Developing a Low-Cost Device for Estimating Air–Water ΔpCO2 in Coastal Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Developing the SEACOW
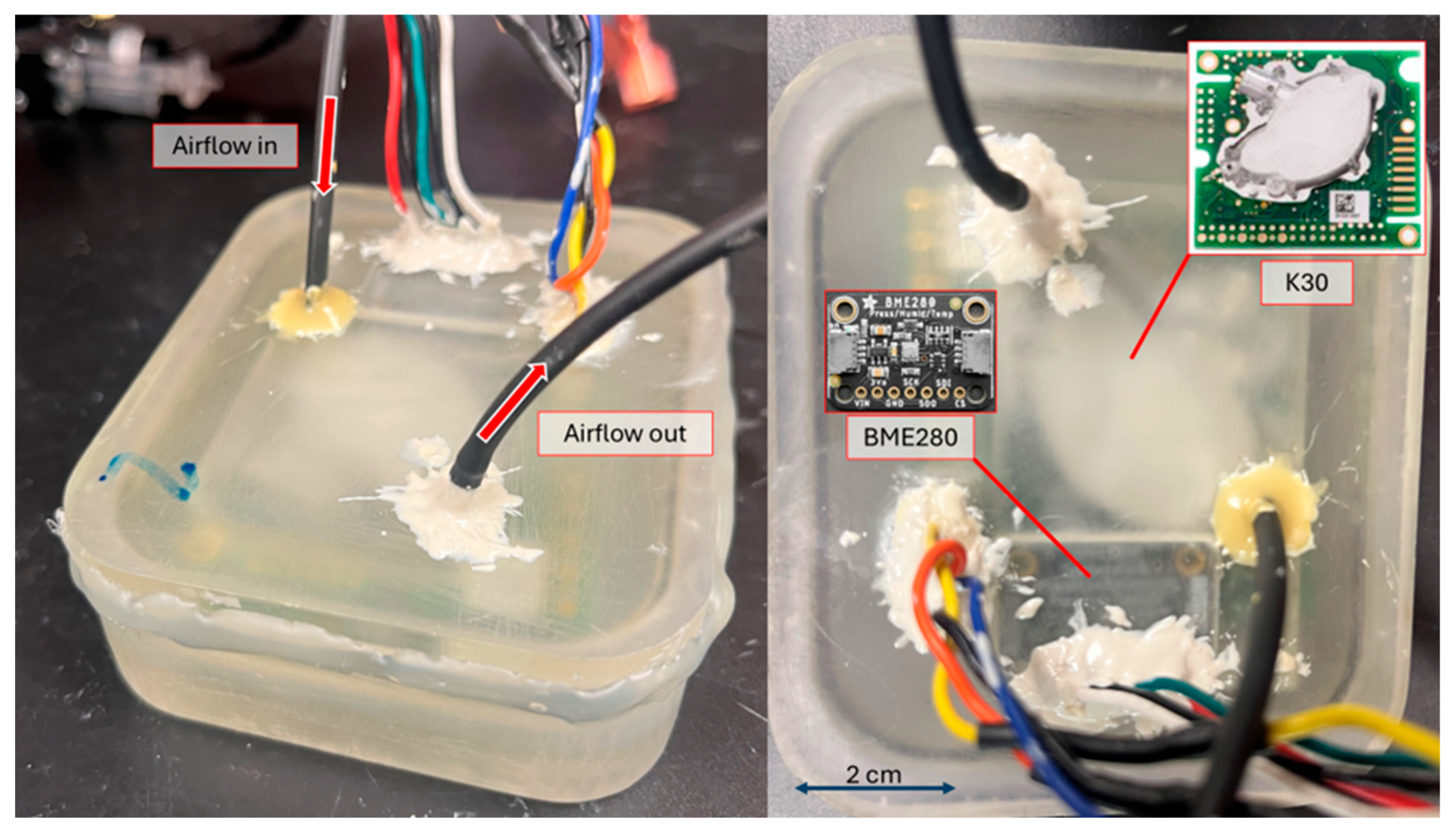
2.1.1. Internal Components
2.1.2. Electronics and Software
2.1.3. Outer Housing
2.2. Characterizing the SEACOW
2.2.1. Air-Side Accuracy
2.2.2. Response Time
2.2.3. Humidity and Pressure Correction
2.3. Laboratory Seagrass Experiment
3. Results
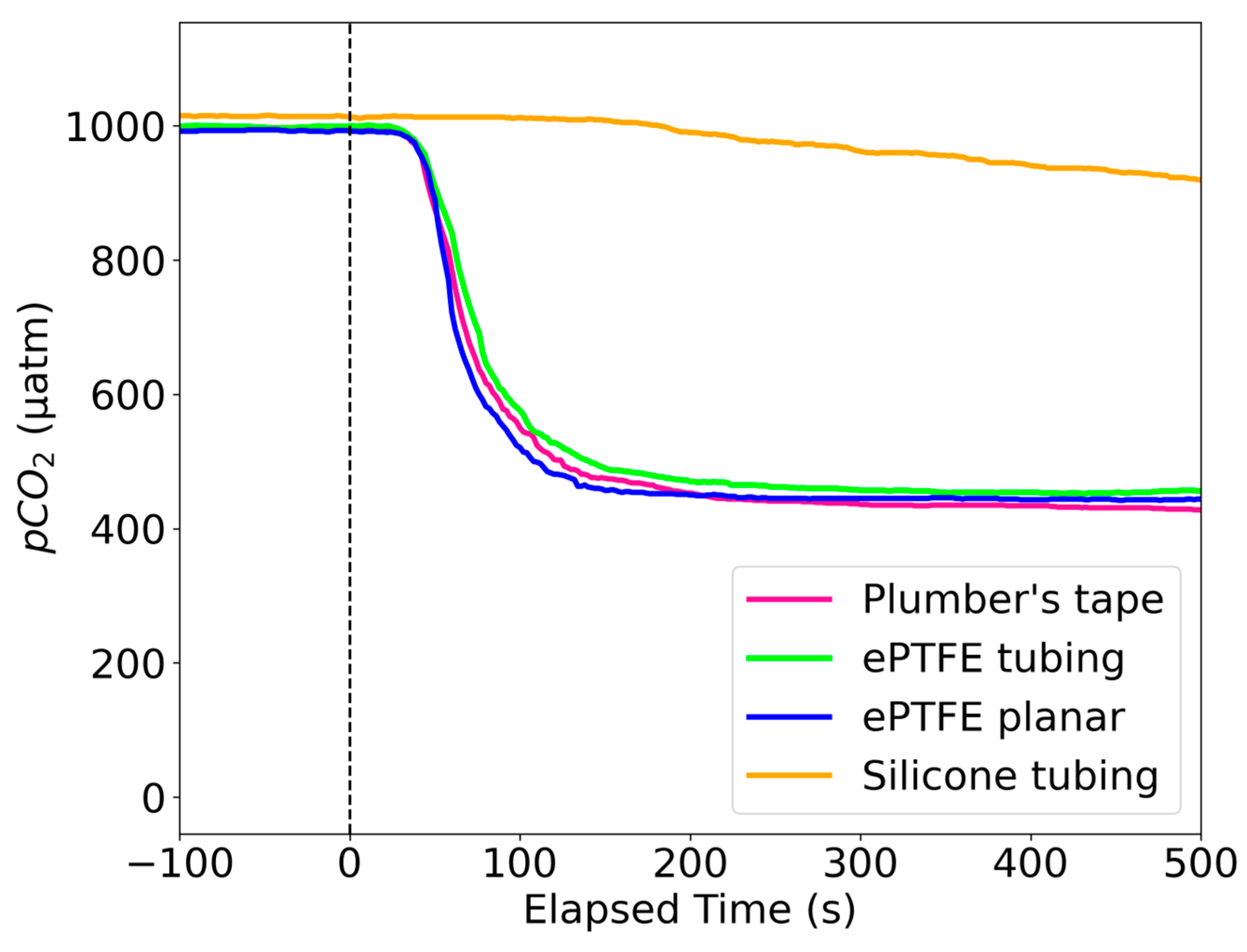
3.1. Response Times of Different Membranes
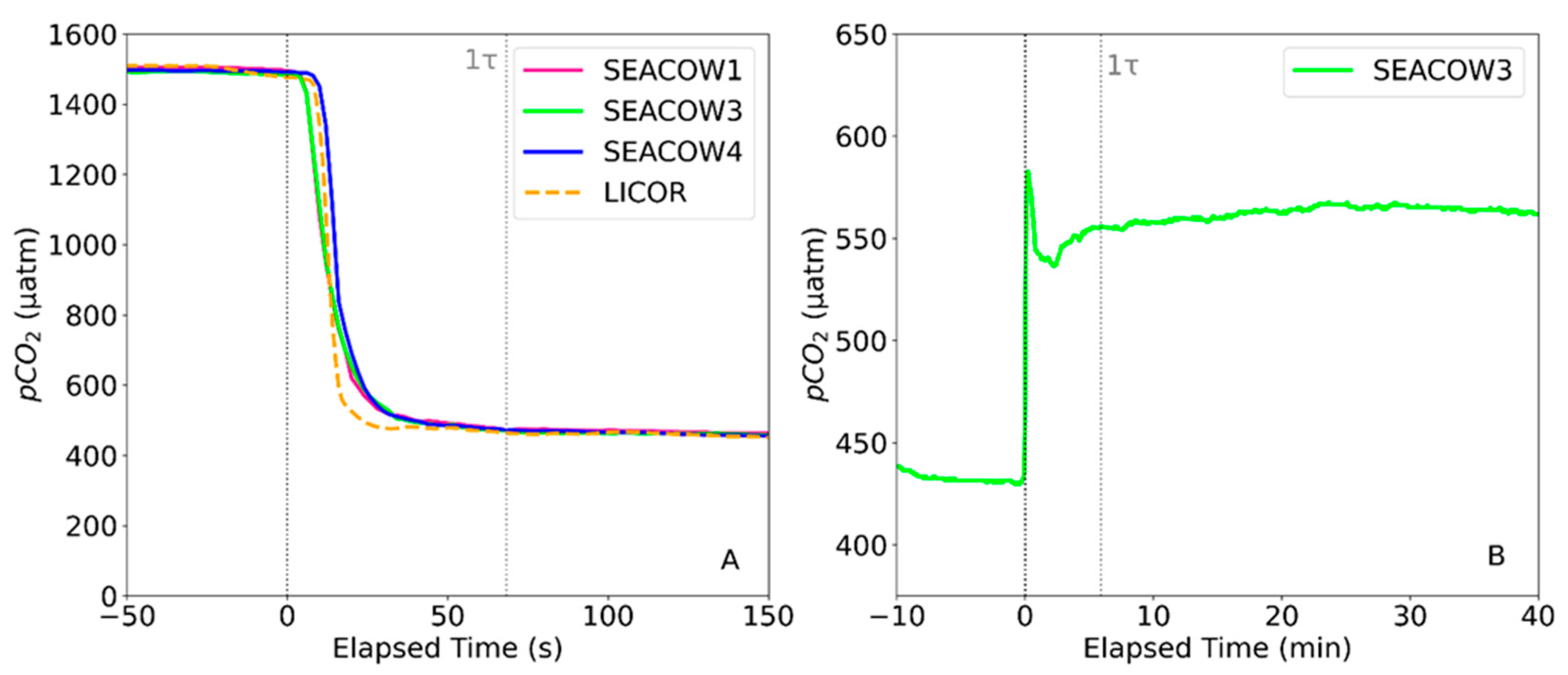
3.2. Air- and Water-Side Response Times
3.3. Air-Side Accuracy
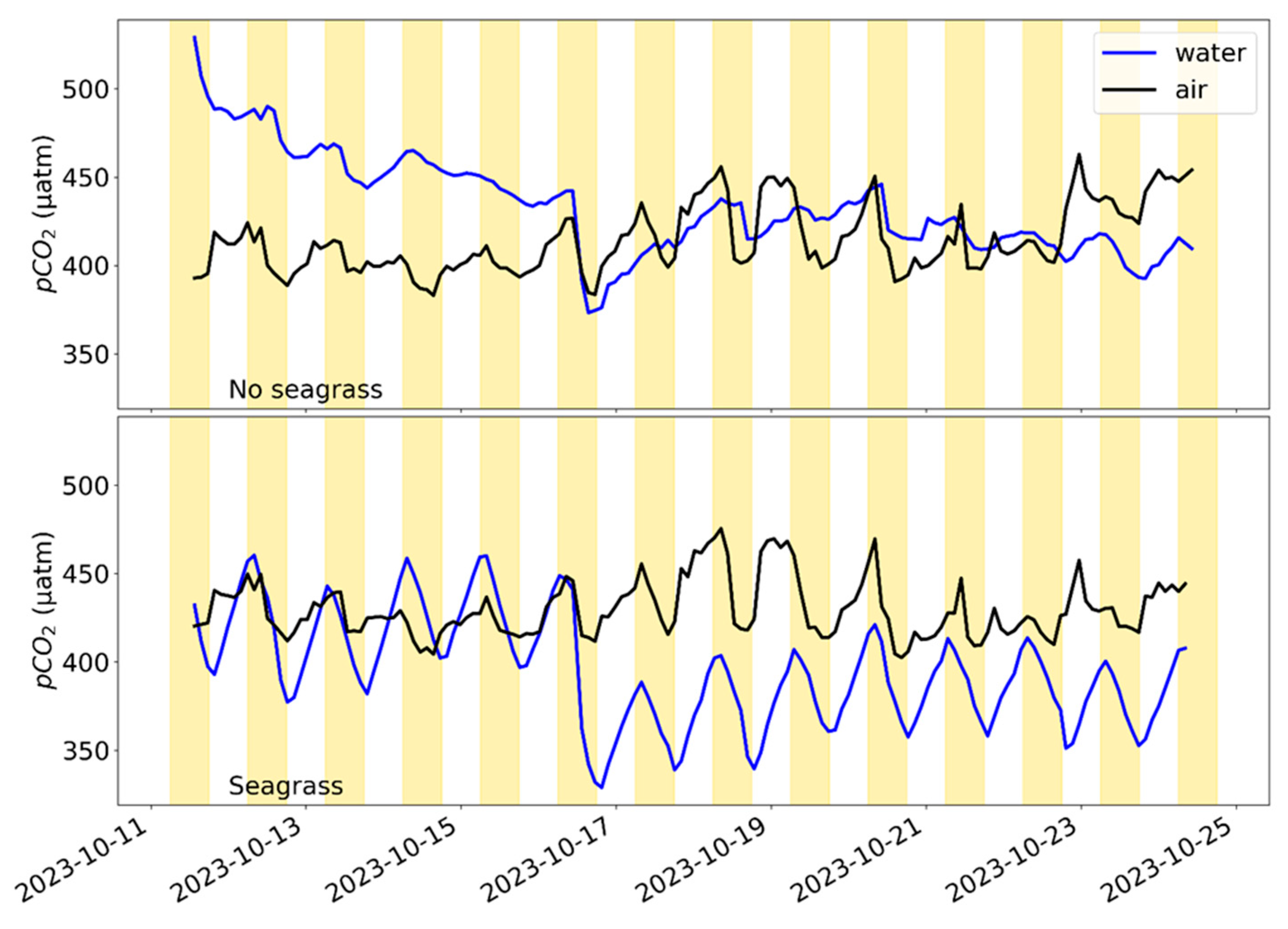
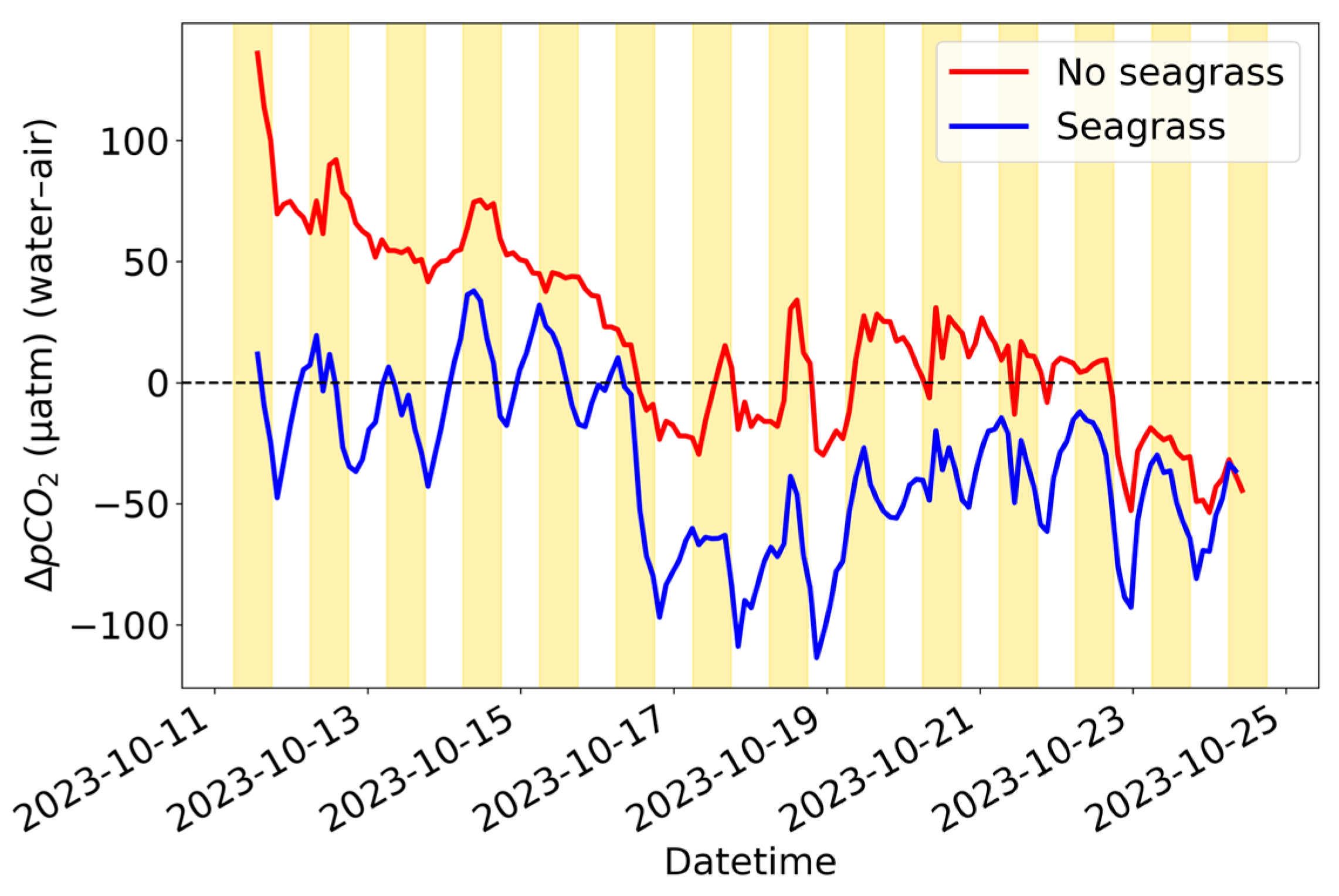
3.4. Seagrass Tank Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEACOW | System for Exchange of Atmospheric CO2 with Water |
| ePTFE/PTFE | Expanded Polytetrafluoroethylene |
| NDIR | Non-dispersive infrared |
| UART | Universal asynchronous receiver transmitter |
| DI | Deionized |
| atm | Atmosphere |
Appendix A
- Particle Boron (BRN404XKIT): This is the microcontroller that carries out the commands from the firmware. The Boron has an onboard cellular modem with LTE capabilities, allowing it to upload data directly to a Google spreadsheet during its deployments. It is powered using a rechargeable 3.7 V Li-ion battery.
- Lee Co 3-way solenoid valves (LHLA0531211H): These control the flow of air to switch between measuring the air side and the water side of the instrument.
- Sparkfun motor driver (ROB-14450): This controls the solenoid valves.
- Blue Robotics temperature sensor (BR-100317): This measures the water temperature.
- Adafruit BME 280 sensor (2652): This measures the temperature, pressure, and humidity inside of the K30 housing.
- Adafruit TMP 117 sensor (4821): The measures the temperature on the air side of the instrument.
- Senseair K30 CO2 sensor (030-8-0006): This is the NDIR sensor that is measuring the pCO2.
- Adafruit Powerboost (1944): This converts the 3.3 V output of the Boron to a 5 V output, which is the minimum voltage required for the K30 to function. It also turns on and off the K30 during sleeping periods.
- Adafruit Adalogger Featherwing (2922): The datalogger stores data onto its SD card.
- Diaphragm gas pump (UNMP 05): This moves the air throughout the closed loop system.
- Sparkfun MOSFET power control kit (COM-12959): This allows us to turn the pump on and off in between samples.

Appendix B
- T = temperature = 296.15 K;
- R = 0.0821 (L atm mol−1 K−1);
- P = room pressure = 1 atm;
- C = the desired CO2 concentration in ppm;
- MFCN2 = the mass flow controller for N2 is set at 5 Lpm.
Appendix C
| Set Point (µatm) | SEACOW1 Average Reading (µatm) | SEACOW3 Average Reading (µatm) | SEACOW4 Average Reading (µatm) | LI-850 Average Reading (µatm) | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | ||
| 0 | 94 ± 0.78 | −19 ± 0.37 | 6 ± 0.91 | 25 ± 0.89 | 51 ± 0.73 | −30 ± 0.54 | 1.44 ± 0.09 |
| 250 | 369 ± 0.57 | 230 ± 0.52 | 208 ± 0.70 | 233 ± 0.79 | 298 ± 1.82 | 219 ± 1.36 | 238.38 ± 0.20 |
| 500 | 653 ± 0.61 | 484 ± 0.52 | 445 ± 0.88 | 472 ± 0.80 | 561 ± 1.08 | 474 ± 1.00 | 490.90 ± 0.32 |
| 750 | 937 ± 0.87 | 738 ± 0.76 | 690 ± 0.98 | 719 ± 0.94 | 825 ± 2.42 | 726 ± 2.21 | 744.75 ± 0.50 |
| 1000 | 1229 ± 0.61 | 997 ± 0.49 | 941 ± 1.37 | 968 ± 1.30 | 1097 ± 1.42 | 985 ± 1.30 | 999.32 ± 0.59 |
| 1500 | 1798 ± 0.84 | 1505 ± 0.75 | 1464 ± 1.42 | 1493 ± 1.47 | 1633 ± 2.04 | 1496 ± 1.91 | 1508.16 ± 0.72 |
| Dry calibration curves: SEACOW1: K30corrected = 0.89 (K30raw) − 86 (R2 = 1); SEACOW3: K30corrected = 1 (K30raw) + 20 (R2 = 0.99); SEACOW4: K30corrected = 0.95 (K30raw) − 45 (R2 = 1). | |||||||
References
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Li, H.; Luijkx, I.T.; Olsen, A. Global carbon budget 2024. Earth Syst. Sci. Data Discuss. 2024, 2024, 1–133. [Google Scholar] [CrossRef]
- Dai, M.; Su, J.; Zhao, Y.; Hofmann, E.E.; Cao, Z.; Cai, W.-J.; Gan, J.; Lacroix, F.; Laruelle, G.G.; Meng, F.; et al. Carbon Fluxes in the Coastal Ocean: Synthesis, Boundary Processes, and Future Trends. Annu. Rev. Earth Planet. Sci. 2022, 50, 593–626. [Google Scholar] [CrossRef]
- Bauer, J.E.; Cai, W.-J.; Raymond, P.A.; Bianchi, T.S.; Hopkinson, C.S.; Regnier, P.A.G. The changing carbon cycle of the coastal ocean. Nature 2013, 504, 61–70. [Google Scholar] [CrossRef]
- Takahashi, T.; Olafsson, J.; Goddard, J.G.; Chipman, D.W.; Sutherland, S. Seasonal variation of CO2 and nutrients in the high-latitude surface oceans: A comparative study. Glob. Biogeochem. Cycles 1993, 7, 843–878. [Google Scholar] [CrossRef]
- Lefèvre, N.; Watson, A.J.; Cooper, D.J.; Weiss, R.F.; Takahashi, T.; Sutherland, S.C. Assessing the seasonality of the oceanic sink for CO2 in the northern hemisphere. Glob. Biogeochem. Cycles 1999, 13, 273–286. [Google Scholar] [CrossRef]
- Gregor, L.; Shutler, J.; Gruber, N. High-resolution variability of the ocean carbon sink. Glob. Biogeochem. Cycles 2024, 38, e2024GB008127. [Google Scholar] [CrossRef]
- Herr, D.; Landis, E. Coastal Blue Carbon Ecosystems. Opportunities for Nationally Determined Contributions; Policy Brief; IUCN: Gland, Switzerland; TNC: Washington, DC, USA, 2016. [Google Scholar]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; Mcglathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Macreadie, P.; Baird, M.; Trevathan-Tackett, S.; Larkum, A.; Ralph, P. Quantifying and modelling the carbon sequestration capacity of seagrass meadows—A critical assessment. Mar. Pollut. Bull. 2014, 83, 430–439. [Google Scholar] [CrossRef]
- Resplandy, L.; Hogikyan, A.; Müller, J.; Najjar, R.; Bange, H.W.; Bianchi, D.; Weber, T.; Cai, W.J.; Doney, S.; Fennel, K. A synthesis of global coastal ocean greenhouse gas fluxes. Glob. Biogeochem. Cycles 2024, 38, e2023GB007803. [Google Scholar] [CrossRef]
- Watson, A.J.; Schuster, U.; Shutler, J.D.; Holding, T.; Ashton, I.G.; Landschützer, P.; Woolf, D.K.; Goddijn-Murphy, L. Revised estimates of ocean-atmosphere CO2 flux are consistent with ocean carbon inventory. Nat. Commun. 2020, 11, 4422. [Google Scholar] [CrossRef]
- Takahashi, T.; Sutherland, S.C.; Wanninkhof, R.; Sweeney, C.; Feely, R.A.; Chipman, D.W.; Hales, B.; Friederich, G.; Chavez, F.; Sabine, C. Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 554–577. [Google Scholar]
- Rosentreter, J.A. Water-air gas exchange of CO2 and CH4 in coastal wetlands. In Carbon Mineralization in Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2022; pp. 167–196. [Google Scholar]
- Wanninkhof, R. Relationship between gas exchange and wind speed over the ocean. J. Geophys. Res. 1992, 97, 7373–7381. [Google Scholar] [CrossRef]
- Wanninkhof, R.; Asher, W.E.; Ho, D.T.; Sweeney, C.; McGillis, W.R. Advances in quantifying air-sea gas exchange and environmental forcing. Annu. Rev. Mar. Sci. 2009, 1, 213–244. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.W.; Snyder, L.; Salisbury, J.E.; Vandemark, D.; Mcdowell, W.H. SIPCO2: A simple, inexpensive surface water pCO2 sensor. Limnol. Oceanogr. Methods 2017, 15, 291–301. [Google Scholar] [CrossRef]
- Wall, C.M. Autonomous In Situ Measurements of Estuarine Surface PCO2: Instrument Development and Initial Estuarine Observations. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2014. [Google Scholar]
- Graziani, S.; Beaubien, S.E.; Bigi, S.; Lombardi, S. Spatial and temporal pCO2 marine monitoring near Panarea Island (Italy) using multiple low-cost GasPro sensors. Environ. Sci. Technol. 2014, 48, 12126–12133. [Google Scholar] [CrossRef]
- Ge, X.; Kostov, Y.; Henderson, R.; Selock, N.; Rao, G. A low-cost fluorescent sensor for pCO2 measurements. Chemosensors 2014, 2, 108–120. [Google Scholar] [CrossRef]
- Pan, C.; Patel, V.; Gewirtzman, J.; Richardson, I.; Dubey, R.; Caylor, K.; Dollar, A.; Forbes, E. Fluxbot: The Next Generation-Design and Validation of a Wireless, Open-Source Mechatronic CO2 Flux Sensing Chamber. In Proceedings of the 7th ACM SIGCAS/SIGCHI Conference on Computing and Sustainable Societies, New Delhi, India, 8–11 July 2024; pp. 85–96. [Google Scholar]
- Sabine, C.; Sutton, A.; Mccabe, K.; Lawrence-Slavas, N.; Alin, S.; Feely, R.; Jenkins, R.; Maenner, S.; Meinig, C.; Thomas, J.; et al. Evaluation of a New Carbon Dioxide System for Autonomous Surface Vehicles. J. Atmos. Ocean. Technol. 2020, 37, 1305–1317. [Google Scholar] [CrossRef]
- Nicholson, D.P.; Michel, A.P.M.; Wankel, S.D.; Manganini, K.; Sugrue, R.A.; Sandwith, Z.O.; Monk, S.A. Rapid Mapping of Dissolved Methane and Carbon Dioxide in Coastal Ecosystems Using the ChemYak Autonomous Surface Vehicle. Environ. Sci. Technol. 2018, 52, 13314–13324. [Google Scholar] [CrossRef]
- Sutton, A.J.; Sabine, C.L.; Maenner-Jones, S.; Lawrence-Slavas, N.; Meinig, C.; Feely, R.A.; Mathis, J.T.; Musielewicz, S.; Bott, R.; Mclain, P.D.; et al. A high-frequency atmospheric and seawater pCO2 data set from 14 open-ocean sites using a moored autonomous system. Earth Syst. Sci. Data 2014, 6, 353–366. [Google Scholar] [CrossRef]
- Johnson, M.S.; Billett, M.F.; Dinsmore, K.J.; Wallin, M.; Dyson, K.E.; Jassal, R.S. Direct and continuous measurement of dissolved carbon dioxide in freshwater aquatic systems-method and applications. Ecohydrology 2009, 3, 68–78. [Google Scholar] [CrossRef]
- Yasuda, T.; Yonemura, S.; Tani, A. Comparison of the Characteristics of Small Commercial NDIR CO2 Sensor Models and Development of a Portable CO2 Measurement Device. Sensors 2012, 12, 3641–3655. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Zeng, N.; Karion, A.; Dickerson, R.R.; Ren, X.; Turpie, B.N.; Weber, K.J. Evaluation and environmental correction of ambient CO2 measurements from a low-cost NDIR sensor. Atmos. Meas. Tech. 2017, 10, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Senseair AB. Modbus on Senseair K30, Senseair K33 and eSENSE; Document TDE2336; Senseair AB: Delsbo, Sweden, 2023; 19p. [Google Scholar]
- LI-COR. Maintenance on LI-850 and LI-830 Analyzers. Available online: https://www.licor.com/support/LI-850/topics/maintenance.html (accessed on 16 July 2024).
- Mazzotti, F.J.; Pearlstine, L.G.; Chamberlain, R.; Barnes, T.; Chartier, K.; DeAngelis, D. Stressor Response Models for Seagrasses, Halodule Wrightii and Thalassia Testudnium; University of Florida, Florida Lauderdale Research and Education Center: Fort Lauderdale, FL, USA, 2007; p. 19. [Google Scholar]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide To Best Practices for Ocean CO2 Measurements; North Pacific Marine Science Organization: Sidney, BC, Canada, 2007. [Google Scholar]
- Van Heuven, S.; Pierrot, D.; Rae, J.; Lewis, E.; Wallace, D. MATLAB Program Developed for CO2 System Calculations; Carbon Dioxide Information Analysis Center (CDIAC): Oak Ridge, TN, USA, 2011. [Google Scholar]
- Farquhar, E.; Bresnahan, P.; Tydings, M.; Portelli, D. COAST-Lab/SEACOW_Public: V1.0.1. 2025. Available online: https://zenodo.org/records/15122776 (accessed on 2 April 2025).
- Fujita, K.; Hikami, M.; Suzuki, A.; Kuroyanagi, A.; Sakai, K.; Kawahata, H.; Nojiri, Y. Effects of ocean acidification on calcification of symbiont-bearing reef foraminifers. Biogeosciences 2011, 8, 2089–2098. [Google Scholar] [CrossRef]
- Körtzinger, A.; Send, U.; Lampitt, R.; Hartman, S.; Wallace, D.W.; Karstensen, J.; Villagarcia, M.; Llinás, O.; DeGrandpre, M. The seasonal pCO2 cycle at 49 N/16.5 W in the northeastern Atlantic Ocean and what it tells us about biological productivity. J. Geophys. Res. Ocean. 2008, 113, C04020. [Google Scholar] [CrossRef]
- Bastviken, D.; Sundgren, I.; Natchimuthu, S.; Reyier, H.; Gålfalk, M. Cost-efficient approaches to measure carbon dioxide (CO2) fluxes and concentrations in terrestrial and aquatic environments using mini loggers. Biogeosciences 2015, 12, 3849–3859. [Google Scholar] [CrossRef]
- Robison, A.L.; Koenig, L.E.; Potter, J.D.; Snyder, L.E.; Hunt, C.W.; McDowell, W.H.; Wollheim, W.M. Lotic-SIPCO2: Adaptation of an open-source CO2 sensor system and examination of associated emission uncertainties across a range of stream sizes and land uses. Limnol. Oceanogr. Methods 2024, 22, 191–207. [Google Scholar] [CrossRef]
- Lee, D.J.J.; Kek, K.T.; Wong, W.W.; Mohd Nadzir, M.S.; Yan, J.; Zhan, L.; Poh, S.C. Design and optimization of wireless in-situ sensor coupled with gas–water equilibrators for continuous pCO2 measurement in aquatic environments. Limnol. Oceanogr. Methods 2022, 20, 500–513. [Google Scholar] [CrossRef]


| Characterization Parameter | Value |
|---|---|
| Accuracy | ±2.5% of LI-850’s readings |
| Air-side 5τ time | 5.7 min |
| Water-side 5τ time | ~30 min |
| Power draw | 185 mW |
| Drierite budget | 162 g per 5 days |
| Temperature range | 5–40 °C |
| Cost in parts | ~1400 USD |
| Github design files [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farquhar, E.B.; Bresnahan, P.J.; Tydings, M.; Jarvis, J.C.; Whitehead, R.F.; Portelli, D. Developing a Low-Cost Device for Estimating Air–Water ΔpCO2 in Coastal Environments. Sensors 2025, 25, 3547. https://doi.org/10.3390/s25113547
Farquhar EB, Bresnahan PJ, Tydings M, Jarvis JC, Whitehead RF, Portelli D. Developing a Low-Cost Device for Estimating Air–Water ΔpCO2 in Coastal Environments. Sensors. 2025; 25(11):3547. https://doi.org/10.3390/s25113547
Chicago/Turabian StyleFarquhar, Elizabeth B., Philip J. Bresnahan, Michael Tydings, Jessie C. Jarvis, Robert F. Whitehead, and Dan Portelli. 2025. "Developing a Low-Cost Device for Estimating Air–Water ΔpCO2 in Coastal Environments" Sensors 25, no. 11: 3547. https://doi.org/10.3390/s25113547
APA StyleFarquhar, E. B., Bresnahan, P. J., Tydings, M., Jarvis, J. C., Whitehead, R. F., & Portelli, D. (2025). Developing a Low-Cost Device for Estimating Air–Water ΔpCO2 in Coastal Environments. Sensors, 25(11), 3547. https://doi.org/10.3390/s25113547







