 July 2012. [Google Scholar]
- Naqvi, K.; Verstovsek, S.; Kantarjian, H.; Ravandi, F. A potential role of ruxolitinib in leukemia. Expert Opin. Investig. Drugs 2011, 20, 1159–1166. [Google Scholar] [CrossRef]
- Rodgers, J.D.; Shepard, S. Heteroaryl Substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus Kinase Inhibitors. U.S. Patent Number US7598257B2, 6 October 2009. [Google Scholar]
- Li, H.Y.; Rodgers, J.D. Salts of the Janus kinase Inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile. U.S. Patent Number US8722693B2, 13 May 2014. [Google Scholar]
- Zakharia, Y.; Zakharia, K.; Rixe, O. Axitinib: From preclinical development to future clinical perspectives in renal cell carcinoma. Expert Opin. Drug Discov. 2015, 10, 925–935. [Google Scholar] [CrossRef]
- Bender, S.L.; Borchardt, A.J.; Collins, M.R.; Hua, Y.; Johnson, M.D.; Johnson, T.O., Jr.; Luu, H.T.; Palmer, C.L.; Reich, S.H.; Tempczyk-Russell, A.M.; et al. Indazole Compounds and Pharmaceutical Compositions for Inhibiting Protein Kinases, and Methods for Their Use. U.S. Patent Number US6534524B1, 18 March 2003. [Google Scholar]
- Campeta, A.M.; Chekal, B.P.; Singer, R.A. Crystalline Forms of 6-[2-(methylcarbamoyl) phenylsulfanyl]-3-E-[2-(pyridin-2-yl)ethenyondazole Suitable for the Treatment of Abnormal Cell Growth in Mammals. U.S. Patent Number US8791140B2, 29 July 2014. [Google Scholar]
- Quintás-Cardama, A.; Kantarjian, H.; Cortes, J. Bosutinib for the treatment of chronic myeloid leukemia in chronic phase. Drugs Today (Barc). 2012, 48, 177–188. [Google Scholar] [CrossRef]
- Berger, D.M.; Floyd, M.B.; Frost, P.; Hamann, P.R.; Tsou, H.R.; Wissner, A.; Zhang, N. Substituted 3-cyanoquinolines. U.S. Patent Number USRE42376E, 17 May 2011. [Google Scholar]
- Feigelson, G.; Strong, H.; Wen, H.; Tesconi, M.S. Crystalline Forms of 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3 Quinolinecarbonitrile and Methods of Preparing the Same. U.S. Patent Number US7767678B2, 3 August 2010. [Google Scholar]
- Miura, K.; Satoh, M.; Kinouchi, M.; Yamamoto, K.; Hasegawa, Y.; Philchenkov, A.; Kakugawa, Y.; Fujiya, T. The preclinical development of regorafenib for the treatment of colorectal cancer. Expert Opin. Drug Discov. 2014, 9, 1087–1101. [Google Scholar] [CrossRef]
- Boyer, S.; Dumas, J.; Riedl, B.; Wilhelm, S. Fluoro Substituted omega-carboxyaryl Diphenyl Urea for the Treatment and Prevention of Diseases and Conditions. U.S. Patent Number US8637553B2, 28 January 2014. [Google Scholar]
- Grunenberg, A.; Keil, B.; Stiehl, J.; Tenbieg, K. 4-[4-({[4-chloro-3-(trifluoromethyl) phenyl] carbamoyl} amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide Monohydrate. U.S. Patent Number US9957232B2, 1 May 2018. [Google Scholar]
- Kaur, K.; Kalra, S.; Kaushal, S. Systematic review of tofacitinib: A new drug for the management of rheumatoid arthritis. Clin. Ther. 2014, 36, 1074–1086. [Google Scholar] [CrossRef]
- Blumenkopf, T.A.; Flanagan, M.E.; Munchhof, M.J. Pyrrolo [2,3-d]pyrimidine Compounds. U.S. Patent Number USRE41783E, 28 September 2010. [Google Scholar]
- Flanagan, M.E.; Li, Z.J. Crystalline 3-{4-methyl-3-[methyl-(7H- pyrrolo [2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo-propionitrile Citrate. U.S. Patent Number US6965027B2, 15 November 2005. [Google Scholar]
- Grassi, P.; Verzoni, E.; Ratta, R.; Mennitto, A.; de Braud, F.; Procopio, G. Cabozantinib in the treatment of advanced renal cell carcinoma: Design, development, and potential place in the therapy. Drug Des. Devel. Ther. 2016, 10, 2167–2172. [Google Scholar] [CrossRef][Green Version]
- Chan, D.S.M.; Forsyth, T.P.; Khoury, R.G.; Leahy, J.W.; Mann, L.W.; Nuss, J.M.; Parks, J.J.; Wang, Y.; Xu, W.; Bannen, L.C.; et al. c-Met Modulators and Methods of Use. U.S. Patent Number US7579473B2, 28 May 2009. [Google Scholar]
- Brown, A.S.C.; Gallagher, W.P.; Lamb, P. (L)-malate Salt of N-(4-{[6,7-bis (methyloxy) quinolin-4-yl] oxy} phenyl)-N′-(4-fluorophenyl) cyclopropane-1, 1-dicarboxamide. U.S. Patent Number US8877776B2, 4 November 2014. [Google Scholar]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human malignancies. Onco. Targets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef]
- Dalgarno, D.C.; Huang, W.S.; Qi, J.; Sawyer, T.K.; Shakespeare, W.C.; Sundaramoorthi, R.; Wang, Y.; Zhu, X.; Zou, D.; Metcalf, C.A., III; et al. Substituted Acetylenic imidazo[1,2-b]pyridazine Compounds as Kinase Inhibitors. U.S. Patent Number US8114874B2, 14 February 2012. [Google Scholar]
- Chaber, J.J.; Murray, C.K.; Rozamus, L.W.; Sharma, P. Crystalline Forms of 3-(imidazo[1,2-b] pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl) methyl]-3-(trifluoromethyl)phenyl}benzamide and Its Mono Hydrochloride Salt. U.S. Patent Number US9493470B2, 15 November 2016. [Google Scholar]
- Jeanson, A.; Boyer, A.; Greillier, L.; Tomasini, P.; Barlesi, F. Therapeutic potential of trametinib to inhibit the mutagenesis by inactivating the protein kinase pathway in non-small cell lung cancer. Expert Rev. Anticancer Ther. 2019, 19, 11–17. [Google Scholar] [CrossRef]
- Abe, H.; Hayakawa, K.; Hori, Y.; Iida, T.; Kawasaki, H.; Kikuchi, S.; Kurachi, H.; Nanayama, T.; Sakai, T.; Takahashi, M.; et al. Pyrimidine Compound and Medical Use Thereof. U.S. Patent Number US7378423B2, 17 May 2008. [Google Scholar]
- Knispel, S.; Zimmer, L.; Kanaki, T.; Ugurel, S.; Schadendorf, D.; Livingstone, E. The safety and efficacy of dabrafenib and trametinib for the treatment of melanoma. Expert Opin. Drug Saf. 2018, 17, 73–87. [Google Scholar] [CrossRef]
- Rheault, T.R. Benzene Sulfonamide Thiazole and Oxazole Compounds. U.S. Patent Number US7994185B2, 9 August 2011. [Google Scholar]
- Brückl, W.; Tufman, A.; Huber, R.M. Advanced non-small cell lung cancer (NSCLC) with activating EGFR mutations: First-line treatment with afatinib and other EGFR TKIs. Expert Rev. Anticancer Ther. 2017, 17, 143–155. [Google Scholar] [CrossRef]
- Himmelsbach, F.; Blech, S.; Langkopf, E.; Jung, B.; Baum, A.; Solca, F. Quinazoline Derivatives and Pharmaceutical Compositions Containing Them. U.S. Patent Number USRE43431E1, 29 May 2012. [Google Scholar]
- Kulinna, C.; Rall, W.; Schnaubelt, J.; Sieger, P.; Soyka, R. Process for Preparing Amino Crotonyl Compounds. U.S. Patent Number US8426586B2, 23 April 2013. [Google Scholar]
- Liu, L.; Shi, B.; Wang, X.; Xiang, H. Strategies to overcome resistance mutations of Bruton’s tyrosine kinase inhibitor ibrutinib. Future Med. Chem. 2018, 10, 343–356. [Google Scholar] [CrossRef]
- Honigberg, L.; Pan, Z.; Verner, E. Inhibitors of Bruton’s Tyrosine Kinase. U.S. Patent Number US8735403B2, 27 May 2014. [Google Scholar]
- Goldman, E.; Purro, N.; Smyth, M.; Wirth, D.D. Crystalline Forms of a Bruton’s Tyrosine Kinase Inhibitor. U.S. Patent Number US9296753B2, 29 March 2016. [Google Scholar]
- De Pas, T.; Pala, L.; Catania, C.; Conforti, F. Molecular and clinical features of second-generation anaplastic lymphoma kinase inhibitors: Ceritinib. Future Oncol. 2017, 13, 2629–2644. [Google Scholar] [CrossRef]
- Michellys, P.Y.; Pei, W.; Marsilje, T.H.; Chen, B.; Uno, T. Compounds and Compositions as Protein Kinase Inhibitors. U.S. Patent Number US8039479B2, 18 October 2011. [Google Scholar]
- Feng, L.; Gong, B.; Karpinski, P.H.; Waykole, L.M. Crystalline Forms of 5-chloro-N2-(2-isopropoxy-5-methyl-4-piperidin-4-yl-phenyl)-N4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2, 4-diamine. U.S. Patent Number US9309229B2, 12 April 2016. [Google Scholar]
- Zirlik, K.; Veelken, H. Idelalisib. Recent Results Cancer Res. 2018, 212, 243–264. [Google Scholar] [CrossRef]
- Fowler, K.W.; Huang, D.; Kesicki, E.A.; Oliver, A.; Ooi, H.C.; Puri, K.D.; Ruan, F.; Treiberg, J. Quinazolinones as Inhibitors of Human Phosphatidylinositol 3-kinase Delta. U.S. Patent Number USRE44638E, 10 December 2013. [Google Scholar]
- Carra, E.; Evarts, J.B.; Gerber, M.; Shi, B.; Sujino, K.; Tran, D.; Wang, F. Polymorphic Forms of (S)-2-(1-(9H-purin-6-ylamino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one. U.S. Patent Number US9469643B2, 18 October 2016. [Google Scholar]
- Khalique, S.; Banerjee, S. Nintedanib in ovarian cancer. Expert Opin. Investig. Drugs 2017, 26, 1073–1081. [Google Scholar] [CrossRef]
- Heckel, A.; Hilberg, F.; Redemann, N.; Roth, G.J.; Spevak, W.; Tontsch, G.U.; Van, M.J.; Walter, R. Substituted Indolines which Inhibit Receptor Tyrosine Kinases. U.S. Patent Number US6762180B1, 13 July 2004. [Google Scholar]
- Bock, T.; Hilberg, F.; Linz, G.; Rall, W.; Roth, G.J.; Sieger, P. 3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulphonate and the Use Thereof as a Pharmaceutical Composition. U.S. Patent Number US7119093B2, 10 October 2006. [Google Scholar]
- de Dueñas, E.M.; Gavila-Gregori, J.; Olmos-Antón, S.; Santaballa-Bertrán, A.; Lluch-Hernández, A.; Espinal-Domínguez, E.J.; Rivero-Silva, M.; Llombart-Cussac, A. Preclinical and clinical development of palbociclib and future perspectives. Clin. Transl. Oncol. 2018, 20, 1136–1144. [Google Scholar] [CrossRef]
- Barvian, M.; Booth, R.J.; Quinn, J., III; Repine, J.T.; Sheehan, D.J.; Toogood, P.L.; Vanderwel, S.N.; Zhou, H. 2-(pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones. U.S. Patent Number USRE47739E, 26 November 2019. [Google Scholar]
- Chekal, B.P.; Ide, N.D. Solid Forms of a Selective CDK4/6 Inhibitor. U.S. Patent Number US10723730B2, 28 July 2020. [Google Scholar]
- Capozzi, M.; De Divitiis, C.; Ottaiano, A.; von Arx, C.; Scala, S.; Tatangelo, F.; Delrio, P.; Tafuto, S. Lenvatinib, a molecule with versatile application: From preclinical evidence to future development in anti-cancer treatment. Cancer Manag. Res. 2019, 11, 3847–3860. [Google Scholar] [CrossRef]
- Arimoto, I.; Fukuda, Y.; Funahashi, Y.; Haneda, T.; Kamat, J.; Matsui, J.; Matsui, K.; Matsukura, M.; Matsushima, T.; Mimura, F.; et al. Nitrogen-Containing Aromatic Derivatives. U.S. Patent Number US7253286B2, 7 August 2007. [Google Scholar]
- Matsushima, T.; Arimoto, I.; Ayata, Y.; Gotoda, M.; Kamada, A.; Nakamura, T.; Sakaguchi, T.; Suzuki, N.; Yoshizawa, K. Crystalline Form of the Salt of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide or the Solvate of the Salt and a Process for Preparing the Same. U.S. Patent Number US7612208B2, 3 November 2009. [Google Scholar]
- Eagles, J.R.; Jimeno, A. Cobimetinib: Inhibiting MEK1/2 in BRAF V600-mutant melanoma. Drugs Today (Barc) 2016, 52, 593–605. [Google Scholar] [CrossRef]
- Aay, N.; Anand, N.K.; Blazey, C.M.; Bowles, O.J.; Bussenius, J.; Costanzo, S.; Curtis, J.K.; Defina, S.C.; Dubenko, L.; Joshi, A.A.; et al. Azetidines as MEK Inhibitors for the Treatment of Proliferative Diseases. U.S. Patent Number US7803839B2, 28 September 2010. [Google Scholar]
- Brown, A.C. Crystalline Fumarate Salt of (S)-[3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl] [3-hydroxy-3-(piperidin-2-yl) azetidin-1-yl]methanone. U.S. Patent Number US10590102B2, 17 March 2020. [Google Scholar]
- Santarpia, M.; Liguori, A.; Karachaliou, N.; Gonzalez-Cao, M.; Daffinà, M.G.; D’Aveni, A.; Marabello, G.; Altavilla, G.; Rosell, R. Osimertinib in the treatment of non-small-cell lung cancer: Design, development and place in therapy. Lung Cancer (Auckl) 2017, 8, 109–125. [Google Scholar] [CrossRef]
- Butterworth, S.; Finlay, M.R.V.; Redfearn, H.M.; Ward, R.A. 2-(2,4,5-substituted-anilino) pyrimidine Compounds. U.S. Patent Number US8946235B2, 3 February 2015. [Google Scholar]
- Srinivasamaharaj, S.; Salame, B.K.; Rios-Perez, J.; Kloecker, G.; Perez, C.A. The role of alectinib in the treatment of advanced ALK-rearranged non-small-cell lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 1227–1233. [Google Scholar] [CrossRef]
- Asoh, K.; Emura, T.; Furuichi, N.; Hong, W.; Ishii, N.; Ito, T.; Kawada, H.; Kinoshita, K.; Morikami, K.; Oikawa, N.; et al. Tetracyclic Compound. U.S. Patent Number US9126931B2, 8 September 2015. [Google Scholar]
- Curigliano, G.; Criscitiello, C.; Esposito, A.; Intra, M.; Minucci, S. Pharmacokinetic drug evaluation of ribociclib for the treatment of metastatic, hormone-positive breast cancer. Expert Opin. Drug Metab. Toxicol. 2017, 13, 575–581. [Google Scholar] [CrossRef]
- Brain, C.T.; Sung, M.J.E.; Lagu, B. Pyrrolopyrimidine Compounds and Their Uses. U.S. Patent Number US8415355B2, 9 April 2013. [Google Scholar]
- Calienni, J.V.; Chen, G.P.; Gong, B.; Kapa, P.K.; Saxena, V. Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic Acid Dimethylamide and Processes of Making Thereof. U.S. Patent Number US9193732B2, 24 November 2015. [Google Scholar]
- Ali, R.; Arshad, J.; Palacio, S.; Mudad, R. Brigatinib for ALK-positive metastatic non-small-cell lung cancer: Design, development and place in therapy. Drug Des. Devel. Ther. 2019, 13, 569–580. [Google Scholar] [CrossRef]
- Dalgarno, D.C.; Huang, W.S.; Li, F.; Liu, S.; Qi, J.; Romero, J.A.C.; Shakespeare, W.C.; Thomas, R.M.; Wang, Y.; Zhu, X.; et al. Phosphorous Derivatives as Kinase Inhibitors. U.S. Patent Number US9012462B2, 21 April 2015. [Google Scholar]
- Rozamus, L.W.; Sharma, P. Crystalline Forms of 5-chloro-N4-[-2 (dimethylphosphoryl)phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl) piperidin-1-yl]phenyl}pyrimidine-2,4-diamine. U.S. Patent Number US10385078B2, 20 August 2019. [Google Scholar]
- Kim, E.S. Midostaurin: First Global Approval. Drugs 2017, 77, 1251–1259. [Google Scholar] [CrossRef]
- Caravatti, G.; Fredenhagen, A. Staurosporine Derivatives Substituted at Methylamino Nitrogen. U.S. Patent Number US5093330A, 3 March 1992. [Google Scholar]
- Griffin, J.D.; Manley, P.W. Staurosporine Derivatives as Inhibitors of FLT3 Receptor Tyrosine Kinase Activity. U.S. Patent Number US7973031B2, 5 July 2011. [Google Scholar]
- Deeks, E.D. Neratinib: First Global Approval. Drugs 2017, 77, 1695–1704. [Google Scholar] [CrossRef]
- Rabindran, S.K.; Tsou, H.R.; Wissner, A. Protein Tyrosine Kinase Enzyme Inhibitors. U.S. Patent Number US7399865B2, 15 July 2008. [Google Scholar]
- Markham, A. Copanlisib: First Global Approval. Drugs 2017, 77, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Bullion, A.M.; Campbell, A.M.; Hentemann, M.; Michels, M.; Redman, A.; Rowley, B.R.; Scott, W.; Wood, J. Substituted 2,3-dihydroimidazo[1,2-c]quinazoline Derivatives Useful for Treating Hyper-Proliferative Disorders and Diseases Associated with Angiogenesis. U.S. Patent Number USRE46856E, 22 May 2018. [Google Scholar]
- Militzer, H.C.; Müller, H.; Peters, J.G. Substituted 2,3-dihydroimidazo[1,2-c]quinazoline Salts. U.S. Patent Number US10383876B2, 20 August 2019. [Google Scholar]
- Kim, E.S. Abemaciclib: First Global Approval. Drugs 2017, 77, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- De Dios, M.A.; De Prado, G.A.; Filadelfa, D.P.C.M.; Garcia, P.M.C.; Gelbert, L.M.; Knobeloch, J.M.; Martin, D.L.N.E.M.; Martin, O.F.M.D.; Martinez, P.J.A. Protein Kinase Inhibitors. U.S. Patent Number US7855211B2, 21 December 2010. [Google Scholar]
- Markham, A.; Dhillon, S. Acalabrutinib: First Global Approval. Drugs 2018, 78, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Barf, T.A.; Man, P.A.D.A.; Oubrie, A.A.; Rewinkel, J.B.M.; Sterrenburg, J.G.; Jans, C.G.J.M.; Raaijmakers, H. 4-imidazopyridazin-1-yl-benzamides and 4-imidazotriazin-1-yl-benzamides as Btk Inhibitors. U.S. Patent Number US9290504B2, 22 March 2016. [Google Scholar]
- Aret, E.; Barf, T.; Blatter, F.; Evarts, J.; Ingallinera, T.; Krejsa, C. Crystal forms of (S)-4-(8-amino-3-(1-(but-2-ynoyl) pyrrolidin-2-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(pyridin-2-yl)benzamide. U.S. Patent Number US9796721B2, 24 October 2017. [Google Scholar]
- Lin, C.W.; Sherman, B.; Moore, L.A.; Laethem, C.L.; Lu, D.W.; Pattabiraman, P.P.; Rao, P.V.; deLong, M.A.; Kopczynski, C.C. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Elong, M.A.; Royalty, S.M.; Sturdivant, J.M. Dual Mechanism Inhibitors for the Treatment of Disease. U.S. Patent Number US8394826B2, 12 March 2013. [Google Scholar]
- Kopczynski, C.; Lin, C.W.; Sturdivant, J.M.; deLong, M.A. Combination Therapy. U.S. Patent Number US9415043B2, 16 August 2016. [Google Scholar]
- Markham, A. Baricitinib: First Global Approval. Drugs 2017, 77, 697–704. [Google Scholar] [CrossRef]
- Rodgers, J.D.; Shepard, S. Azetidine and Cyclobutane Derivatives as JAK Inhibitors. U.S. Patent Number US8158616B2, 17 April 2012. [Google Scholar]
- Tran, B.; Cohen, M.S. The discovery and development of binimetinib for the treatment of melanoma. Expert Opin. Drug Discov. 2020, 15, 745–754. [Google Scholar] [CrossRef]
- Wallace, E.M.; Lyssikatos, J.P.; Marlow, A.L.; Hurley, T.B. N3 Alkylated Benzimidazole Derivatives as MEK Inhibitors. U.S. Patent Number US7777050B2, 17 August 2010. [Google Scholar]
- Rell, C.M.; Liu, W.; Misun, M.; Nichols, P.; Niederer, D.A.; Pachinger, W.H.; Stengel, P.J.; Wolf, M.C.; Zimmermann, D. Preparation of and Formulation Comprising a MEK Inhibitor. U.S. Patent Number US9562016B2, 7 February 2017. [Google Scholar]
- Shirley, M. Dacomitinib: First Global Approval. Drugs 2018, 78, 1947–1953. [Google Scholar] [CrossRef]
- Fakhoury, S.A.; Lee, H.T.; Reed, J.E.; Schlosser, K.M.; Sexton, K.E.; Tecle, H.; Winters, R.T. 4-phenylamino-quinazolin-6-yl-amides. U.S. Patent Number US7772243B2, 10 August 2010. [Google Scholar]
- Koelblinger, P.; Thuerigen, O.; Dummer, R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr. Opin. Oncol. 2018, 30, 125–133. [Google Scholar] [CrossRef]
- Huang, S.; Jin, X.; Liu, Z.; Poon, D.; Tellew, J.; Wan, Y.; Wang, X.; Xie, Y. Compounds and Compositions as Protein Kinase Inhibitors. U.S. Patent Number US8501758B2, 6 August 2013. [Google Scholar]
- Markham, A. Fostamatinib: First Global Approval. Drugs 2018, 78, 959–963. [Google Scholar] [CrossRef]
- Bhamidipati, S.; Singh, R.; Stella, V.J.; Sun, T. Prodrugs of 2,4-pyrimidinediamine Compounds and Their Uses. U.S. Patent Number US7449458B2, 11 November 2008. [Google Scholar]
- Bhamidipati, S.; Masuda, E.; Singh, R.; Sun, T. Prodrugs of 2,4-pyrimidinediamine Compounds and Their Uses. U.S. Patent Number US8163902B2, 24 April 2012. [Google Scholar]
- Blair, H.A. Duvelisib: First Global Approval. Drugs 2018, 78, 1847–1853. [Google Scholar] [CrossRef]
- Chan, K.; Li, L.; Liu, Y.; Ren, P.; Rommel, C.; Wilson, T.E. Substituted Isoquinolin-1(2H)-ones, and Methods of Use Thereof. U.S. Patent Number US8193182B2, 5 June 2012. [Google Scholar]
- Isbester, P.; Kropp, J.; Lane, B.S.; Michael, M.; Pingda, R. Processes for Preparing Isoquinolinones and Solid Forms of Isoquinolinones. U.S. Patent Number USRE46621E, 5 December 2017. [Google Scholar]
- Dhillon, S. Gilteritinib: First Global Approval. Drugs 2019, 79, 331–339. [Google Scholar] [CrossRef]
- Kazuhiko, I.; Yoshinori, I.; Akio, K.; Yutaka, K.; Kazuo, K.; Takahiro, M.; Itsuro, S.; Hiroshi, T. Diamino Heterocyclic Carboxamide Compound. U.S. Patent Number US8969336B2, 3 March 2015. [Google Scholar]
- Scott, L.J. Larotrectinib: First Global Approval. Drugs 2019, 79, 201–206. [Google Scholar] [CrossRef]
- Andrews, S.W.; Haas, J.; Jiang, Y.; Zhang, G. Method of treatment using substituted pyrazolo[1,5-a] pyrimidine compounds. U.S. Patent Number US9127013B2, 8 September 2015. [Google Scholar]
- Alisha, B.; Juengst, D.; Shah, K. Crystalline Form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide Hydrogen Sulfate. U.S. Patent Number US10172861B2, 8 January 2019. [Google Scholar]
- Syed, Y.Y. Lorlatinib: First Global Approval. Drugs 2019, 79, 93–98. [Google Scholar] [CrossRef]
- Bailey, S.; Burke, B.J.; Collins, M.R.; Cui, J.J.; Deal, J.G.; Hoffman, R.L.; Huang, Q.; Johnson, T.W.; Kania, R.S.; Kath, J.C. Macrocyclic Derivatives for the Treatment of Diseases. U.S. Patent Number US8680111B2, 25 March 2014. [Google Scholar]
- Birch, M.J.; Pencheva, K.D. Crystalline Form of Lorlatinib Free Base. U.S. Patent Number US10420749B2, 24 September 2019. [Google Scholar]
- Al-Salama, Z.T.; Keam, S.J. Entrectinib: First Global Approval. Drugs 2019, 79, 1477–1483. [Google Scholar] [CrossRef]
- Lombardi, B.A.; Marchionni, C.; Menichincheri, M.; Nesi, M.; Orsini, P.; Panzeri, A.; Perrone, E.; Vanotti, E. Substituted Indazole Derivatives Active as Kinase Inhibitors. U.S. Patent Number US8299057B2, 30 October 2012. [Google Scholar]
- Candiani, I.; Ottaiano, G.; Tomasi, A. Crystalline Form of N-[5-(3,5-difluoro-benzyl)-1H-indazol-3-yl]-4-(4-methyl-piperazin-1-yl)-2-(tetrahydro-pyran-4-ylamino)-benzamide. U.S. Patent Number US10738037B2, 11 August 2020. [Google Scholar]
- Duggan, S.; Keam, S.J. Upadacitinib: First Approval. Drugs 2019, 79, 1819–1828. [Google Scholar] [CrossRef]
- Frank, K.E.; Friedman, M.; George, D.M.; Stewart, K.D.; Wallace, G.A.; Wishart, N. Tricyclic Compounds. U.S. Patent Number USRE47221E, 5 February 2019. [Google Scholar]
- Allian, A. Processes for the Preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-alpha]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and Solid State Forms Thereof. U.S. Patent Number US9951080B2, 24 April 2018. [Google Scholar]
- Markham, A. Alpelisib: First Global Approval. Drugs 2019, 79, 1249–1253. [Google Scholar] [CrossRef]
- Caravatti, G.; Fairhurst, R.A.; Furet, P.; Guagnano, V.; Imbach, P. Pyrrolidine-1,2-dicarboxamide Derivatives. U.S. Patent Number US8227462B2, 24 July 2012. [Google Scholar]
- Hanna, K.S. Erdafitinib to treat urothelial carcinoma. Drugs Today (Barc.) 2019, 55, 495–501. [Google Scholar] [CrossRef]
- Akkari, R.; Berdini, V.; Besong, G.E.; Embrechts, W.C.J.; Freyne, E.J.E.; Gilissen, R.A.H.J.; Hamlett, C.C.F.; Johnson, C.N.; Lacrampe, J.F.A.; Meerpoel, L.; et al. Pyrazolyl Quinoxaline Kinase Inhibitors. U.S. Patent Number US8895601B2, 25 November 2014. [Google Scholar]
- Monestime, S.; Lazaridis, D. Pexidartinib (TURALIO™): The first FDA-indicated systemic treatment for Tenosynovial Giant Cell Tumor. Drugs R D 2020, 20, 189–195. [Google Scholar] [CrossRef]
- Bremer, R.; Ibrahim, P.N.; Zhang, J. Compounds Modulating c-fms and/or c-kit Activity and Uses Therefor. U.S. Patent Number US9169250B2, 27 October 2015. [Google Scholar]
- Ibrahim, P.N.; Visor, G.C. Solid Forms of a Compound Modulating Kinases. U.S. Patent 2017. [Google Scholar]
- Blair, H.A. Fedratinib: First Approval. Drugs 2019, 79, 1719–1725. [Google Scholar] [CrossRef]
- Cao, J.; Hood, J.D.; Lohse, D.L.; Mcpherson, A.; Noronha, G.; Pathak, V.P.; Renick, J.; Soll, R.M.; Zeng, B.; Mak, C.C. Bi-aryl meta-pyrimidine Inhibitors of Kinases. U.S. Patent Number US7528143B2, 5 May 2009. [Google Scholar]
- Syed, Y.Y. Zanubrutinib: First Approval. Drugs 2020, 80, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z. Substituted pyrazolo[1,5-a]pyrimidines as Bruton’s Tyrosine Kinase Modulators. U.S. Patent Number US9447106B2, 20 September 2016. [Google Scholar]
- Dhillon, S. Avapritinib: First Approval. Drugs 2020, 80, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Hodous, B.L.; Kim, J.L.; Wilson, D.; Wilson, K.J.; Zhang, Y. Compositions Useful for Treating Disorders Related to Kit. U.S. Patent Number US9944651B2, 17 April 2018. [Google Scholar]
- Markham, A.; Keam, S.J. Selumetinib: First Approval. Drugs 2020, 80, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.M.; Lyssikatos, J.P.; Marlow, A.L.; Hurley, T.B. N3 Alkylated Benzimidazole Derivatives as MEK Inhibitors. U.S. Patent Number US7425637B2, 16 September 2008. [Google Scholar]
- Chuang, T.H.; Demattei, J.; Dickinson, P.A.; Ford, J.G.; Pervez, M.; Roberts, R.J.; Sharma, S.G.; Squire, C.J.; Storey, R.A. Hydrogen Sulfate Salt. U.S. Patent Number US9156795B2, 13 October 2015. [Google Scholar]
- Hoy, S.M. Pemigatinib: First Approval. Drugs 2020, 80, 923–929. [Google Scholar] [CrossRef]
- He, C.; Lu, L.; Wu, L.; Yao, W.; Zhang, C. Substituted Tricyclic Compounds as FGFR Inhibitors. U.S. Patent Number US9611267B2, 4 April 2017. [Google Scholar]
- Lee, A. Tucatinib: First Approval. Drugs 2020, 80, 1033–1038. [Google Scholar] [CrossRef]
- Greschuk, J.M.; Hennings, D.D.; Liu, W.; Lyssikatos, J.P.; Marmsaeter, F.P.; Zhao, Q. N4-phenyl-quinazoline-4-amine Derivatives and Related Compounds as ErbB type I Receptor Tyrosine Kinase Inhibitors for the Treatment of Hyperproliferative Diseases. U.S. Patent Number US8648087B2, 11 February 2014. [Google Scholar]
- Dhillon, S. Capmatinib: First Approval. Drugs 2020, 80, 1125–1131. [Google Scholar] [CrossRef]
- He, C.; Metcalf, B.; Qian, D.Q.; Xu, M.; Yao, W.; Zhang, C.; Zhuo, J. Imidazotriazines and Imidazopyrimidines as Kinase Inhibitors. U.S. Patent Number US7767675B2, 3 August 2010. [Google Scholar]
- Liu, P.; Pan, Y.; Qiao, L.; Weng, L.; Zhou, J. Salts of 2-fluoro-N-methyl-4-[7-(quinolin-6-yl-methyl)-imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide and Processes Related to Preparing the Same. U.S. Patent Number US8420645B2, 16 April 2013. [Google Scholar]
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef]
- Andrews, S.W.; Aronow, S.; Blake, J.F.; Brandhuber, B.J.; Cook, A.; Haas, J.; Jiang, Y.; Kolakowski, G.R.; McFaddin, E.A.; McKenney, M.L.; et al. Substituted Pyrazolo[1,5-a]pyridine Compounds as RET Kinase Inhibitors. U.S. Patent Number US10112942B2, 30 October 2018. [Google Scholar]
- Metcalf, A.T.; Fry, D.; McFaddin, E.A.; Kolakowski, G.R.; Haas, J.; Tang, T.P.; Jiang, Y. Crystalline Forms. U.S. Patent Number US10584124B2, 10 March 2020. [Google Scholar]
- Dhillon, S. Ripretinib: First Approval. Drugs 2020, 80, 1133–1138. [Google Scholar] [CrossRef]
- Flynn, D.L.; Kaufman, M.D.; Petillo, P.A. Dihydronaphthyridines and Related Compounds Useful as Kinase Inhibitors for the Treatment of Proliferative Diseases. U.S. Patent Number US8461179B1, 11 June 2013. [Google Scholar]
- Markham, A. Pralsetinib: First Approval. Drugs 2020, 80, 1865–1870. [Google Scholar] [CrossRef]
- Brubaker, J.D.; DiPietro, L.V.; Kim, J.L.; Wilson, D.W.; Wilson, K.J. Inhibitors of RET. U.S. Patent Number US10030005B2, 24 July 2018. [Google Scholar]
- Dhillon, S. Trilaciclib: First Approval. Drugs 2021, 81, 867–874. [Google Scholar] [CrossRef]
- Strum, J.C.; Tavares, F.X. CDK Inhibitors. U.S. Patent Number US8598186B2, 3 December 2013. [Google Scholar]
- Markham, A. Tepotinib: First Approval. Drugs 2020, 80, 829–833. [Google Scholar] [CrossRef]
- Dorsch, D.; Steiber, F.; Schadt, O.; Blaukat, A. Pyridazinone Derivatives. U.S. Patent Number US8580781B2, 12 November 2013. [Google Scholar]
- Schadt, O.; Dorsch, D.; Steiber, F.; Blaukat, A. Pyrimidinyl Pyridazinone Derivatives. U.S. Patent Number US8329692B2, 11 December 2012. [Google Scholar]
- Dhillon, S.; Keam, S.J. Umbralisib: First Approval. Drugs 2021, 81, 857–866. [Google Scholar] [CrossRef]
- Muthuppalaniappan, M.; Nagarathnam, D.; Vakkalanka, S.K. Selective PI3K Delta Inhibitors. U.S. Patent 2020. [Google Scholar]
- Vakkalanka, S.K. Forms of a PI3K Delta Selective Inhibitor for Use in Pharmaceutical Formulations. U.S. Patent 2019. [Google Scholar]
- Tivozanib Hydrochloride. Am. J. Health Syst. Pharm. 2021, zxab199. [CrossRef]
- Kubo, K.; Sakai, T.; Nagao, R.; Fujiwara, Y.; Isoe, T.; Hasegawa, K. Quinoline Derivatives and Quinazoline Derivatives Having Azolyl Group. U.S. Patent Number US6821987B2, 23 November 2004. [Google Scholar]
- Kubo, K.; Sakai, T.; Nagao, R.; Fujiwara, Y.; Isoe, T.; Hasegawa, K. Quinoline Derivatives and Quinazoline Derivatives Having Azolyl Group. U.S. Patent Number US7211587B2, 1 May 2007. [Google Scholar]
- Matsunaga, N.; Yoshida, S.; Yoshino, A.; Nakajima, T. N-{2-chloro-4-[(6,7-dimethoxy-4-quinolyl)oxy]phenyl}-N′-(5-methyl-3-isoxazolyl)urea Salt in Crystalline Form. U.S. Patent Number US7166722B2, 23 January 2007. [Google Scholar]
- Botrus, G.; Raman, P.; Oliver, T.; Bekaii-Saab, T. Infigratinib (BGJ398): An investigational agent for the treatment of FGFR-altered intrahepatic cholangiocarcinoma. Expert Opin. Investig. Drugs 2021, 30, 309–316. [Google Scholar] [CrossRef]
- Ding, Q.; Gray, N.S.; Li, B.; Liu, Y.; Sim, T.; Uno, T.; Zhang, G.; Soldermann, C.P.; Breitenstein, W.; Bold, G.; et al. Compounds and Compositions as Protein Kinase Inhibitors. U.S. Patent Number US8552002B2, 8 October 2013. [Google Scholar]
- Berghausen, J.; Kapa, P.K.; McKenna, J.; Slade, J.; Wu, R.; Du, Z.; Stowasswer, F. Crystalline Forms of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl) -phenylamino]-pyrimidin-4-yl}-1-methyl-urea and Salts Thereof. U.S. Patent Number US9067896B2, 30 June 2015. [Google Scholar]
- Kanev, G.K.; de Graaf, C.; de Esch, I.J.P.; Leurs, R.; Würdinger, T.; Westerman, B.A.; Kooistra, A.J. The Landscape of Atypical and Eukaryotic Protein Kinases. Trends Pharmacol. Sci. 2019, 40, 818–832. [Google Scholar] [CrossRef]
- 2020 Medicines in Development–Cancer. Available online: https://www.phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/MID-Reports/MID-Cancer2020_Product-List_FINAL.pdf (accessed on 31 May 2021).
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 1–19. [Google Scholar] [CrossRef]
- Couillaud, B.M.; Espeau, P.; Mignet, N.; Corvis, Y. State of the art of pharmaceutical solid forms: From crystal property issues to nanocrystals formulation. Chem. Med. Chem. 2019, 14, 8–23. [Google Scholar] [CrossRef]
- Smalley, K.S.M. Pharmacological research and cancer: A call to arms. Pharmacol. Res. 2019, 146, 104291. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Fojo, T.; Mathisen, M.; Zwelling, L.A. Cancer drugs in the United States: Justum Pretium--the just price. J. Clin. Oncol. 2013, 31, 3600–3604. [Google Scholar] [CrossRef]
- Mishuk, A.U.; Fasina, I.; Qian, J. Impact of U.S. federal and state generic drug policies on drug use, spending, and patient outcomes: A systematic review. Res. Soc. Adm. Pharm. 2020, 16, 736–745. [Google Scholar] [CrossRef]

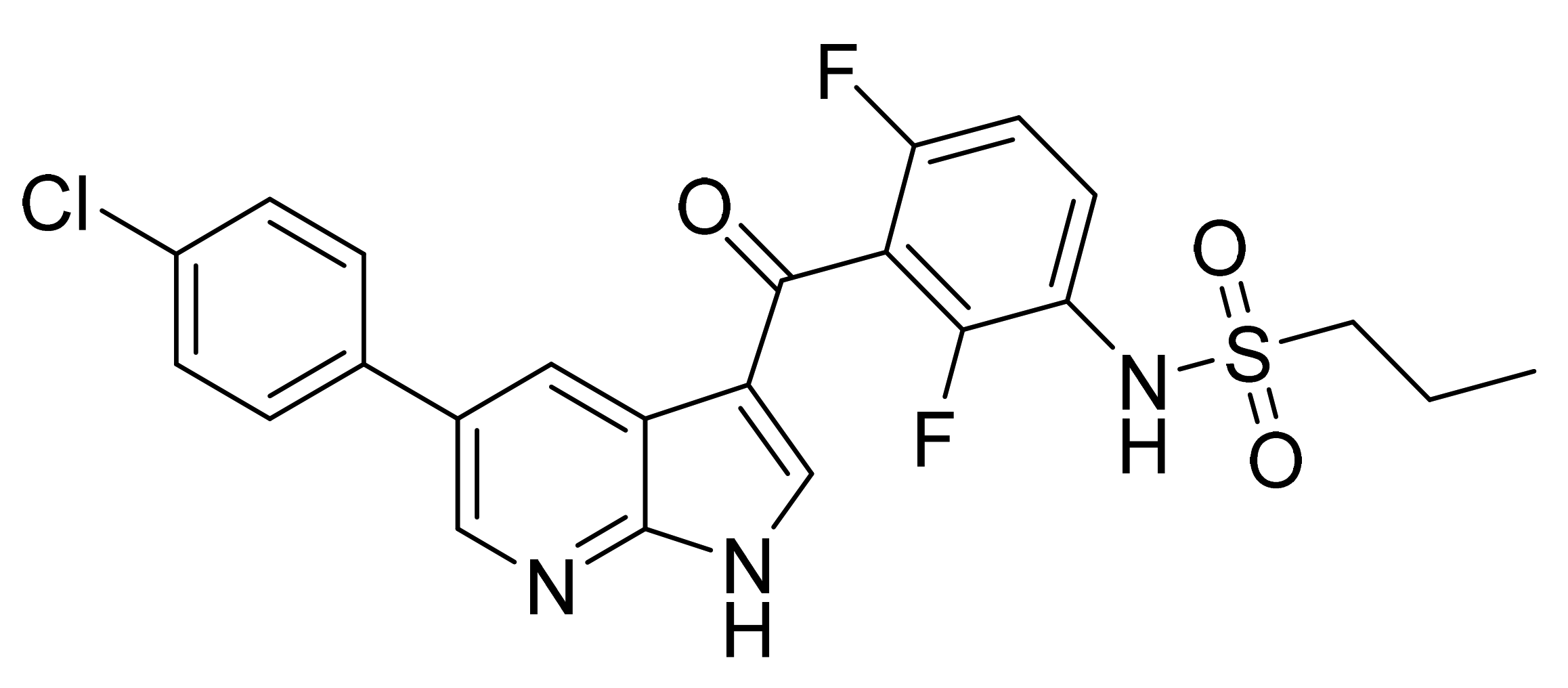
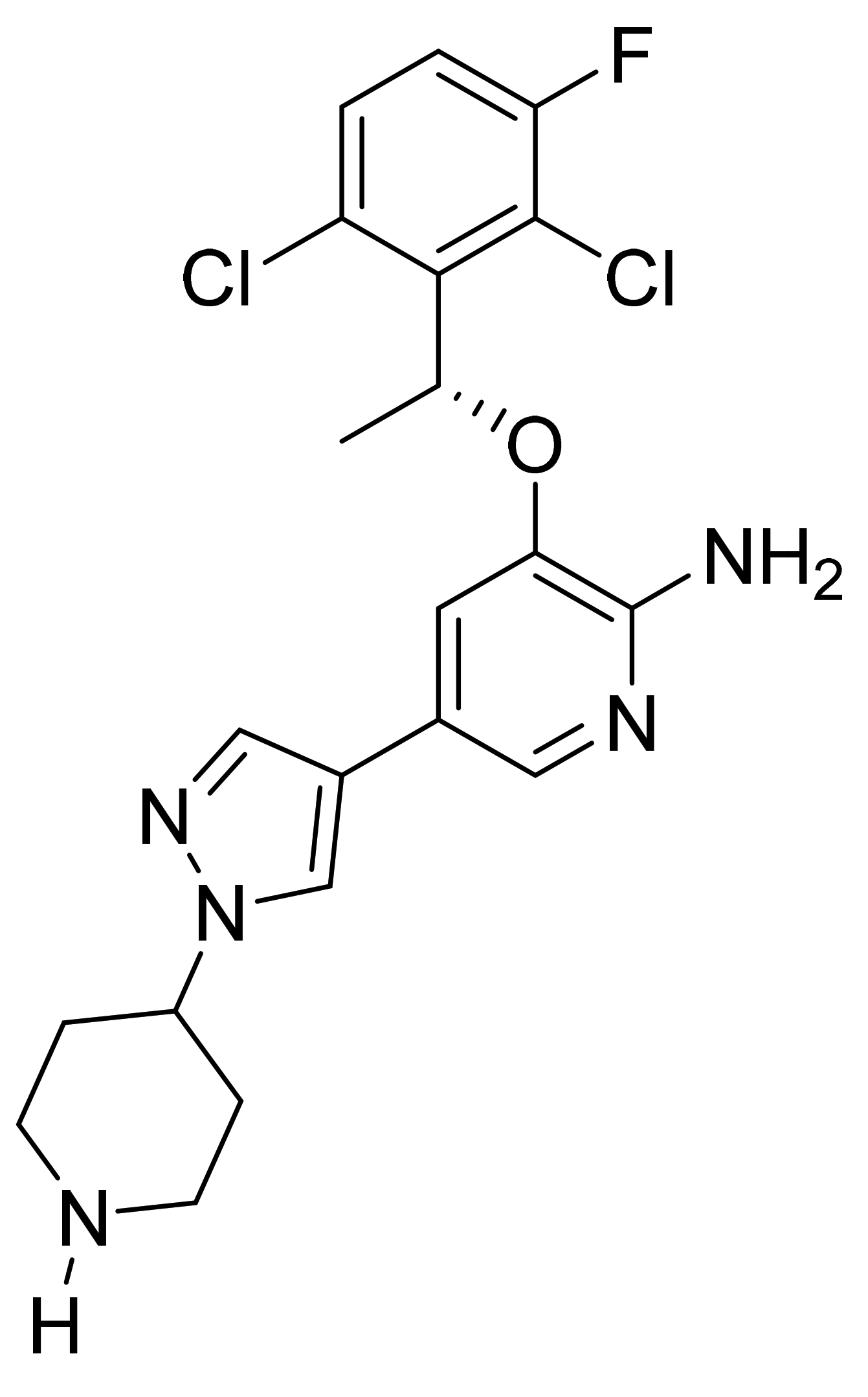
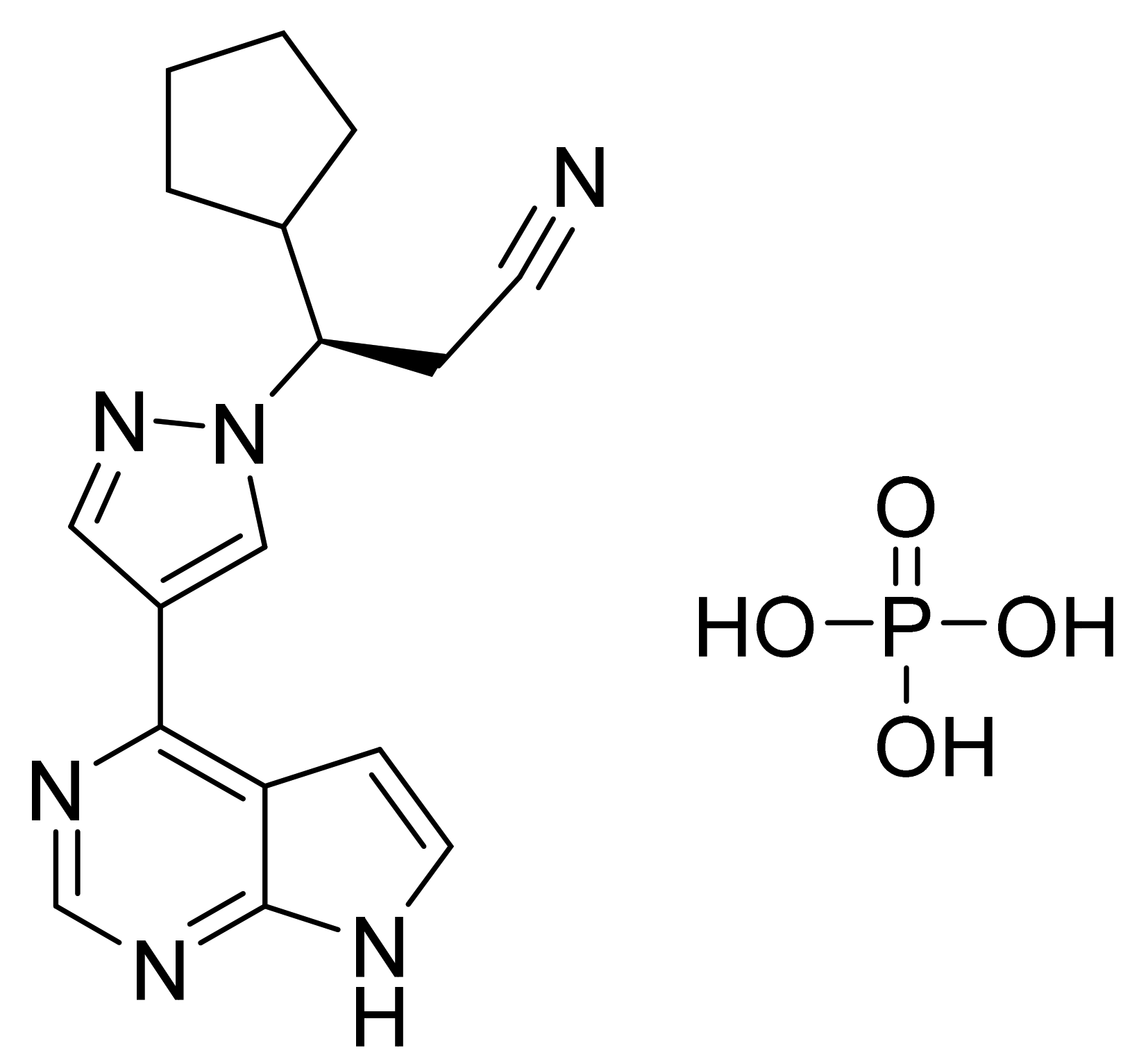
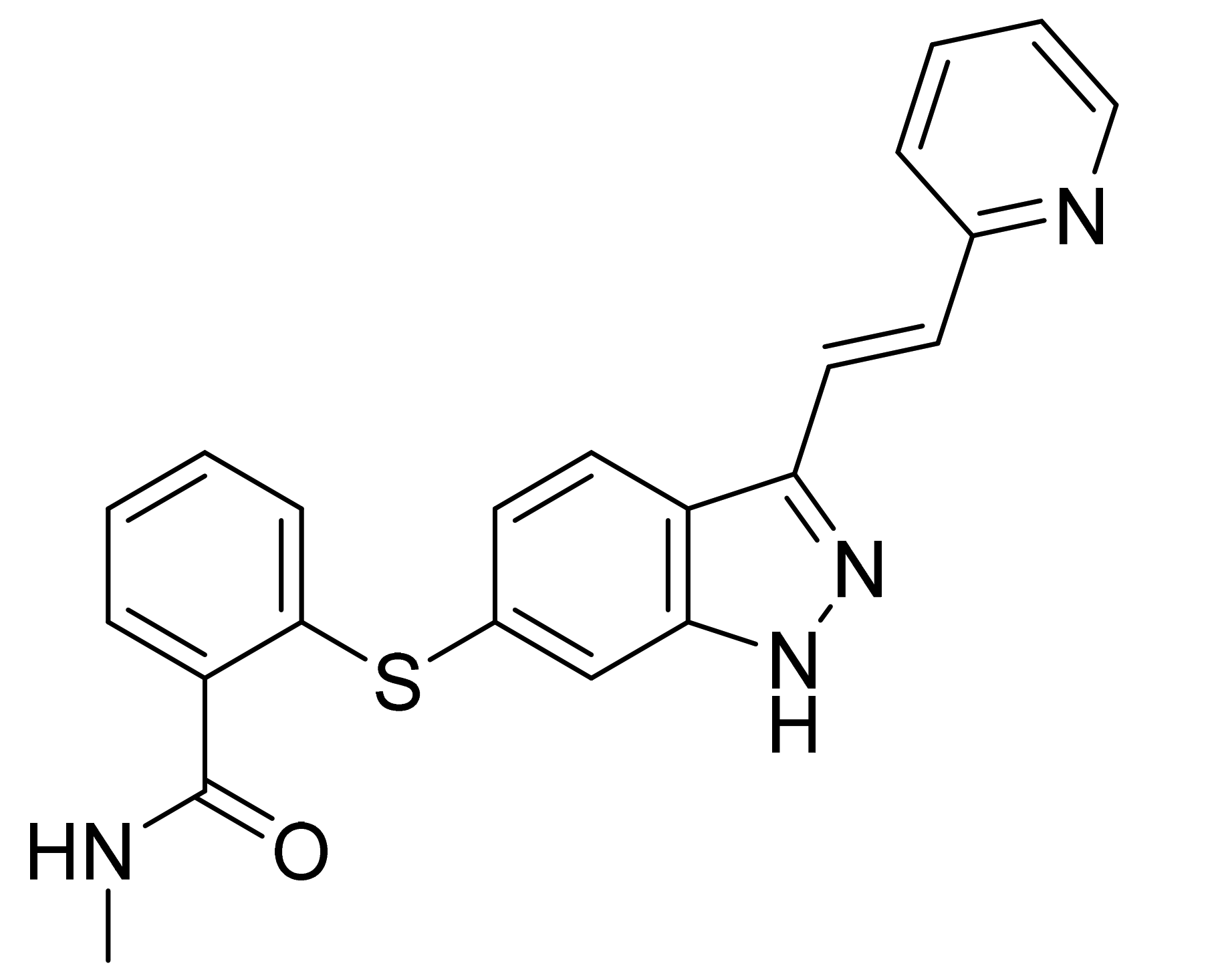
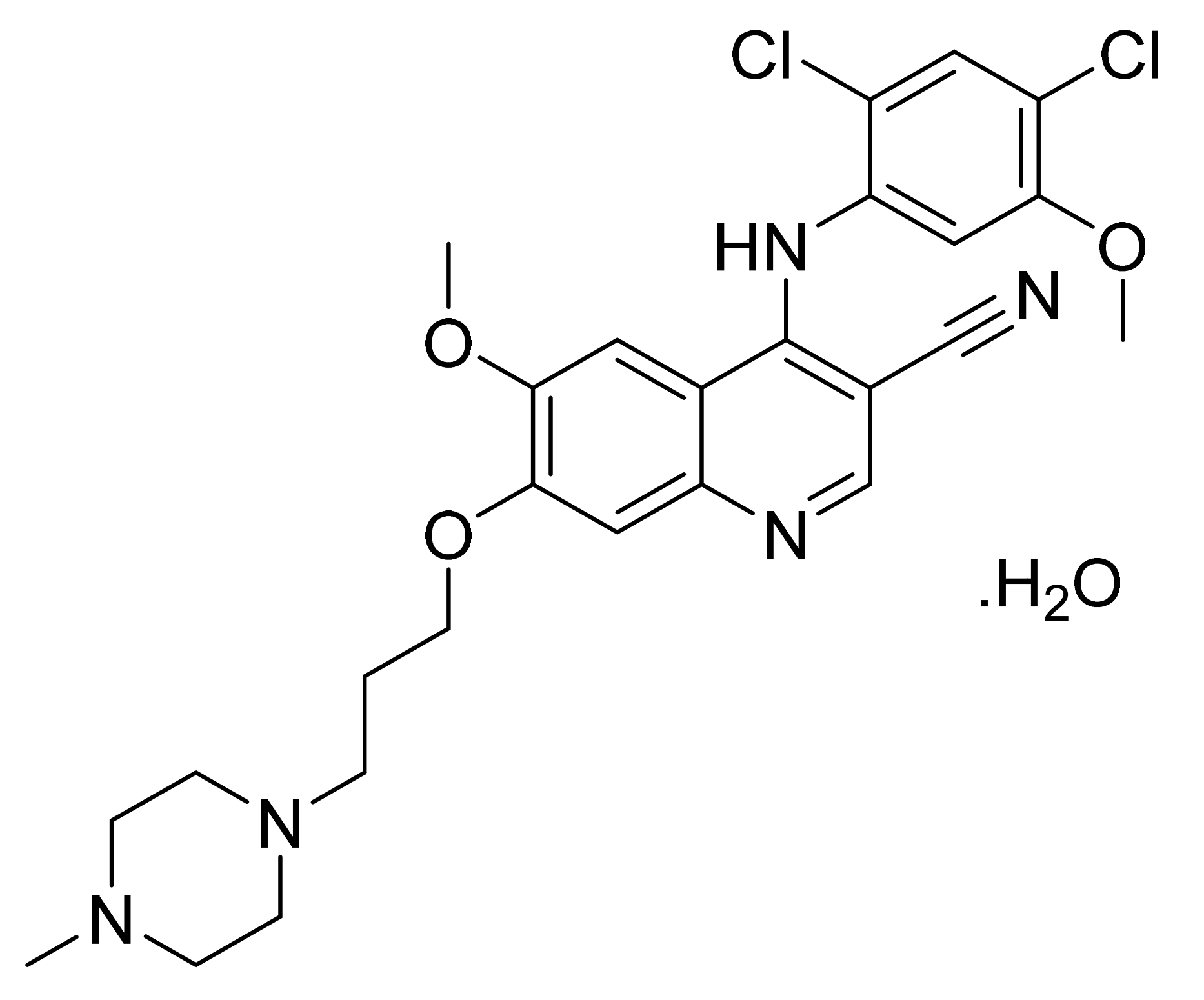
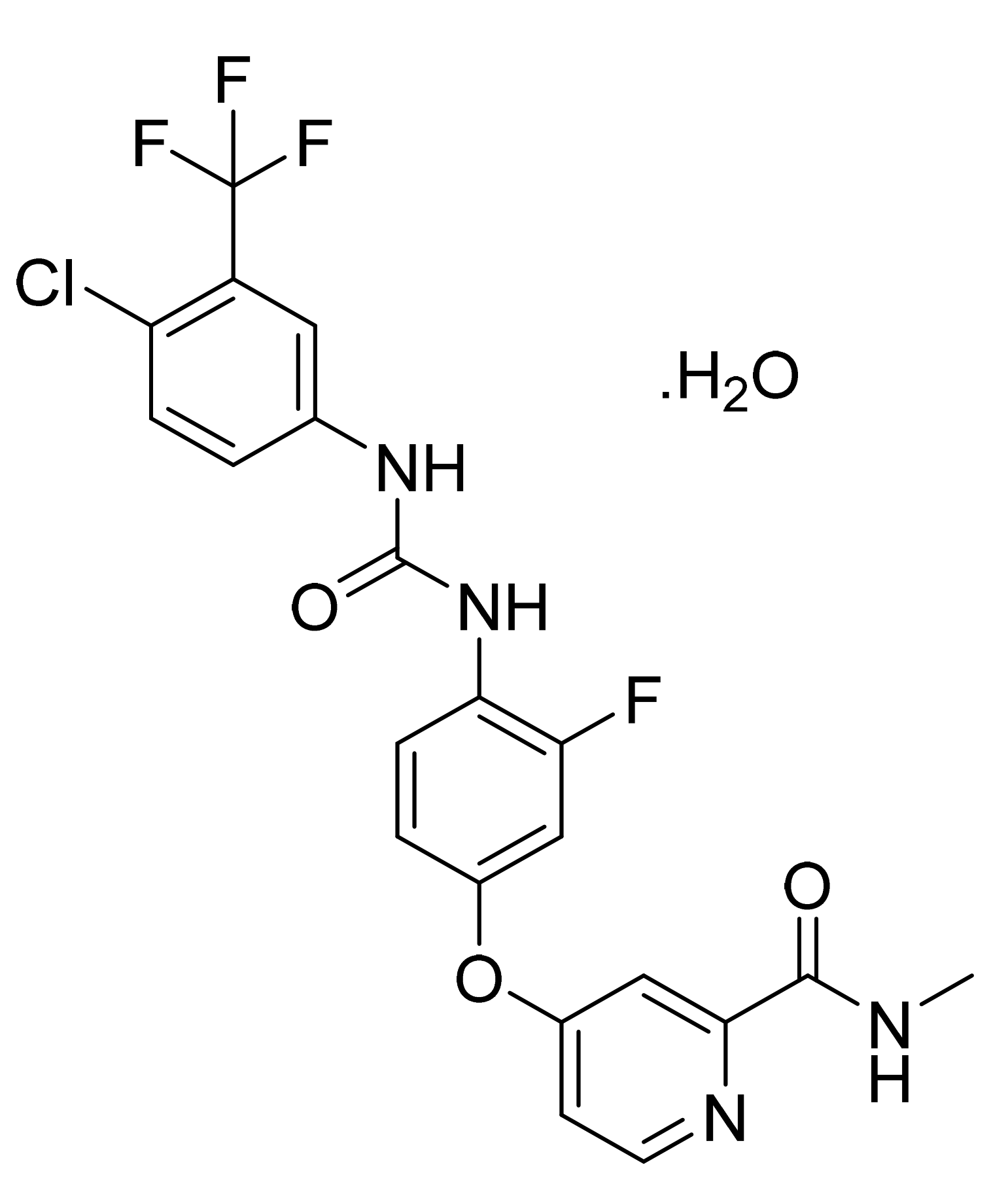
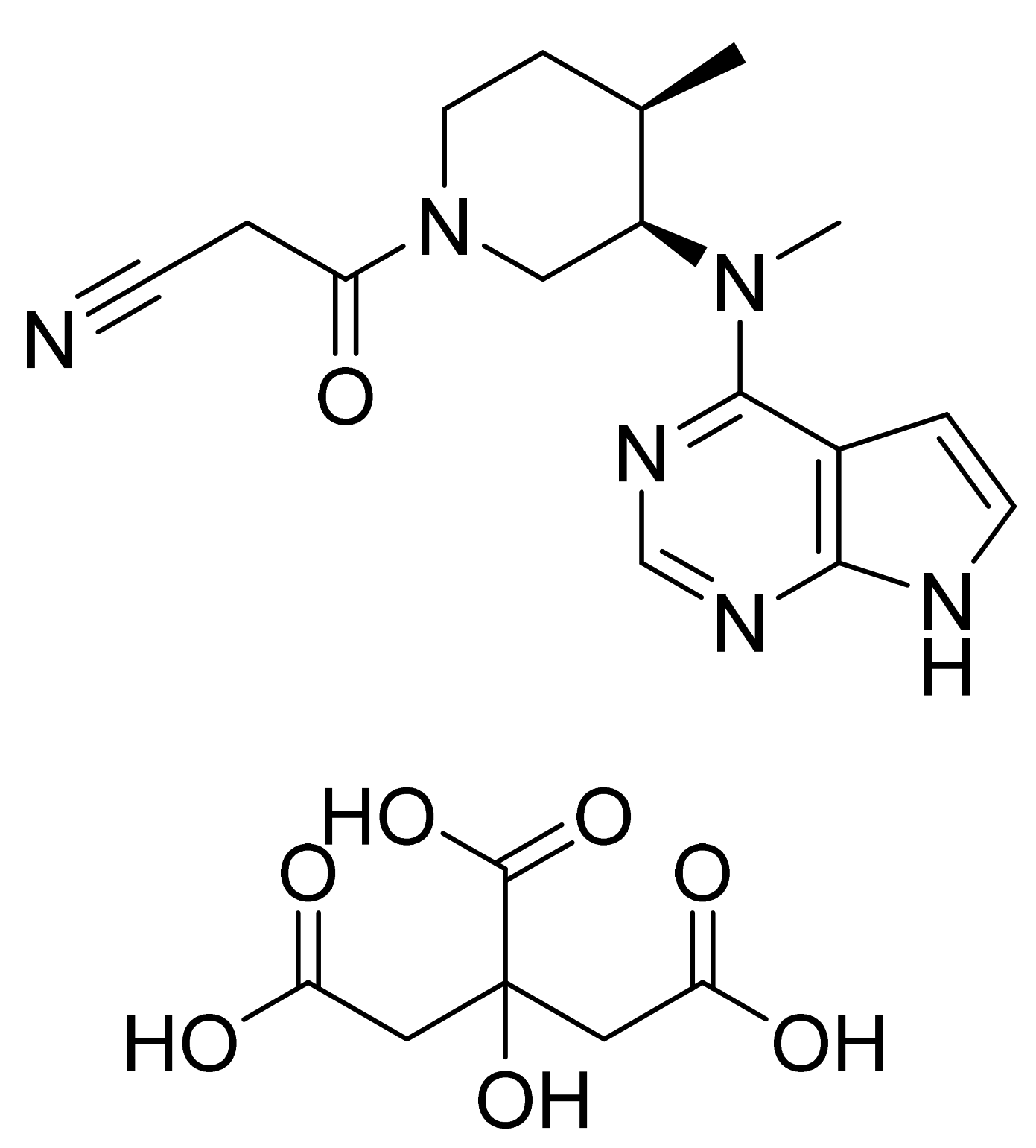
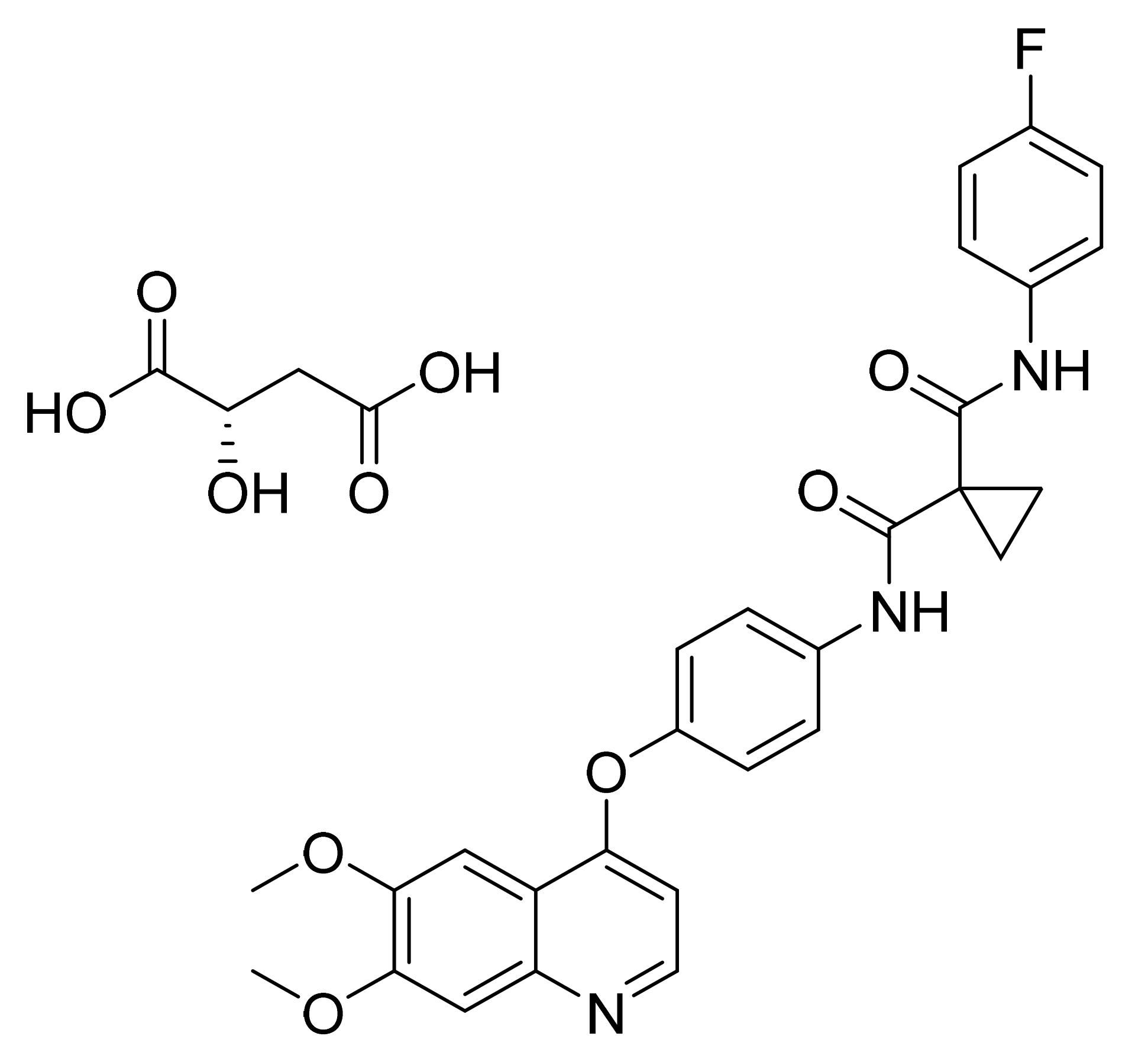
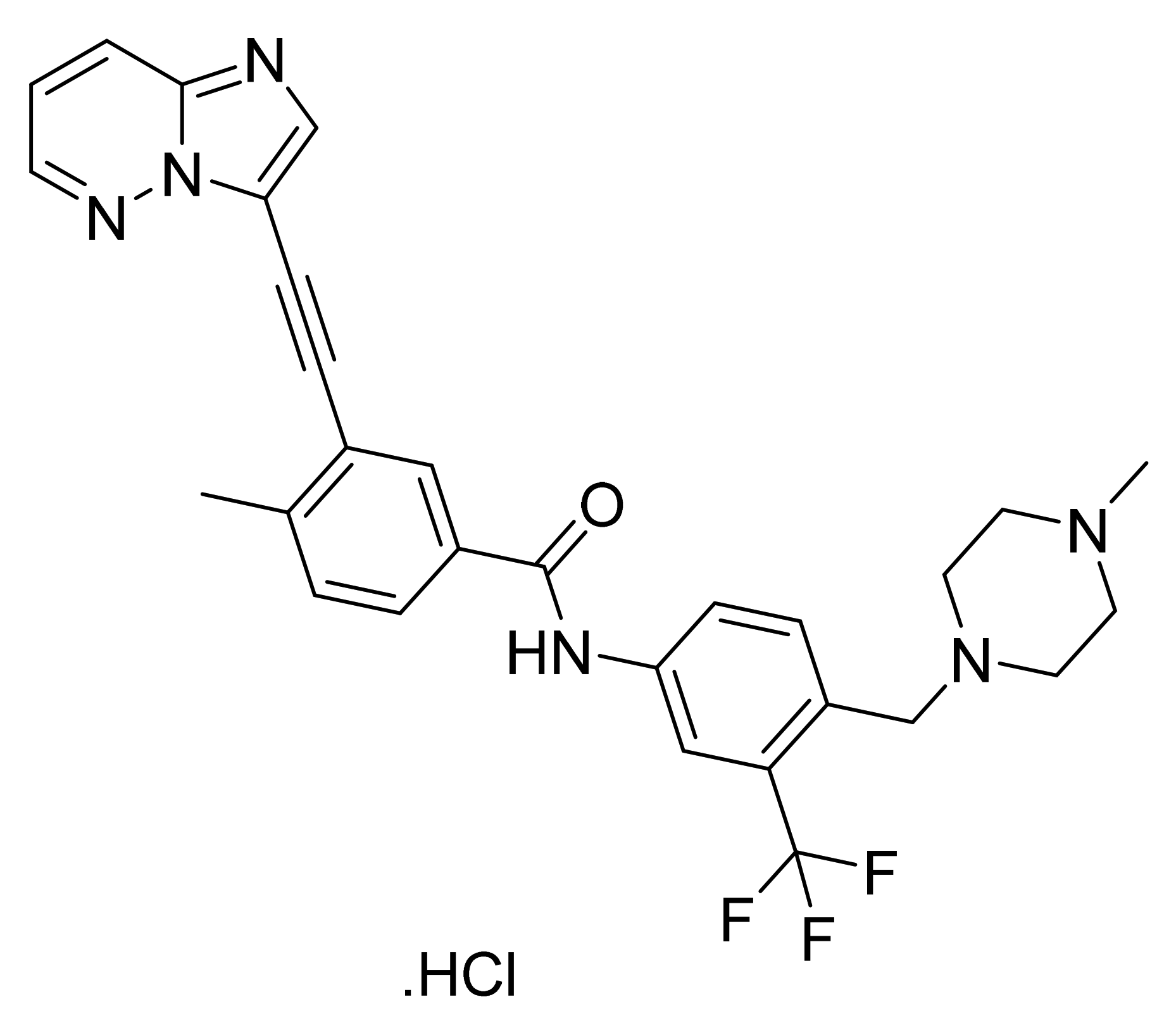
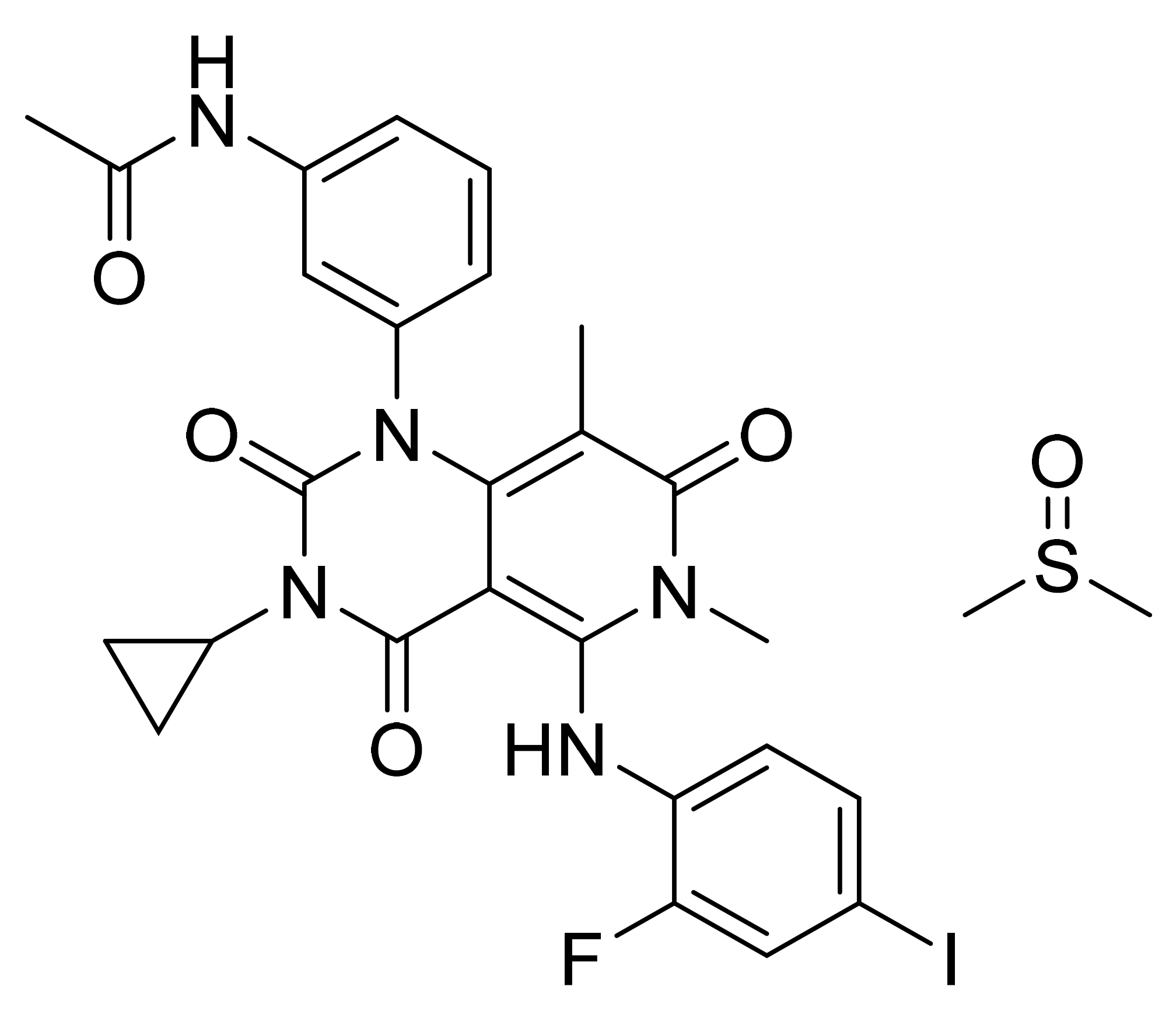

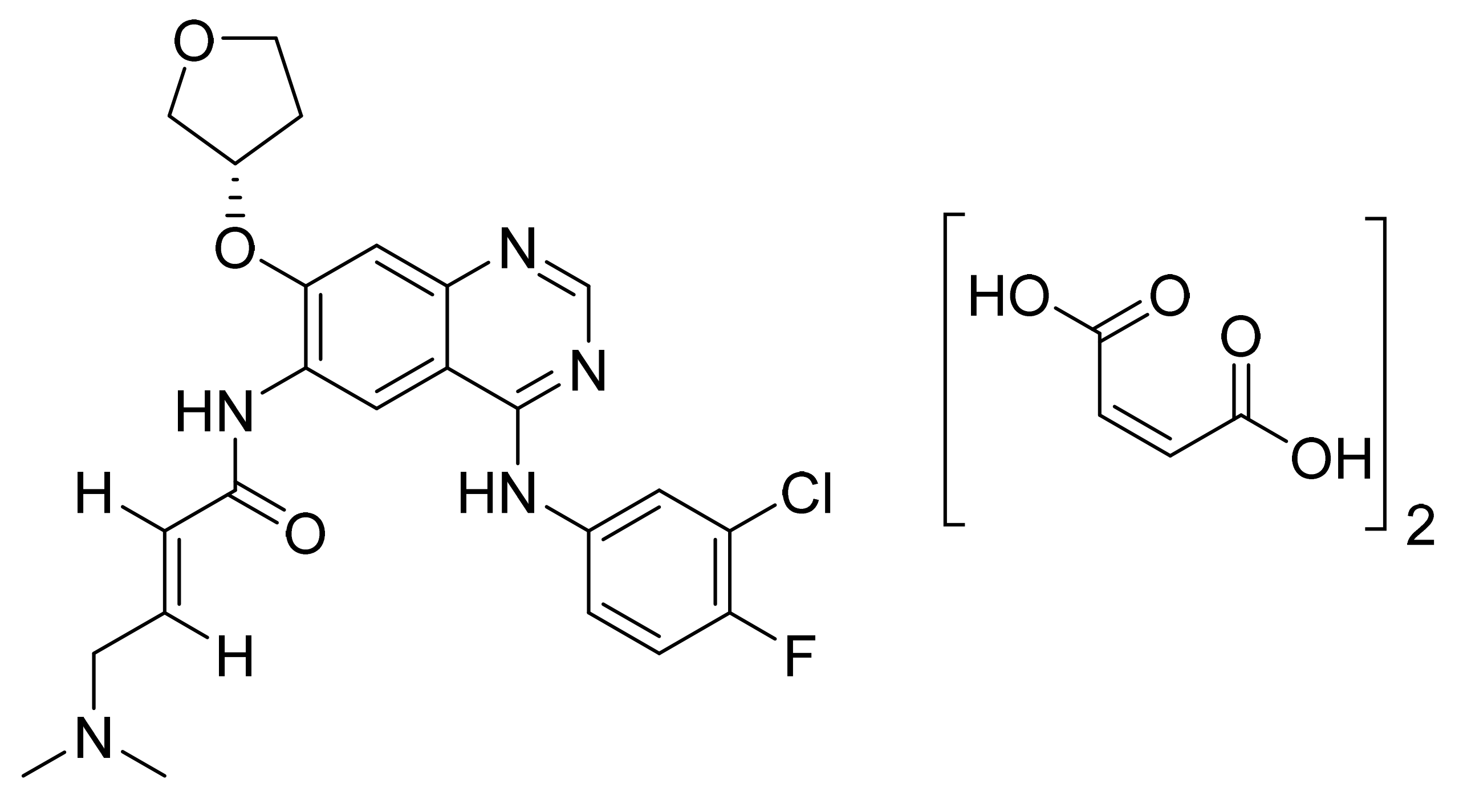
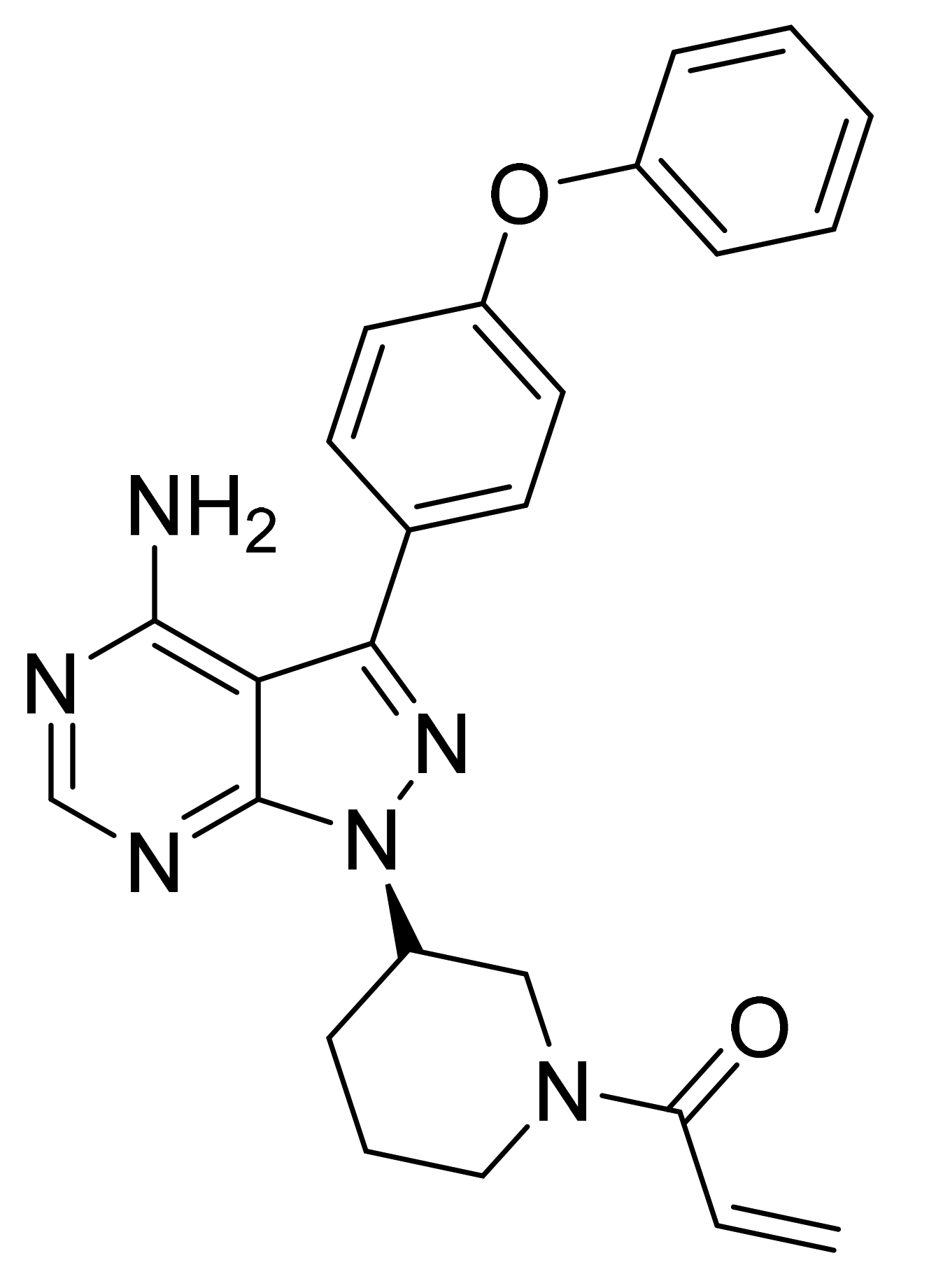
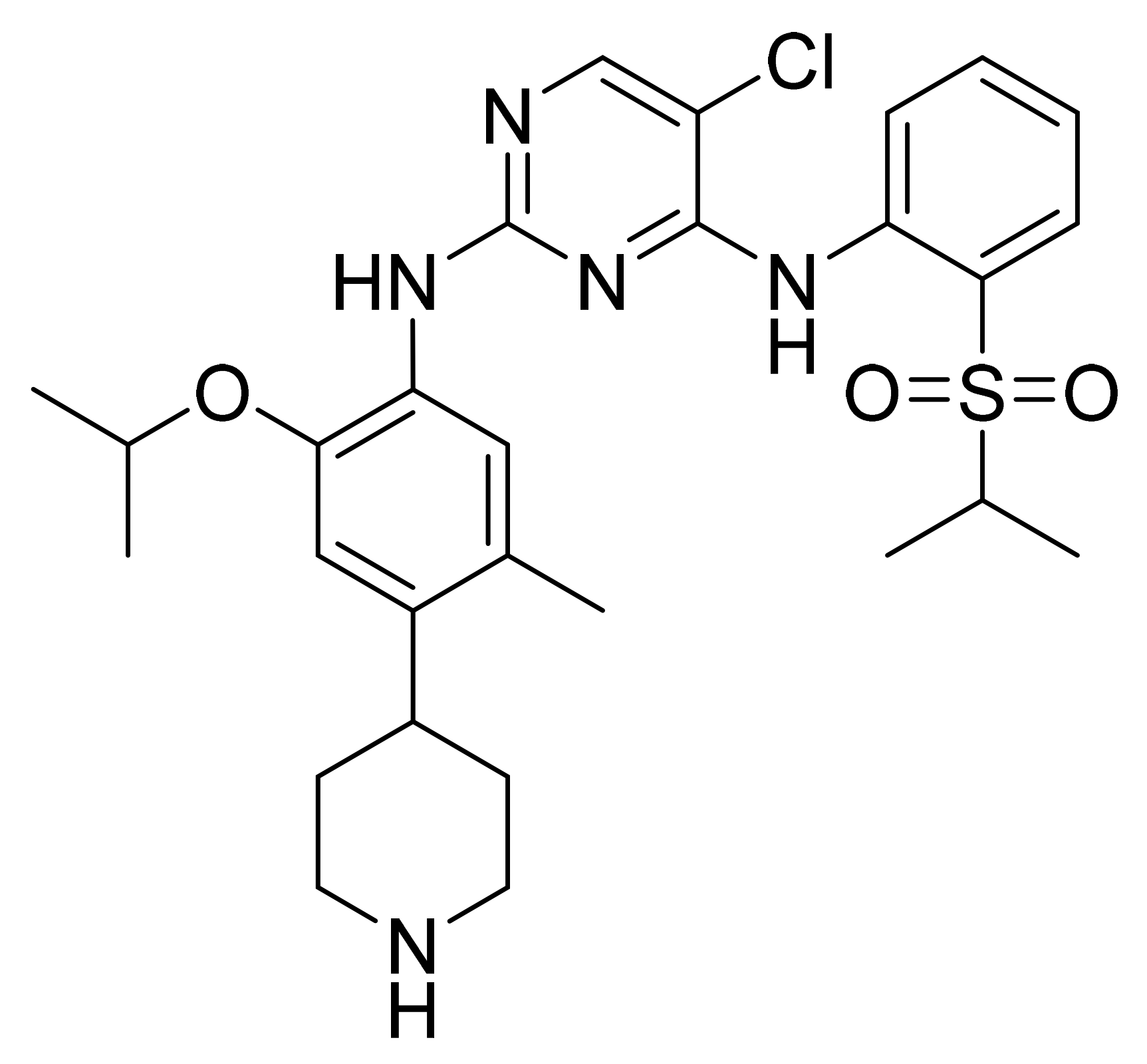
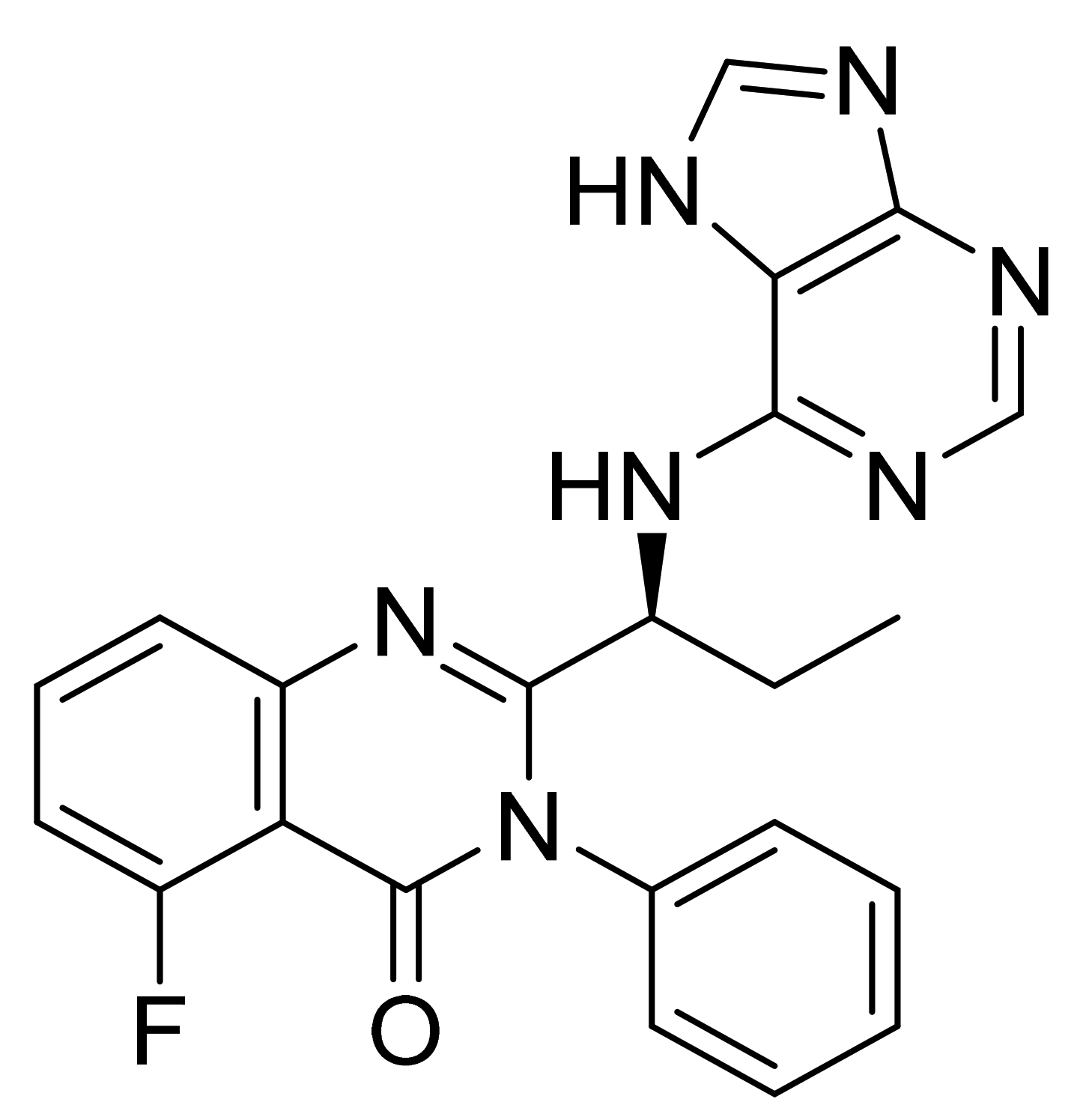
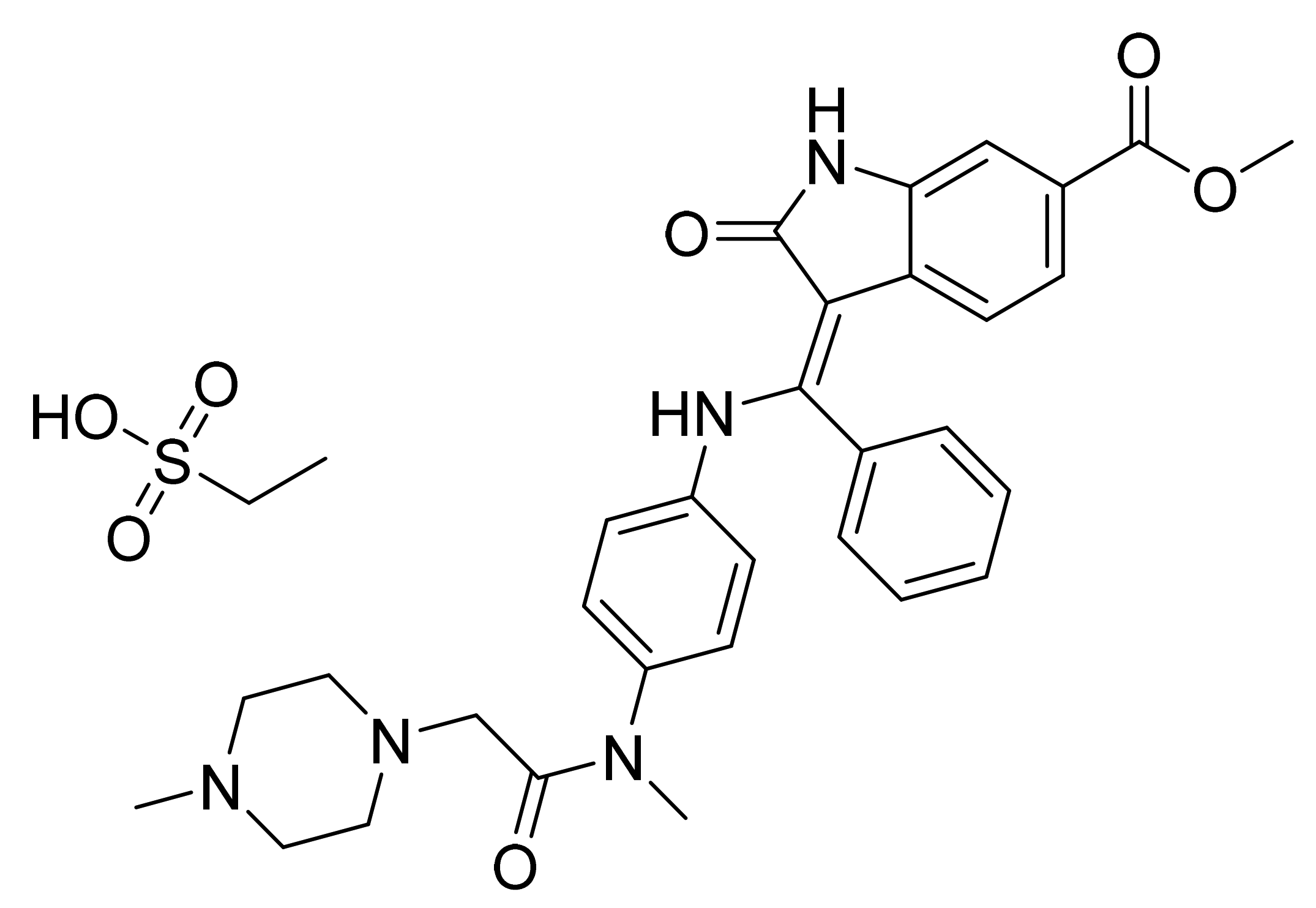

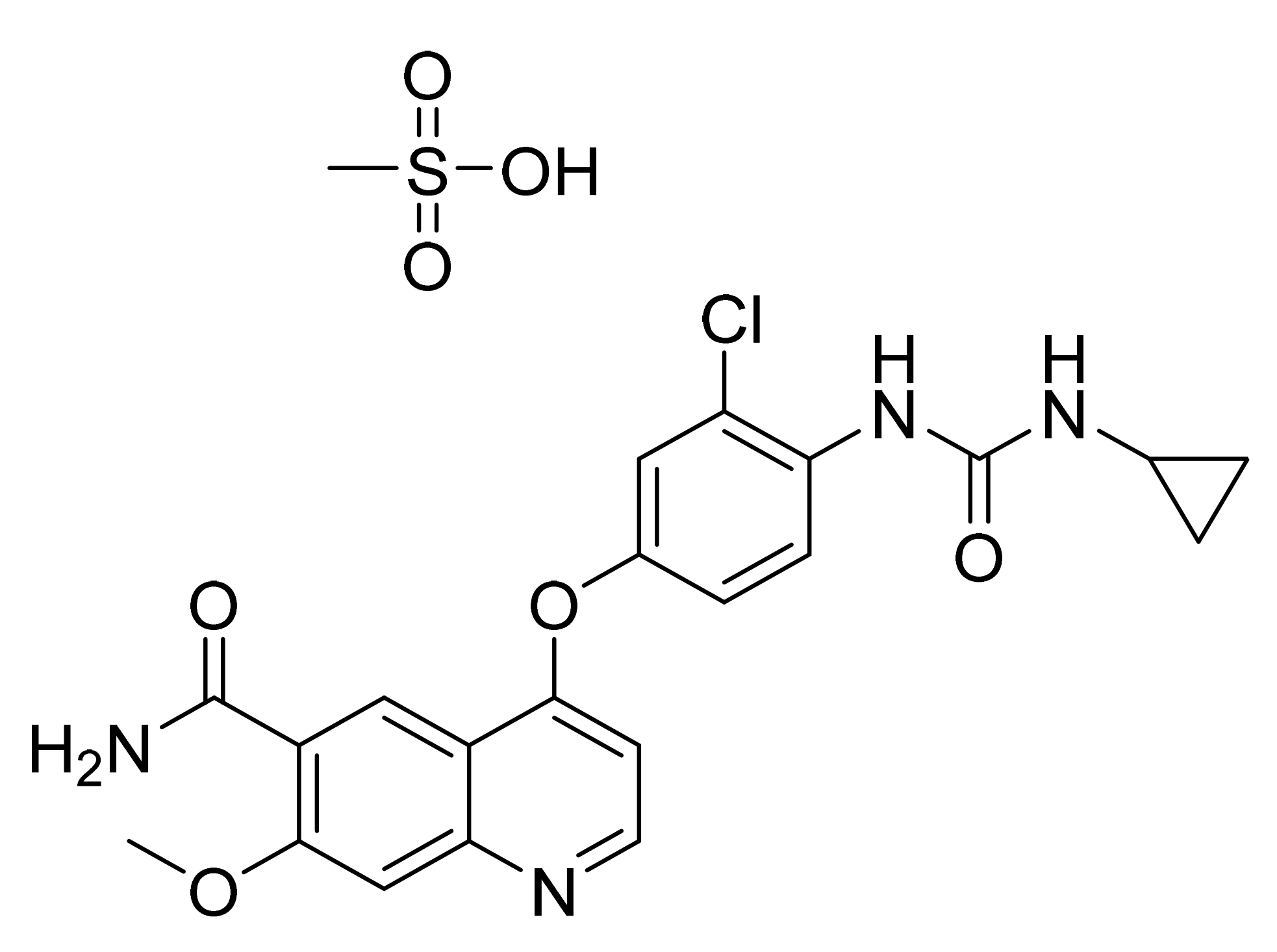

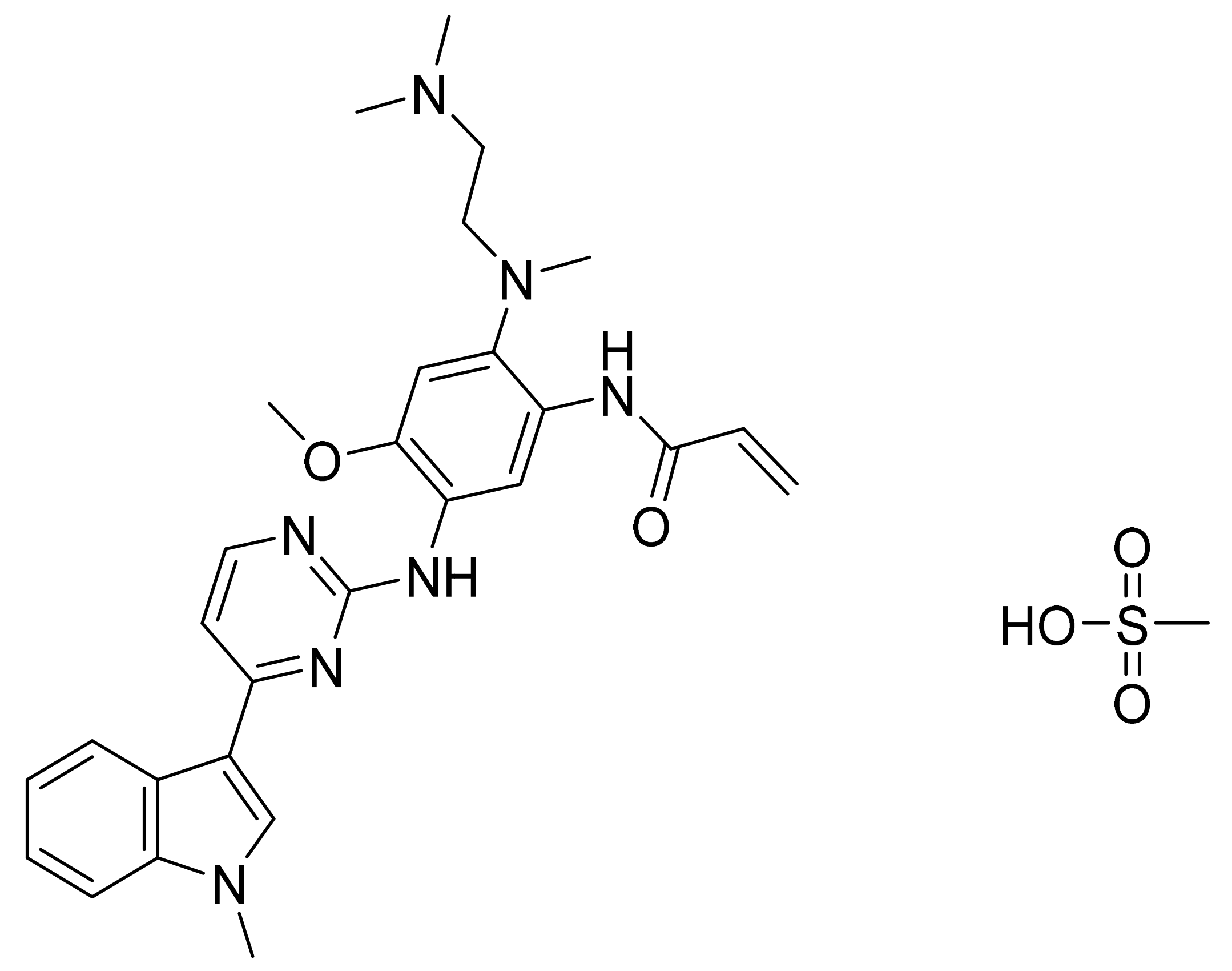
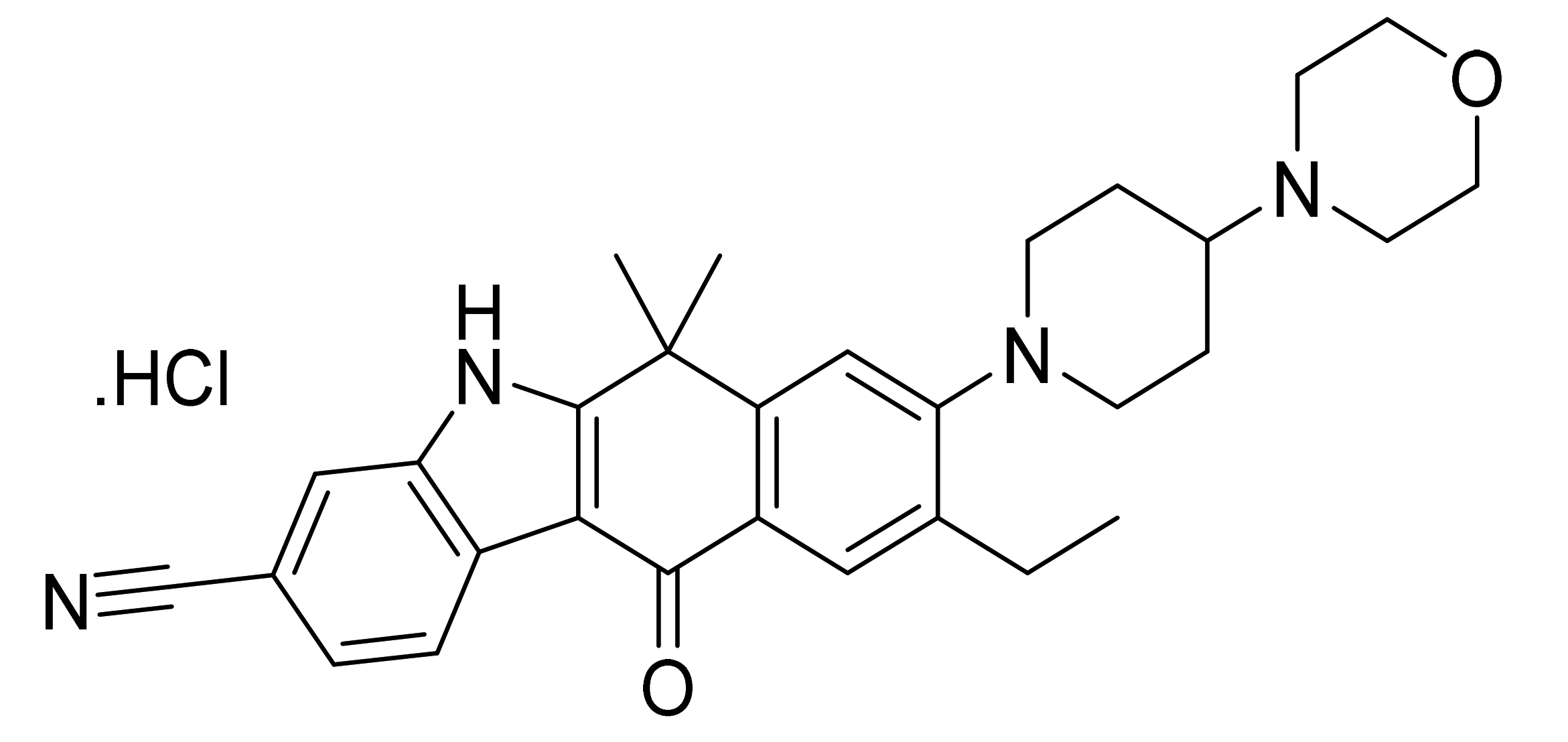
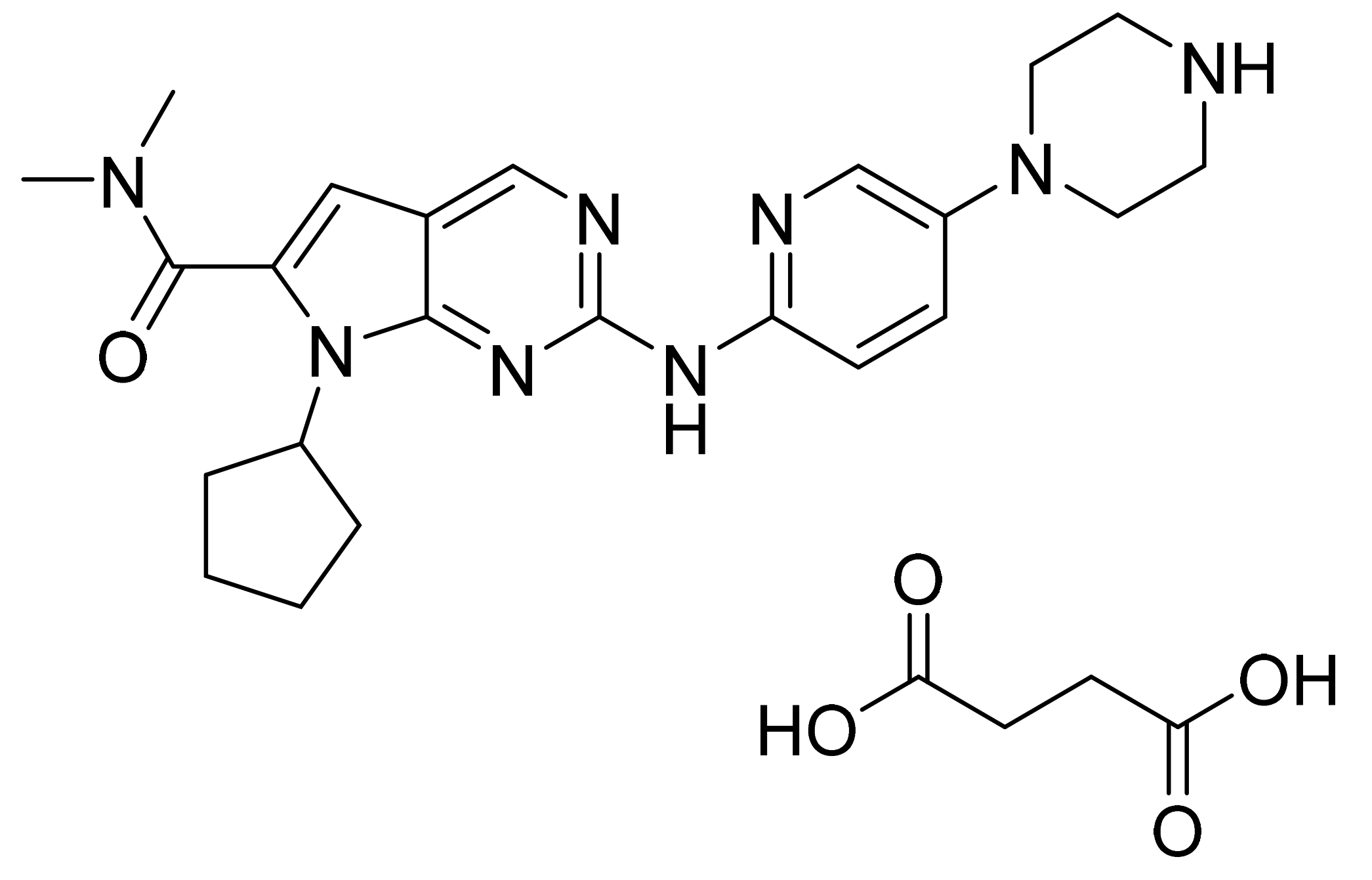
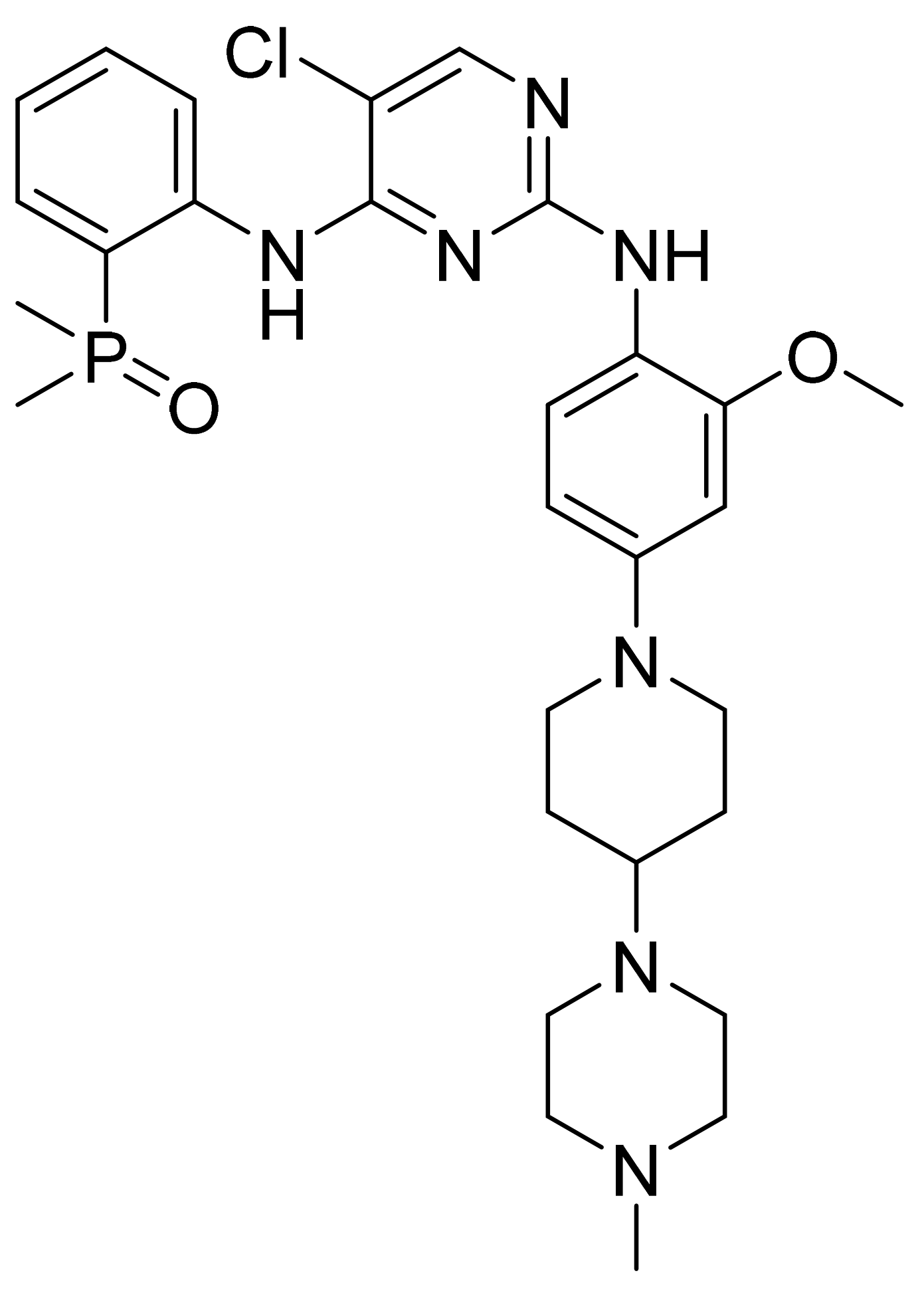




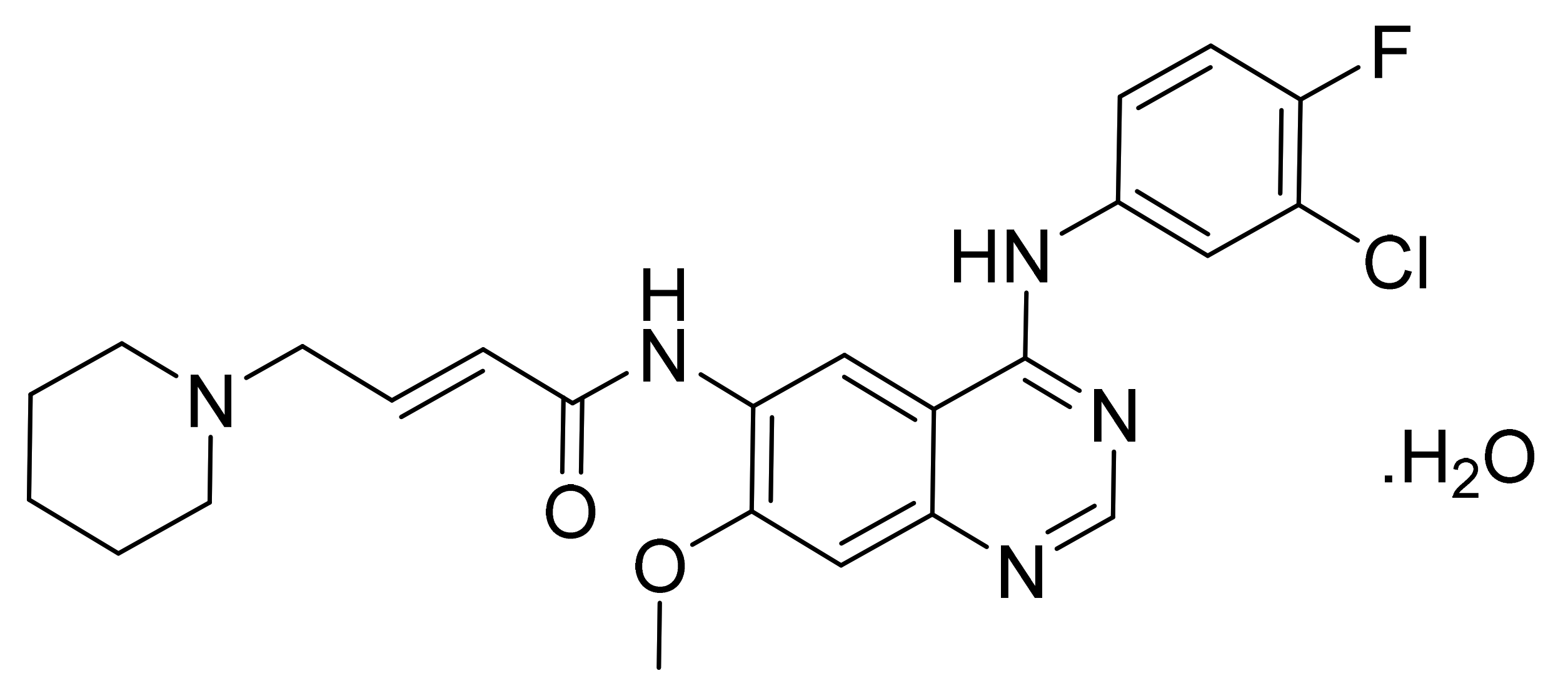
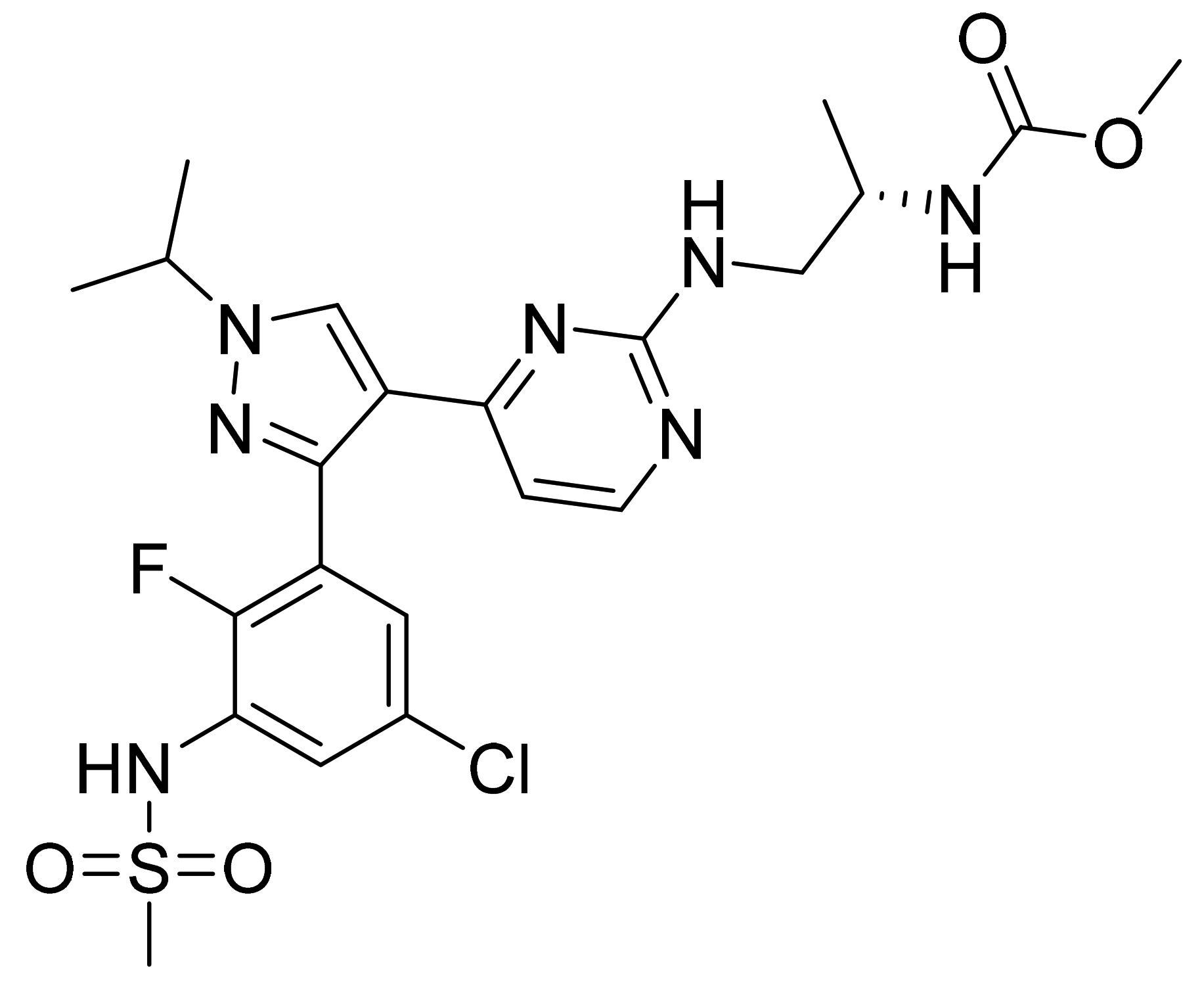
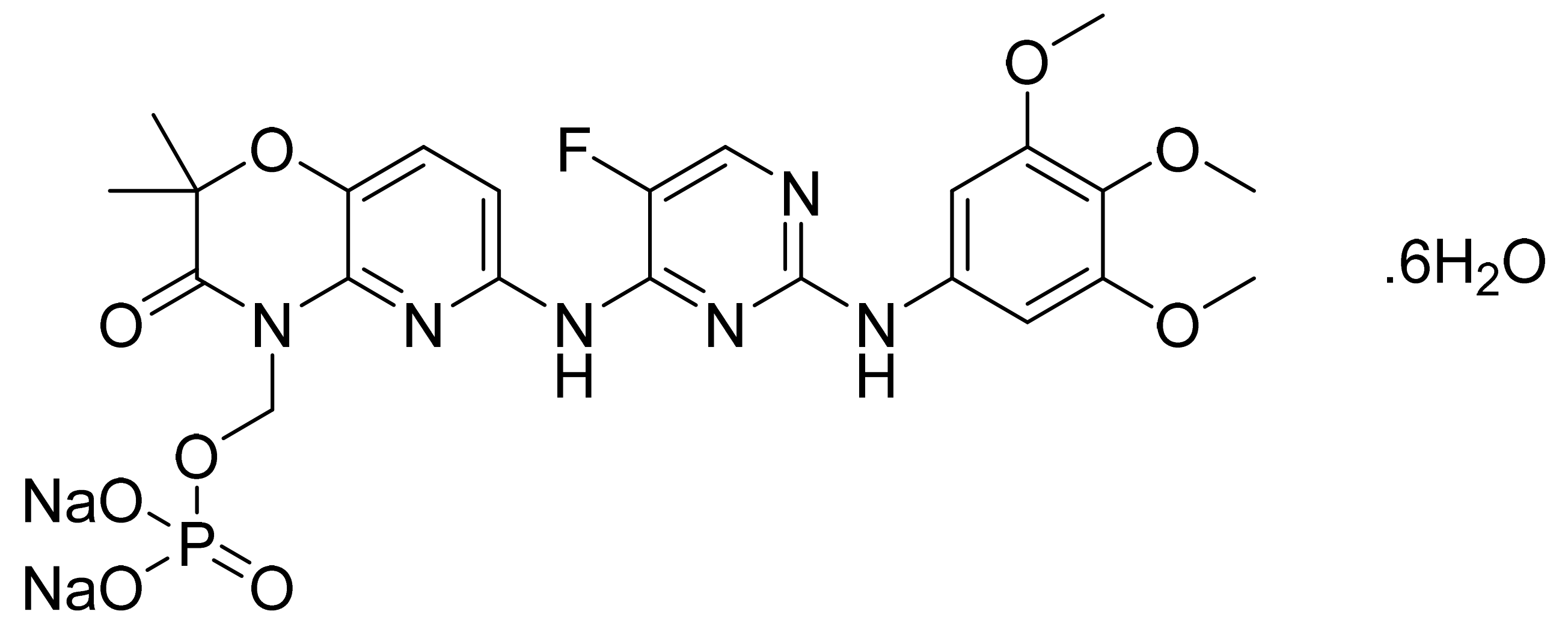
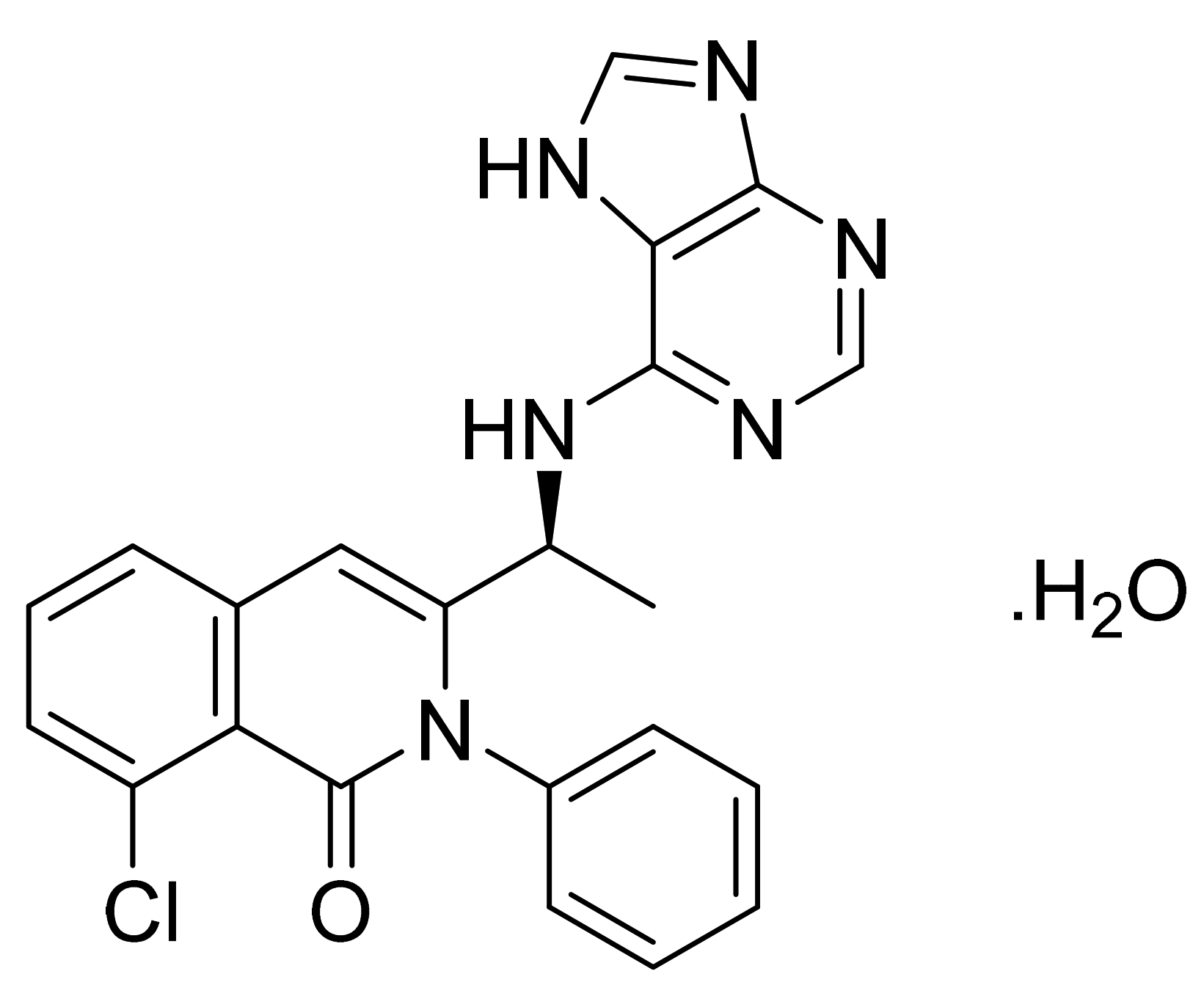

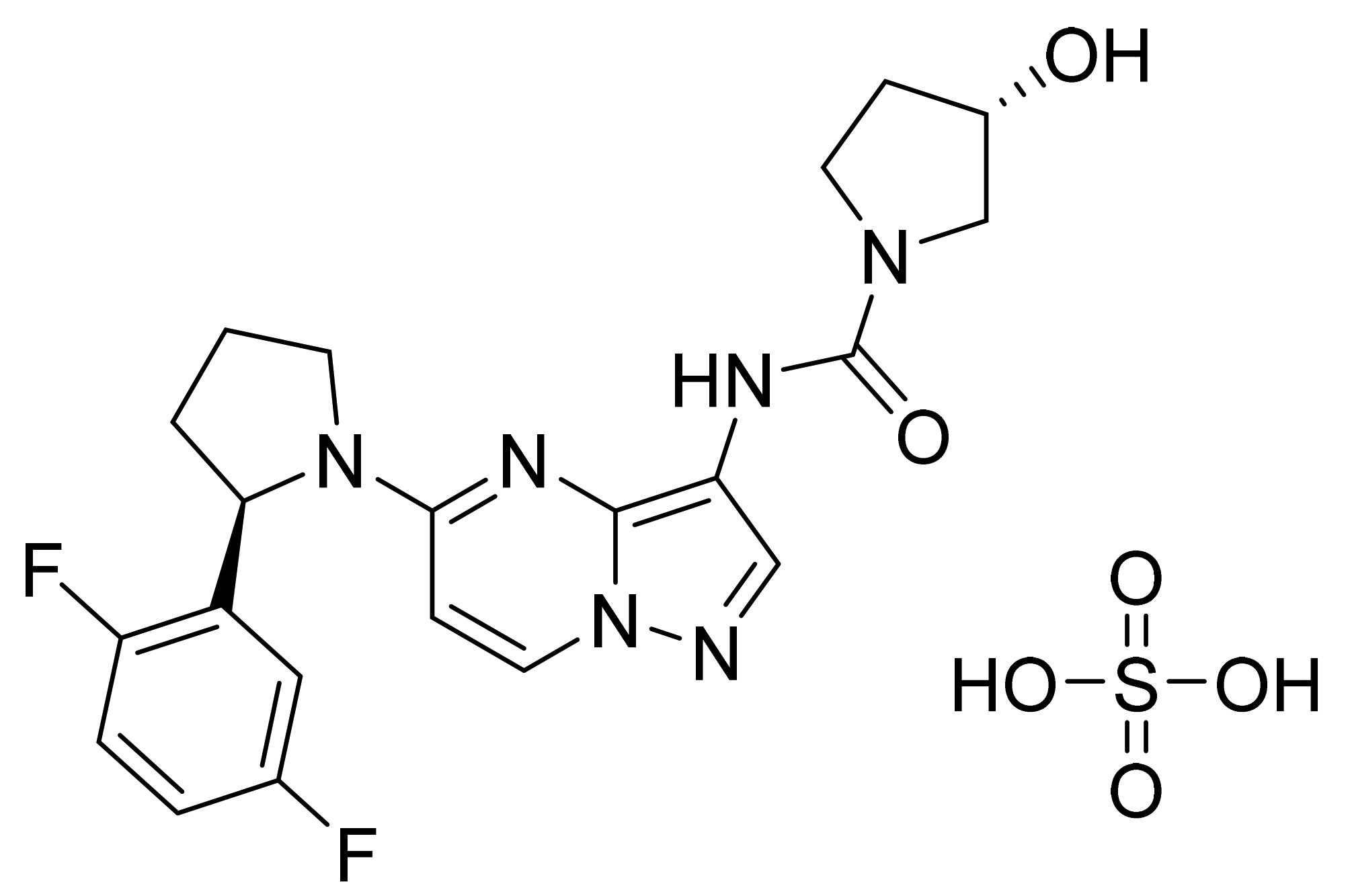
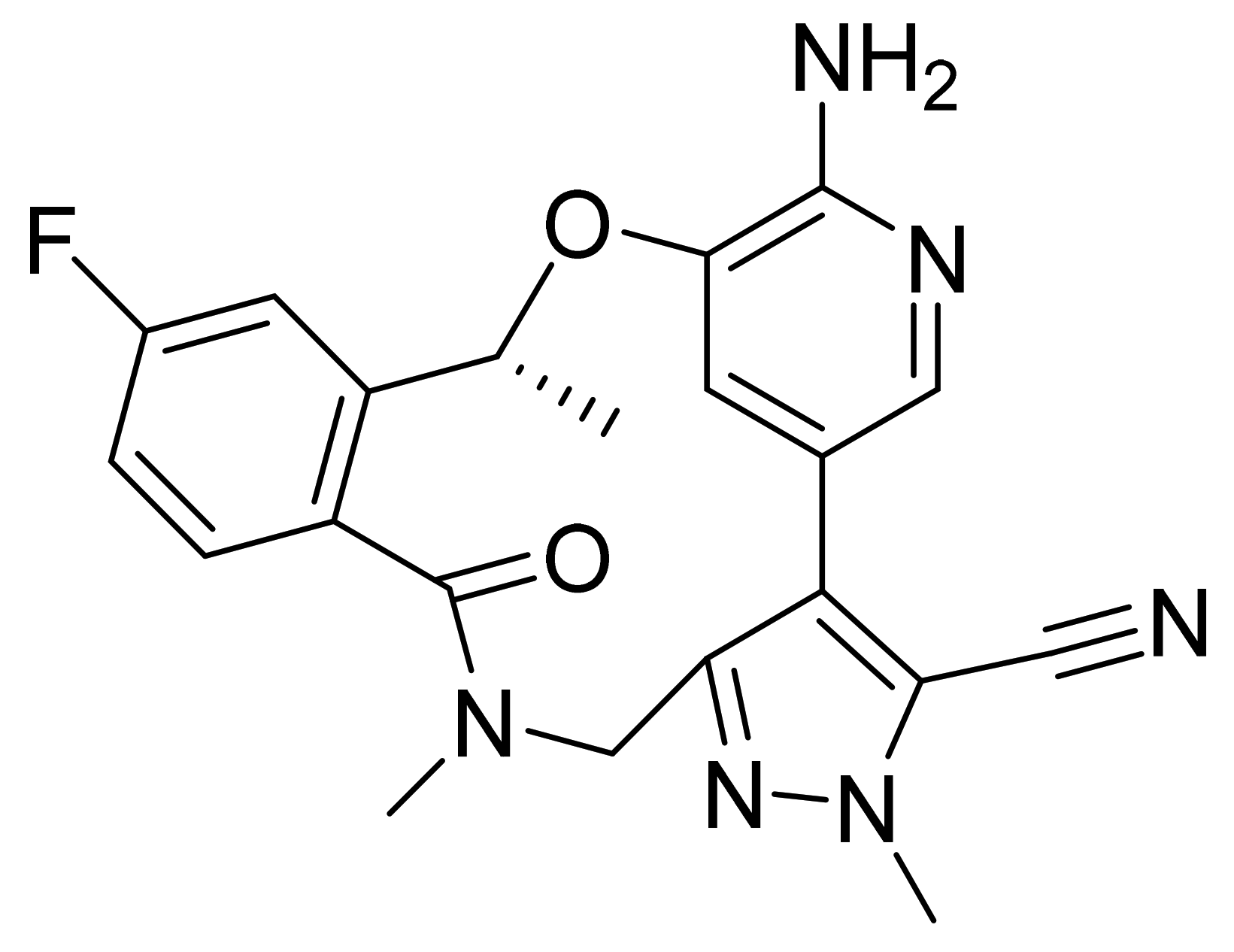
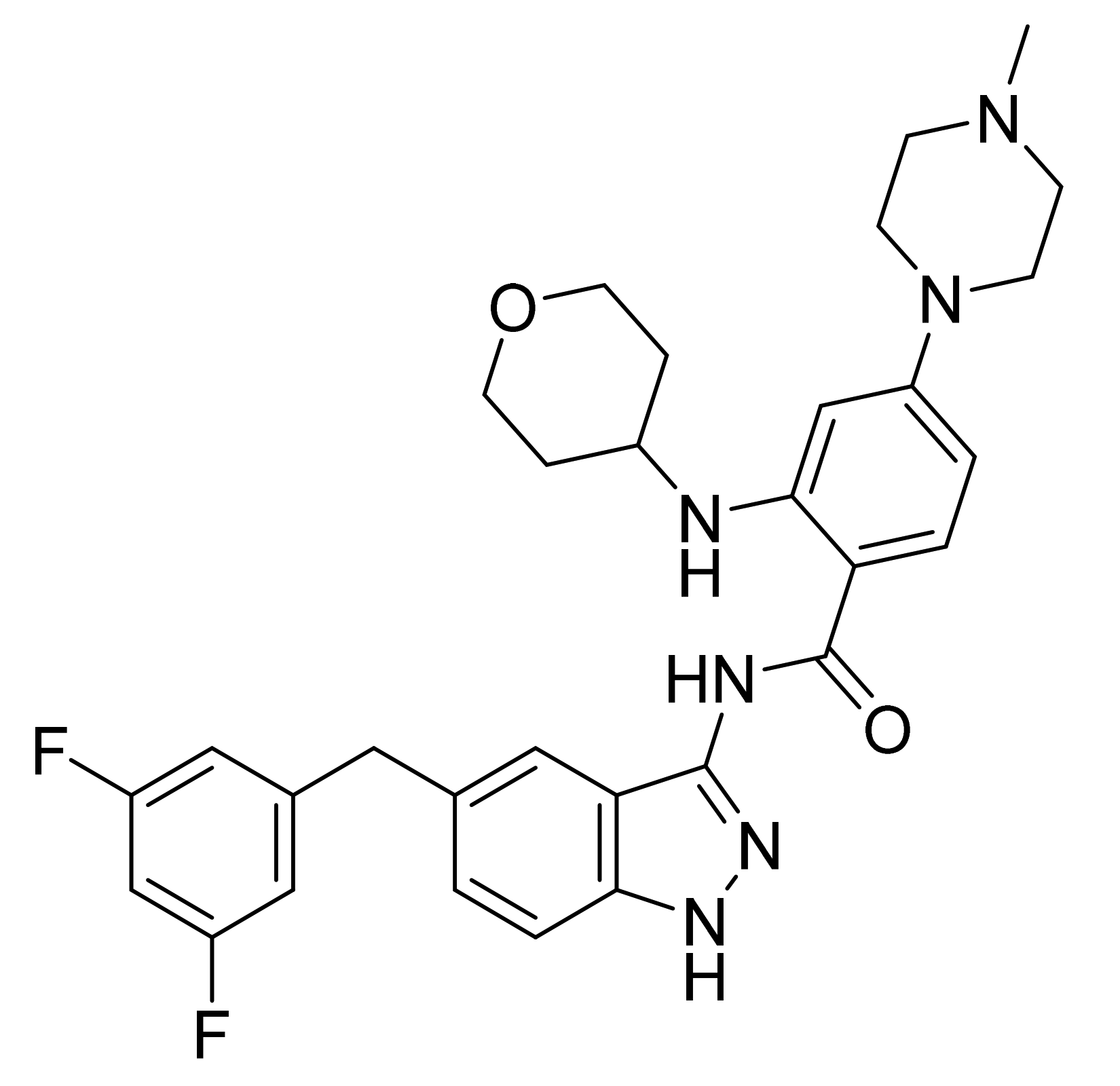
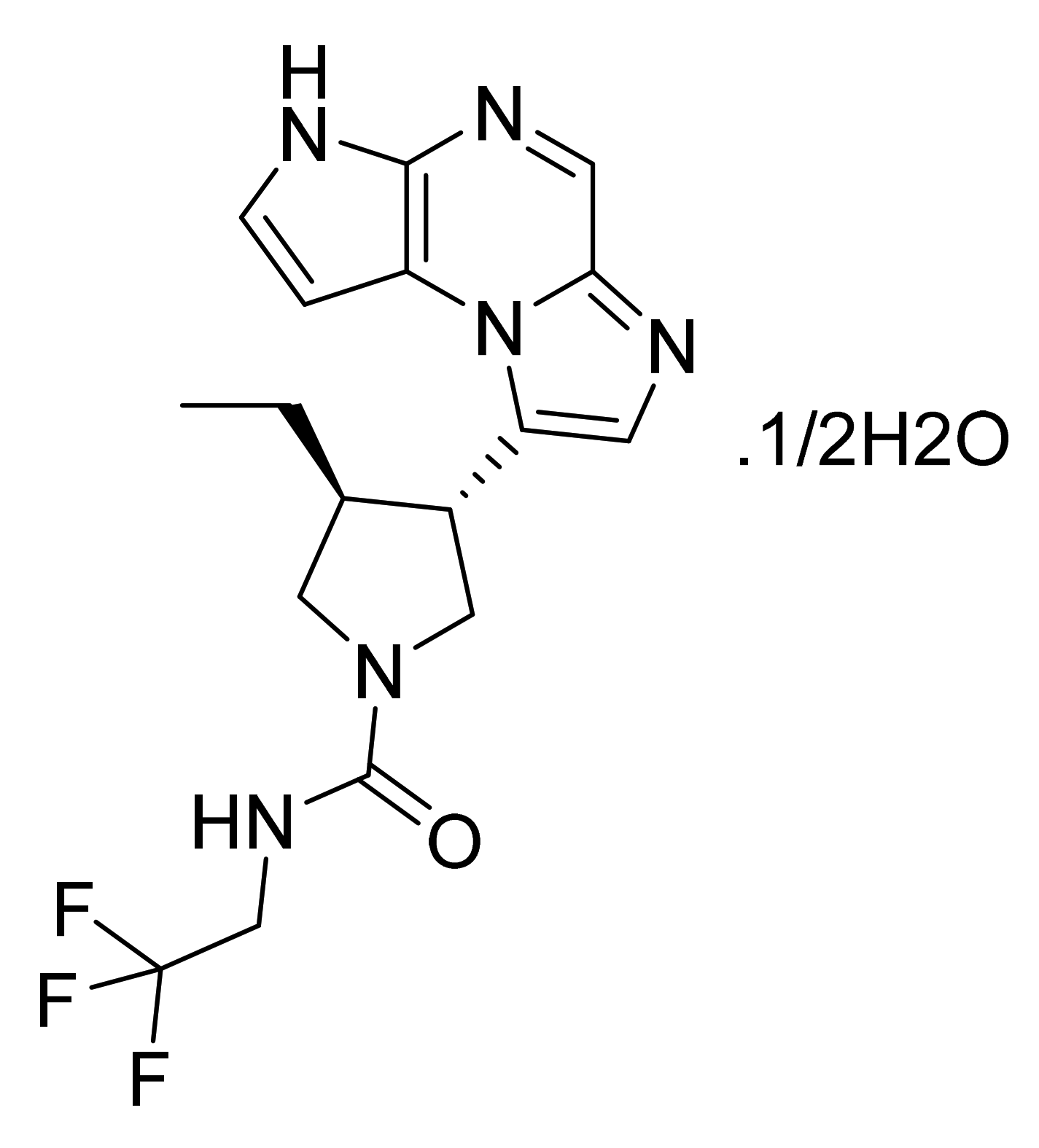





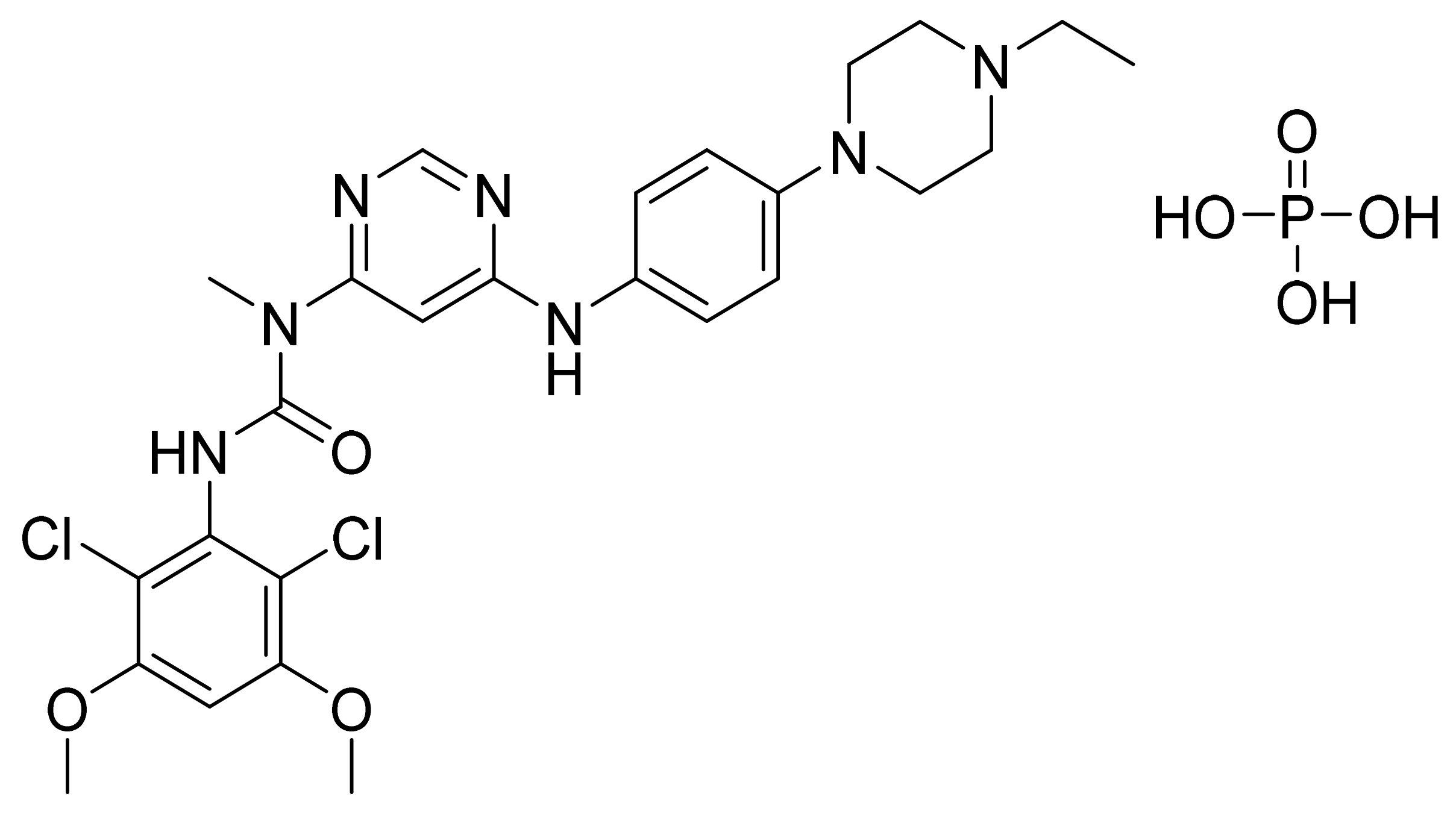
| Marketed Active Ingredient (Proprietary Name, Applicant) | Approved Dosage Form (Strength) | Approval Date (Marketing Status) | Primary Target # | Approved Indication |
|---|---|---|---|---|
| Imatinib mesylate (Gleevec, Novartis Pharmaceuticals) | Tablet (100 and 400 mg of imatinib free base) | 18 April 2003 (Prescription) | BCR-Abl | Many cancer types, including CML, Ph+-ALL, CEL, and GISTs |
| Capsule (50 and 100 mg of imatinib free base) | 10 May 2001 (Discontinued) | |||
| Gefitinib (Iressa, AstraZeneca Pharmaceuticals) | Tablet (250 mg) | 13 July 2015 (Prescription) | EGFR | NSCLC |
| Tablet (250 mg) | 5 May 2003 (Discontinued) | |||
| Erlotinib hydrochloride (Tarceva, OSI Pharmaceuticals) | Tablet (25, 100, and 150 mg of erlotinib free base) | 18 November 2004 (Prescription) | EGFR | Metastatic NSCLC and pancreatic cancer |
| Sorafenib tosylate (Nexavar, Bayer Healthcare Pharmaceuticals) | Tablet (200 mg of sorafenib free base) | 20 December 2005 (Prescription) | VEGFR/BRAF | HCC, RCC, and DTC |
| Sunitinib malate (Sutent, CP Pharmaceuticals International) | Capsule (12.5, 25, 37.5, and 50 mg of sunitinib free base) | 26 January 2006 (Prescription, 12.5, 25, and 50 mg) 31 March 2009 (Prescription, 37.5 mg) | VEGFR/PDGFR | GIST, RCC, and pNET |
| Dasatinib (Sprycel, Bristol Myers Squibb) | Tablet (20, 50, 70, 80, 100, and 140 mg) | 28 June 2006 (Prescription) | BCR-Abl/ABL2 | Ph+-CML |
| Lapatinib ditosylate (Tykerb, Novartis Pharmaceuticals) | Tablet (250 mg of lapatinib free base) | 13 March 2007 (Prescription) | HER-1/HER-2/EGFR | Breast cancer |
| Temsirolimus (Torisel, PF Prism CV) | IV Solution (25 mg/mL) | 30 May 2007 (Prescription) | FKBP12/mTOR | ARCC |
| Everolimus (Afinitor, Zortress, AfinitorDisperz, Novartis Pharmaceutical) | Tablet (2.5 mg, 5 mg, 7.5 mg, and 10 mg) | 30 March 2009 (5 mg and 10 mg) 9 July 2010 (2.5 mg) 30 March 2012 (5 mg) (All are prescription products) | FKBP12/mTOR | pNET and RCC |
| Tablet (0.25 mg, 0.5 mg, 0,75 mg, and 1 mg) | 20 April 2010 (0.25, 0.5, and 0.75 mg) 10 August 2018 (1 mg) (All are prescription products) | |||
| Tablet for suspension (2 mg, 3 mg, and 5 mg) | 29 August 2012 (Prescription) | |||
| Nilotinib hydrochloride (Tasigna, Novartis Pharmaceuticals) | Capsule (50, 150, and 200 mg of nilotinib base) | 29 October 2007 (200 mg Tablet) 17 June 2010 (150 mg Tablet) 22 March 2018 (50 mg Tablet) (All are prescription products) | BCR-Abl | Ph+-CML |
| Pazopanib hydrochloride (Votrient, Novartis Pharmaceuticals) | Tablet (200, and 400 mg of pazopanib base) | 19 October 2009 (200 mg tablet, Prescription) (400 mg tablet has been discontinued) | VEGFR/PDGFR | RCC and STS |
| Vandetanib (Caprelsa, Genzyme Corp) | Tablet (100 mg and 300 mg) | 6 April 2011 (Prescription) | VEGFR/EGFR | MTC |
| Vemurafenib (Zelboraf, Hoffmann La Roche) | Tablet (240 mg) | 17 August 2011 (Prescription) | B-Raf | Melanoma with BRAF V600E mutation |
| Crizotinib (Xalkori, PF Prism CV) | Capsule (200 mg and 250 mg) | 26 August 2011 (Prescription) | ALK/HGFR | NSCLC |
| Ruxolitinib phosphate (Jakafi, Incyte Corp) | Tablet (5 mg, 10 mg, 15 mg, 20 mg, and 25 mg of ruxolitinib free base) | 16 November 2011 (Prescription) | JAK1/2/3 and Tyk2 | Myelofibrosis and polycythemia vera |
| Axitinib (Inlyta, PF Prism CV) | Tablet (1 mg and 5 mg) | 27 January 2012 (Prescription) | VEGFR/PDGFR | RCC |
| Bosutinib monohydrate (Bosulif, PF Prism CV) | Tablet (100 mg, 400 mg, and 500 mg of bosutinib free base) | 4 September 2012 (100 and 500 mg) 27 October 2017 (400 mg) (All are prescription products) | BCR-Abl | Ph+-CML |
| Regorafenib (Stivarga, Bayer Healthcare Pharmaceuticals) | Tablet (40 mg) | 27 September 2012 (Prescription) | VEGFR/TIE | Colorectal cancer, GIST, HCC, RCC and STS |
| Tofacitinib citrate (Xeljanz, Pfizer) | Solution (1 mg/mL of tofacitinib free base) | 25 September 2020 (Prescription) | JAK1/2/3 and Tyk2 | Rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and juvenile idiopathic arthritis |
| Tablet (5 mg and 10 mg of tofacitinib free base) | 6 November 2012 (5 mg) 30 May 2018 (10 mg) (All are prescription products) | |||
| Extended-release tablet (11 mg and 22 mg of tofacitinib free base) | 23 February 2016 (11 mg) 12 December 2019 (22 mg) (All are prescription products) | |||
| Cabozantinib S-malate (Cometriq and Cabometyx, Exelixis) | Capsule (20 mg and 80 mg of cabozantinib free base) | 29 November 2012 (Prescription) | RET | MTC, RCC, and HCC |
| Tablet (20 mg, 40 mg and 80 mg of cabozantinib free base) | 25 April 2016 (Prescription) | |||
| Ponatinib hydrochloride (Iclusig, Ariad Pharmaceuticals) | Tablet (10 mg, 15 mg, 30 mg, and 45 mg of ponatinib free base) | 18 December 2020 (10 mg) 14 December 2012 (15 mg and 45 mg) 23 April 2015 (30 mg) (All are prescription products) | BCR-Abl | CML, Ph+-ALL, T315I-positive CML or Ph+-ALL |
| Trametinib dimethyl sulfoxide (Mekinist, Novartis Pharmaceuticals) | Tablet (0.5 mg, 1 mg and 2 mg) | 29 May 2013 (Prescription) (The 1 mg tablet has been discontinued) | MEK1/2 | Metastatic melanoma, NSCLC, and ATC |
| Dabrafenib mesylate (Tafinlar, Novartis Pharmaceuticals) | Capsule (50 mg and 75 mg of dabrafenib free base) | 29 May 2013 (Prescription) | B-Raf | Metastatic melanoma, NSCLC, and ATC |
| Afatinib dimaleate (Gilotrif, Boehringer Ingelheim) | Tablet (20 mg, 30 mg, and 40 mg of afatinib free base) | 12 July 2013 (Prescription) | EGFR/HER2/HER4 | NSCLC |
| Ibrutinib (Imbruvica, Pharmacyclics) | Capsule (70 mg and 140 mg) | 13 November 2013 (140 mg) 20 December 2017 (70 mg) | BTK | MCL, CLL, SLL, and MZL |
| Tablet (140 mg, 280 mg, 420 mg, and 560 mg) | 16 February 2018 | |||
| Ceritinib (Zykadia, Novartis Pharmaceuticals) | Tablet (150 mg) | 18 March 2019 (Prescription) | ALK | NSCLC |
| Capsule (150 mg) | 29 April 2014 (Discontinued) | |||
| Idelalisib (Zydelig, Gilead Sciences) | Tablet (100 mg and 150 mg) | 23 July 2014 (Prescription) | PI3Kδ | CLL, FL, and SLL |
| Nintedanib esylate (Ofev, Boehringer Ingelheim Pharmaceuticals) | Capsule (100 mg and 150 mg of nintedanib free base) | 15 October 2014 (Prescription) | PDGFR/FGFR/VEGFR | IPF, ILDs, and SSc-ILD |
| Palbociclib (Ibrance, Pfizer) | Capsule (75 mg, 100 mg, and 125 mg) | 3 February 2015 (Prescription) | CDK4/6 | Breast cancer |
| Tablet (75 mg, 100 mg, and 125 mg) | 1 November 2019 (Prescription) | |||
| Lenvatinib mesylate (Lenvima, Eisai) | Capsule (4 mg and 10 mg of lenvatinib free base) | 13 February 2015 (Prescription) | VEGFR/RET | Thyroid cancer, RCC, HCC, and endometrial carcinoma |
| Cobimetinib fumarate (Cotellic, Genentech) | Tablet (20 mg of cobimetinib free base) | 10 November 2015 (Prescription) | MEK1/2 | Melanoma |
| Osimertinib mesylate (Tagrisso, AstraZeneca Pharmaceuticals) | Tablet (40 mg and 80 mg of osimertinib free base) | 13 November 2015 (Prescription) | EGFR | NSCLC |
| Alectinib hydrochloride (Alecensa, Hoffmann-La Roche) | Capsule (150 mg of alectinib free base) | 11 December 2015 (Prescription) | ALK/RET | NSCLC |
| Ribociclib succinate (Kisqali, Novartis Pharmaceuticals) | Tablet (200 mg of ribociclib free base) | 13 March 2017 (Prescription) | CDK4/6 | Breast cancer |
| Brigatinib (Alunbrig, Ariad Pharmaceuticals) | Tablet (30 mg, 90 mg, and 180 mg) | 28 April 2017 (30 mg, and 90 mg) 2 October 2017 (180 mg) (All are prescription products) | ALK | NSCLC |
| Midostaurin (Rydapt, Novartis Pharmaceuticals) | Capsule (25 mg) | 28 April 2017 (Prescription) | Flt3 | AML, MCL, and systemic mastocytosis |
| Neratinib maleate (Nerlynx, Puma Biotechnology) | Tablet (40 mg of neratinib free base) | 17 July 2017 (Prescription) | EGFR/HER2 | Breast cancer |
| Copanlisib dihydrochloride (Aliqopa, Bayer Healthcare Pharmaceuticals) | Powder (60 mg/vial) | 14 September 2017 (Prescription) | PI3K-α/β/δ | FL and Non-Hodgkin Lymphoma |
| Abemaciclib (Verzenio, Eli Lilly) | Tablet (50 mg, 100 mg, 150 mg, and 200 mg) | 28 September 2017 (Prescription) | CDK4/6 | Breast cancer |
| Acalabrutinib (Calquence, Astrazeneca) | Capsule (100 mg) | 31 October 2017 (Prescription) | BTK | MCL, CLL, SLL, and urothelial carcinoma |
| Netarsudil mesylate (Rhopressa, Aerie Pharmaceuticals) | Solution/Drops (0.02% of netarsudil free base) | 18 December 2017 (Prescription) | ROCK1/2 | Open-angle glaucoma or ocular hypertension |
| Baricitinib (Olumiant, Eli Lilly) | Tablet (1 mg and 2 mg) | 8 October 2019 (1 mg) 31 May 2018 (2 mg) (All are prescription products) | JAK1/2/3 andTyk | Rheumatoid arthritis |
| Binimetinib (Mektovi, Array Biopharma) | Tablet (15 mg) | 27 June 2018 (Prescription) | MEK1/2 | Melanoma with a BRAF V600 mutation |
| Dacomitinib (Vizimpro, Pfizer) | Tablet (15 mg, 30 mg, and 45 mg) | 27 September 2018 (Prescription) | EGFR/HER1 | NSCLC |
| Encorafenib (Braftovi, Array Biopharma) | Capsule (50 mg, and 75 mg) | 27 June 2018 (Prescription) (50 mg capsules have been discontinued) | B-Raf | Melanoma |
| Fostamatinib disodium (Tubeless, Rigel Pharmaceuticals) | Tablet (100 mg, and 150 mg of fostamatinib free base) | 17 April 2018 (Prescription) | Syk | ITP |
| Duvelisib (Copiktra, Secura Bio) | Capsule (15 mg, and 25 mg) | 24 September 2018 (Prescription) | PI3K-δ/PI3K-γ | CLL, SLL, FL, and hematological malignancies |
| Gilteritinib fumarate (Xospata, Astellas Pharma) | Tablet (40 mg of gilteritinib free base) | 28 November 2018 (Prescription) | Flt3 | AML |
| Larotrectinib sulfate (Vitrakvi, Bayer Healthcare Pharmaceuticals) | Capsule (25 mg, and 100 mg of larotrectinib free base) | 26 November 2018 (Prescription) | TRK | Solid tumors |
| Solution (20 mg of larotrectinib free base per ml) | ||||
| Lorlatinib (Lorbrena, Pfizer) | Tablet (25 mg, and 100 mg) | 2 November 2018 (Prescription) | ALK | NSCLC |
| Entrectinib (Rozlytrek, Genentech) | Capsule (100 mg and 200 mg) | 15 August 2019 (Prescription) | TRK-A, TRK-B, and TRK-C | NSCLC and solid tumors |
| Upadacitinib (Rinvoq, Abbvie) | Extended-release tablet (15 mg) | 16 August 2019 (Prescription) | JAK | Rheumatoid arthritis |
| Alpelisib (Piqray, Novartis Pharmaceuticals) | Tablet (50 mg, 100 mg, and 200 mg) | 24 May 2019 (Prescription) | PI3K | Breast cancer |
| Erdafitinib (Balversa, Janssen Biotech) | Tablet (3 mg, 4 mg, and 5 mg) | 12 April 2019 (Prescription) | FGFR1/2/3/4 | Metastatic urothelial carcinoma (mUC) |
| Pexidartinib hydrochloride (Turalio, Daiichi Sankyo) | Capsule (200 mg of pexidartinib free base) | 2 August 2019 (Prescription) | CSF1R/KIT/Flt3 | TGCT |
| Fedratinib hydrochloride (Inrebic, Impact Biomedicines) | Capsule (100 mg of fedratinib free base) | 16 August 2019 (Prescription) | JAK2 | Myelofibrosis |
| Zanubrutinib (Brukinsa, Beigene) | Capsule (80 mg) | 14 November 2019 (Prescription) | BTK | MCL, CLL, WM, and SLL |
| Avapritinib (Ayvakit, Blueprint Medicines) | Tablet (100 mg, 200 mg, and 300 mg) | 9 January 2020 (Prescription) | PDGFRA/KIT | GIST |
| Selumetinib sulfate (Koselugo, Astrazeneca Pharmaceuticals) | Capsule (10 mg and 25 mg of selumetinib free base) | 10 April 2020 (Prescription) | MAPK/MEK 1,2 | Neurofibromatosis type 1 (NF1) |
| Pemigatinib (Pemazyre, Incyte) | Tablet (4.5 mg, 9 mg, and 135 mg) | 17 April 2020 (Prescription) | FGFR1- 3 | Cholangiocarcinoma |
| Tucatinib (Tukysa, Seagen) | Tablet (50 mg and 150 mg) | 17 April 2020 (Prescription) | HER2 | Breast cancer |
| Capmatinib hydrochloride (Tabrecta, Novartis Pharmaceutical) | Tablet (150 mg and 200 mg of capmatinib free base) | 6 May 2020 (Prescription) | MET | NSCLC |
| Selpercatinib (Retevmo, Loxo Oncology) | Capsule (40 mg and 80 mg) | 8 May 2020 (Prescription) | RET/VEGFR | NSCLC, and MTC |
| Ripretinib (Qinlock, Deciphera Pharmaceuticals) | Tablet (50 mg) | 15 May 2020 (Prescription) | PDGFRA/KIT | GIST |
| Pralsetinib (Gavreto, Blueprint Medicines) | Capsule (100 mg) | 4 September 2020 (Prescription) | RET | NSCLC, and MTC |
| Trilaciclib dihydrochloride (Cosela; G1 Therapeutics Inc.,) | Powder for IV injection (300 mg of Trilaciclib free base per vial) | 12 February 2021 (Prescription) | CDK4 | ES-SCLC |
| Tepotinib hydrochloride monohydrate (Tepmetko; EMD Serono Inc.,) | Tablet (225 mg of Tepotinib free base) | 3 February 2021 (Prescription) | MET | NSCLC |
| Umbralisib tosylate (Ukoniq; TG Therapeutics) | Tablet (200 mg of Umbralisib free base) | 5 February 2021 (Prescription) | PI3Kδ and CK1ε | MZL, and FL |
| Tivozanib hydrochloride monohydrate (Fotivda; Aveo Pharmaceuticals) | Capsule (0.89 mg and 1.34 mg of tivozanib free base) | 10 March 2021 (Prescription) | VEGFR/PDGFR | RCC |
| Infigratinib (Truseltiq; QED Therapeutics) | Capsule (25 and 100 mg) | 28 May 2021 (Prescription) | FGFR | Cholangiocarcinoma |
| S. No. | Drug’s Name | Patent Number | Applicant/Assignee | Expiry Date | Legal Status | Expected Date of Generic Availability in the USA * |
|---|---|---|---|---|---|---|
| 1 | Imatinib | US5521184A | Ciba Geigy | 4 July 2015 | Expired | Generic is available |
| USRE43932E | Novartis | 16 July 2019 | Expired | |||
| 2 | Gefitinib | US5457105A | Zeneca | 19 January 2013 | Expired | July 2022 due to the Orphan Drug Exclusivity |
| US5770599A | Zeneca | 5 May 2017 | Expired | |||
| 3 | Erlotinib | USRE41065E | OSI Pharmaceuticals | 8 May 2019 | Expired | Generic is available |
| US6900221B1 | OSI Pharmaceuticals | 9 May 2021 | Litigation | |||
| 4 | Sorafenib | US7235576B1 | Bayer Pharmaceuticals | 12 January 2020 | Expired | Generic is available |
| US8877933B2 | Bayer IP | 24 December 2027 | Patented | |||
| 5 | Sunitinib | US7125905B2 | Sugen Incorporation | 15 August 2021 | Patented | August 2021 |
| US6573293B2 | Sugen Incorporation | 15 August 2021 | Patented | |||
| 6 | Dasatinib | US6596746B1 | Bristol-Myers Squibb | 28 December 2020 | Expired | Generic is available |
| US7491725B2 | Bristol-Myers Squibb | 28 September 2026 | Patented | |||
| 7 | Lapatinib | US8513262B2 | Glaxo Group | 8 January 2019 | Expired | Generic is available |
| US7157466B2 | Smithkline Beecham | 19 November 2021 | Patented | |||
| 8 | Temsirolimus | USRE44768E | Wyeth | 15 August 2019 | Expired | Generic is available |
| 9 | Everolimus | US5665772A | Sandoz | 9 March 2020 | Expired | Generic is available |
| 10 | Nilotinib | US7169791B2 | Novartis | 4 January 2024 | Patented | February 2029 |
| US8163904B2 | Novartis | 23 February 2029 | Patented | |||
| US8415363B2 | Novartis | 18 January 2027 | Patented | |||
| 11 | Pazopanib | US7105530B2 | Smithkline Beecham | 19 October 2023 | Patented | October 2023 |
| US8114885B2 | Glaxosmithkline | 19 December 2021 | Patented | |||
| 12 | Vandetanib | USRE42353E | Astrazeneca | 27 June 2022 | Patented | June 2022 |
| 13 | Vemurafenib | US8143271B2 | Plexxikon Incorporation | 21 June 2026 | Patented | June 2026 |
| 14 | Crizotinib | US7858643B2 | Agouron Pharmaceuticals | 8 October 2029 | Patented | October 2029 |
| US8217057B2 | Pfizer | 6 November 2029 | Patented | |||
| 15 | Ruxolitinib | US7598257B2 | Incyte Corporation | 24 December 2027 | Patented | June 2028 |
| US8722693B2 | Incyte Corporation | 12 June 2028 | Patented | |||
| 16 | Axitinib | US6534524B1 | Agouron Pharmaceuticals | 29 April 2025 | Patented | April 2025 |
| US8791140B2 | Pfizer | 14 December 2030 | Patented | |||
| 17 | Bosutinib | USRE42376E | Wyeth | 13 April 2024 | Patented | April 2024 |
| US7767678B2 | Wyeth | 23 November 2026 | Patented | |||
| 18 | Regorafenib | US8637553B2 | Bayer Healthcare | 16 February 2031 | Patented | July 2032 |
| US9957232B2 | Bayer Healthcare | 9 July 2032 | Patented | |||
| 19 | Tofacitinib | USRE41783E | Pfizer | 8 December 2025 | Patented | December 2025 |
| US6965027B2 | Pfizer | 25 March 2023 | Patented | |||
| 20 | Cabozantinib | US7579473B2 | Exelixis | 14 August 2026 | Patented | August 2026 |
| US8877776B2 | Exelixis | 8 October 2030 | Patented | |||
| 21 | Ponatinib | US8114874B2 | Ariad Pharmaceuticals | 24 January 2027 | Patented | January 2027 |
| US9493470B2 | Ariad Pharmaceuticals | 12 December 2033 | Patented | |||
| 22 | Trametinib | US7378423B2 | Japan Tobacco | 29 May 2027 | Patented | May 2027 |
| 23 | Dabrafenib | US7994185B2 | Glaxo Smith Kline | 20 January 2030 | Patented | January 2030 |
| 24 | Afatinib | USRE43431E | Boehringer Ingelheim | 13 January 2026 | Patented | January 2026 |
| US8426586B2 | Boehringer Ingelheim | 10 October 2029 | Patented | |||
| 25 | Ibrutinib | US8735403B2 | Pharmacyclics | 28 December 2026 | Patented | December 2026 |
| US9296753B2 | Pharmacyclics | 30 October 2033 | Patented | |||
| 26 | Ceritinib | US8039479B2 | IRM | 29 June 2030 | Patented | June 2030 |
| US9309229B2 | Novartis | 18 January 2032 | Patented | |||
| 27 | Idelalisib | USRE44638E | ICOS Corporation | 5 August 2025 | Patented | August 2025 |
| US9469643B2 | Gilead | 2 September 2033 | Patented | |||
| 28 | Nintedanib | US6762180B1 | Boehringer Ingelheim | 1 October 2025 | Patented | October 2025 |
| US7119093B2 | Boehringer Ingelheim | 21 February 2024 | Patented | |||
| 29 | Palbociclib | USRE47739E | Warner Lambert | 5 March 2027 | Patented | 5 March 2027 |
| US10723730B2 | Pfizer | 8 February 2034 | Patented | |||
| 30 | Lenvatinib | US7253286B2 | Eisai | 19 October 2021 | Patented | October 2021 |
| US7612208B2 | Eisai | 19 September 2026 | Patented | |||
| 31 | Cobimetinib | US7803839B2 | Exelixis | 10 November 2029 | Patented | November 2029 |
| US10590102B2 | Exelixis | 30 June 2036 | Patented | |||
| 32 | Osimertinib | US8946235B2 | Astrazeneca | 8 August 2032 | Patented | August 2032 |
| 33 | Alectinib | US9126931B2 | Chugai Pharmaceutical | 29 May 2031 | Patented | May 2031 |
| 34 | Ribociclib | US8415355B2 | Astex Therapeutics | 19 February 2031 | Patented | 19 February 2031 |
| US9193732B2 | Astex Therapeutics | 9 November 2031 | Patented | |||
| 35 | Brigatinib | US9012462B2 | Ariad Pharmaceuticals | 31 July 2030 | Patented | July 2030 |
| US10385078B2 | Ariad Pharmaceuticals | 10 November 2035 | Patented | |||
| 36 | Midostaurin | US5093330A | Ciba Geigy | 21 July 2009 | Expired | October 2024 |
| US7973031B2 | Novartis | 17 October 2024 | Patented | |||
| 37 | Neratinib | US7399865B2 | Wyeth | 29 December 2025 | Patented | December 2025 |
| 38 | Copanlisib | USRE46856E | Bayer | 22 October 2029 | Patented | March 2032 |
| US10383876B2 | Bayer | 29 March 2032 | Patented | |||
| 39 | Abemaciclib | US7855211B2 | Eli Lilly | 15 December 2029 | Patented | December 2029 |
| 40 | Acalabrutinib | US9290504B2 | Merck | 11 July 2032 | Patented | July 2032 |
| US9796721B2 | Acerta Pharma | 1 July 2036 | Patented | |||
| 41 | Netarsudil | US8394826B2 | Aerie Pharmaceuticals | 10 November 2030 | Patented | March 2034 |
| US9415043B2 | Aerie Pharmaceuticals | 14 March 2034 | Patented | |||
| 42 | Baricitinib | US8158616B2 | Incyte Corporation | 8 June 2030 | Patented | June 2030 |
| 43 | Binimetinib | US7777050B2 | Array Biopharma | 13 March 2023 | Patented | June 2025 based on ODE |
| US9562016B2 | Array Biopharma | 18 October 2033 | Patented | |||
| 44 | Dacomitinib | US7772243B2 | Warner Lambert | 26 August 2028 | Patented | August 2028 |
| 45 | Encorafenib | US8501758B2 | IRM | 4 March 2031 | Patented | March 2031 |
| 46 | Fostamatinib | US7449458B2 | Rigel Pharmaceuticals | 4 September 2026 | Patented | 4 September 2026 |
| US8163902B2 | Rigel Pharmaceuticals | 17 June 2026 | Patented | |||
| 47 | Duvelisib | US8193182B2 | Intellikine | 13 February 2030 | Patented | February 2030 |
| USRE46621E | Infinity Pharmaceuticals | 17 May 2032 | Patented | |||
| 48 | Gilteritinib | US8969336B2 | Astellas Pharma | 27 January 2031 | Patented | January 2031 |
| 49 | Larotrectinib | US9127013B2 | Array Biopharma | 21 October 2029 | Patented | October 2029 |
| US10172861B2 | Array Biopharma | 16 November 2035 | Patented | |||
| 50 | Lorlatinib | US8680111B2 | Pfizer | 5 March 2033 | Patented | March 2033 |
| US10420749B2 | Pfizer | 27 July 2036 | Patented | |||
| 51 | Entrectinib | US8299057B2 | Nerviano Medical Sciences | 1 March 2029 | Patented | March 2029 |
| US10738037B2 | Nerviano Medical Sciences | 18 May 2037 | Patented | |||
| 52 | Upadacitinib | USRE47221E | Abbvie | 1 December 2030 | Patented | December 2030 |
| US9951080B2 | Abbvie | 17 October 2036 | Patented | |||
| 53 | Alpelisib | US8227462B2 | Novartis | 28 September 2030 | Patented | September 2030 |
| 54 | Erdafitinib | US8895601B2 | Astex Therapeutics | 22 May 2031 | Patented | May 2031 |
| 55 | Pexidartinib | US9169250B2 | Plexxikon | 21 November 2027 | Patented | November 2027 |
| US9802932B2 | Plexxikon | 5 May 2036 | Patented | |||
| 56 | Fedratinib | US7528143B2 | Targegen | 16 December 2026 | Patented | December 2026 |
| 57 | Zanubrutinib | US9447106B2 | Beigene | 22 April 2034 | Patented | April 2034 |
| 58 | Avapritinib | US9944651B2 | Blueprint Medicines Corporation | 15 October 2034 | Patented | October 2034 |
| 59 | Selumetinib | US7425637B2 | Array Biopharma | 11 April 2024 | Patented | April 2027 based on ODE |
| US9156795B2 | Array Biopharma | 12 December 2026 | Patented | |||
| 60 | Pemigatinib | US9611267B2 | Incyte Corporation | 30 January 2035 | Patented | January 2035 |
| 61 | Tucatinib | US8648087B2 | Array Biopharma | 12 April 2031 | Patented | April 2031 |
| 62 | Capmatinib | US7767675B2 | Incyte Corporation | 19 November 2027 | Patented | June 2031 |
| US8420645B2 | Incyte Corporation | 5 June 2031 | Patented | |||
| 63 | Selpercatinib | US10112942B2 | Array Biopharma | 10 October 2037 | Patented | October 2037 |
| US10584124B2 | Array Biopharma | 10 October 2038 | Patented | |||
| 64 | Ripretinib | US8461179B1 | Deciphera Pharmaceuticals | 7 June 2032 | Patented | June 2032 |
| 65 | Pralsetinib | US10030005B2 | Blueprint Medicines Corporation | 1 November 2036 | Patented | November 2036 |
| 66 | Trilaciclib | US8598186B2 | G1 Therapeutics | 25 October 2031 | Patented | October 2031 |
| 67 | Tepotinib | US8580781B2 | Merck | 19 March 2030 | Patented | March 2030 |
| US8329692B2 | Merck | 30 October 2029 | Patented | |||
| 68 | Umbralisib | US10570142B2 | Rhizen Pharmaceuticals | 2 July 2033 | Patented | July 2033 |
| US10414773B2 | Rhizen Pharmaceuticals | 26 May 2035 | Patented | |||
| 69 | Tivozanib | US6821987B2 | Kirin Beer Kabushiki Kaisha | 26 April 2022 | Patented | 10 March 2026, based on NCE (Patent term extension is possible) |
| US7211587B2 | Kirin Beer Kabushiki Kaisha | 26 April 2022 | Patented | |||
| US7166722B2 | Kirin Beer Kabushiki Kaisha | 21 October 2023 | Patented | |||
| 70 | Infigratinib | US8552002B2 | Novartis | 13 December 2025 | Patented | 25 May 2026, based on NCE (Patent term extension is possible) |
| US9067896B2 | Novartis | 24 February 2031 | Patented |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Asdaq, S.M.B.; Khan, S.A.; Unnikrishnan Meenakshi, D.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Mohzari, Y.; Alrashed, A.; AlMotairi, M.; et al. Innovations and Patent Trends in the Development of USFDA Approved Protein Kinase Inhibitors in the Last Two Decades. Pharmaceuticals 2021, 14, 710. https://doi.org/10.3390/ph14080710
Imran M, Asdaq SMB, Khan SA, Unnikrishnan Meenakshi D, Alamri AS, Alsanie WF, Alhomrani M, Mohzari Y, Alrashed A, AlMotairi M, et al. Innovations and Patent Trends in the Development of USFDA Approved Protein Kinase Inhibitors in the Last Two Decades. Pharmaceuticals. 2021; 14(8):710. https://doi.org/10.3390/ph14080710
Chicago/Turabian StyleImran, Mohd., Syed Mohammed Basheeruddin Asdaq, Shah Alam Khan, Dhanalekshmi Unnikrishnan Meenakshi, Abdulhakeem S. Alamri, Walaa F. Alsanie, Majid Alhomrani, Yahya Mohzari, Ahmed Alrashed, Mohammed AlMotairi, and et al. 2021. "Innovations and Patent Trends in the Development of USFDA Approved Protein Kinase Inhibitors in the Last Two Decades" Pharmaceuticals 14, no. 8: 710. https://doi.org/10.3390/ph14080710
APA StyleImran, M., Asdaq, S. M. B., Khan, S. A., Unnikrishnan Meenakshi, D., Alamri, A. S., Alsanie, W. F., Alhomrani, M., Mohzari, Y., Alrashed, A., AlMotairi, M., Alkhaldi, E. H., Alorabi, A. K., Alshrari, A. S., Tauseef, M., Abida, Alaqel, S. I., Alam, O., & Bakht, M. A. (2021). Innovations and Patent Trends in the Development of USFDA Approved Protein Kinase Inhibitors in the Last Two Decades. Pharmaceuticals, 14(8), 710. https://doi.org/10.3390/ph14080710








