Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
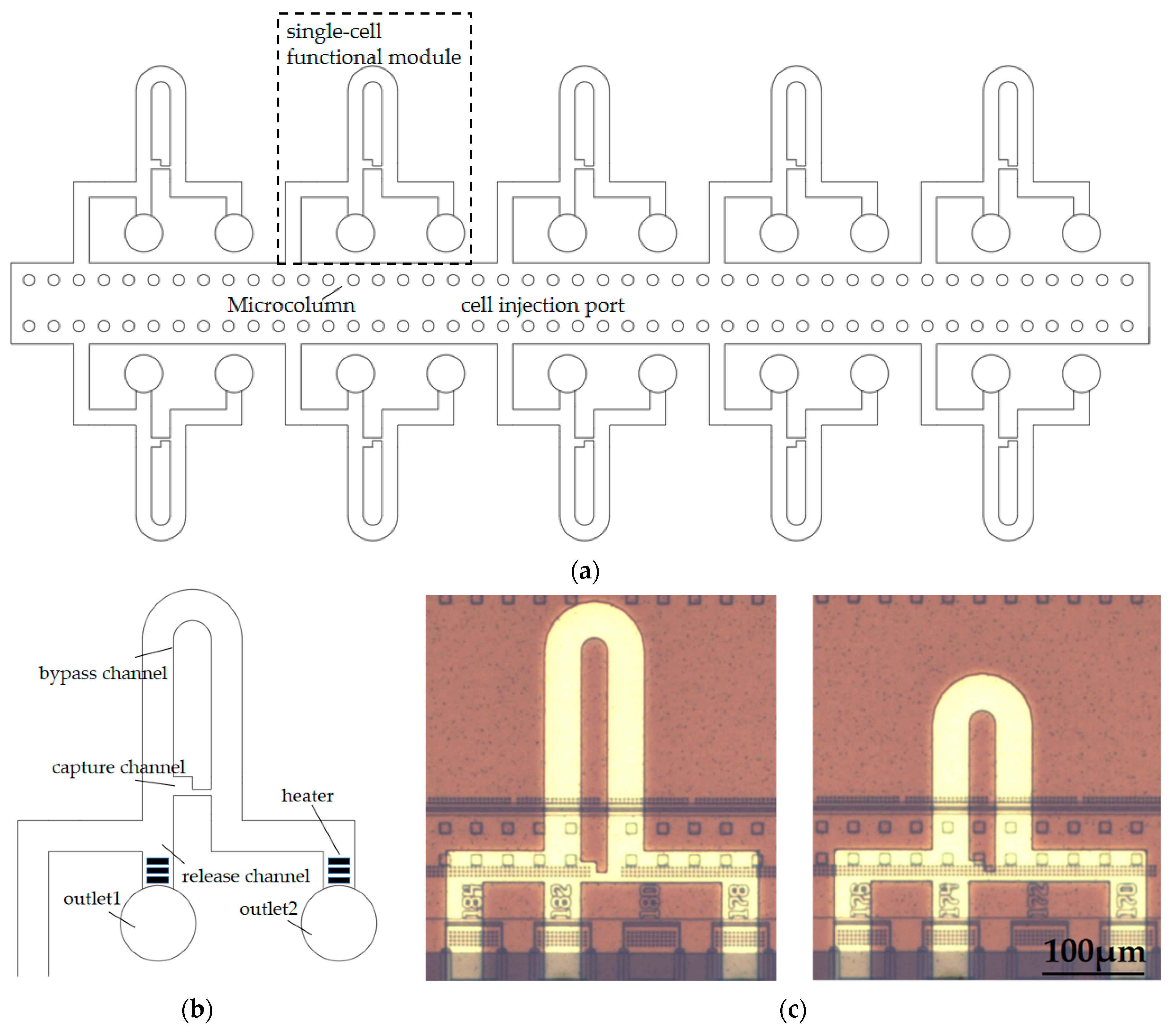
2.2. Microfluidic Chip Design
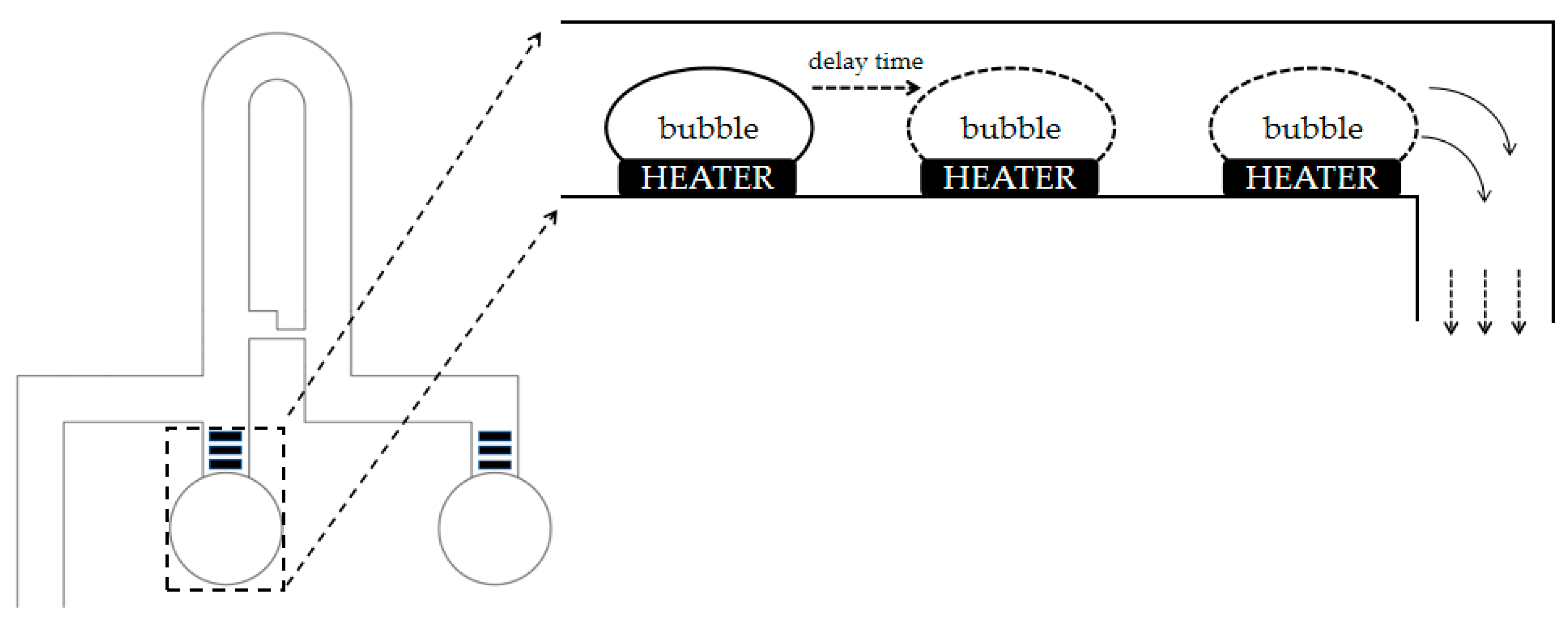
2.3. Thermal Bubble Micropump
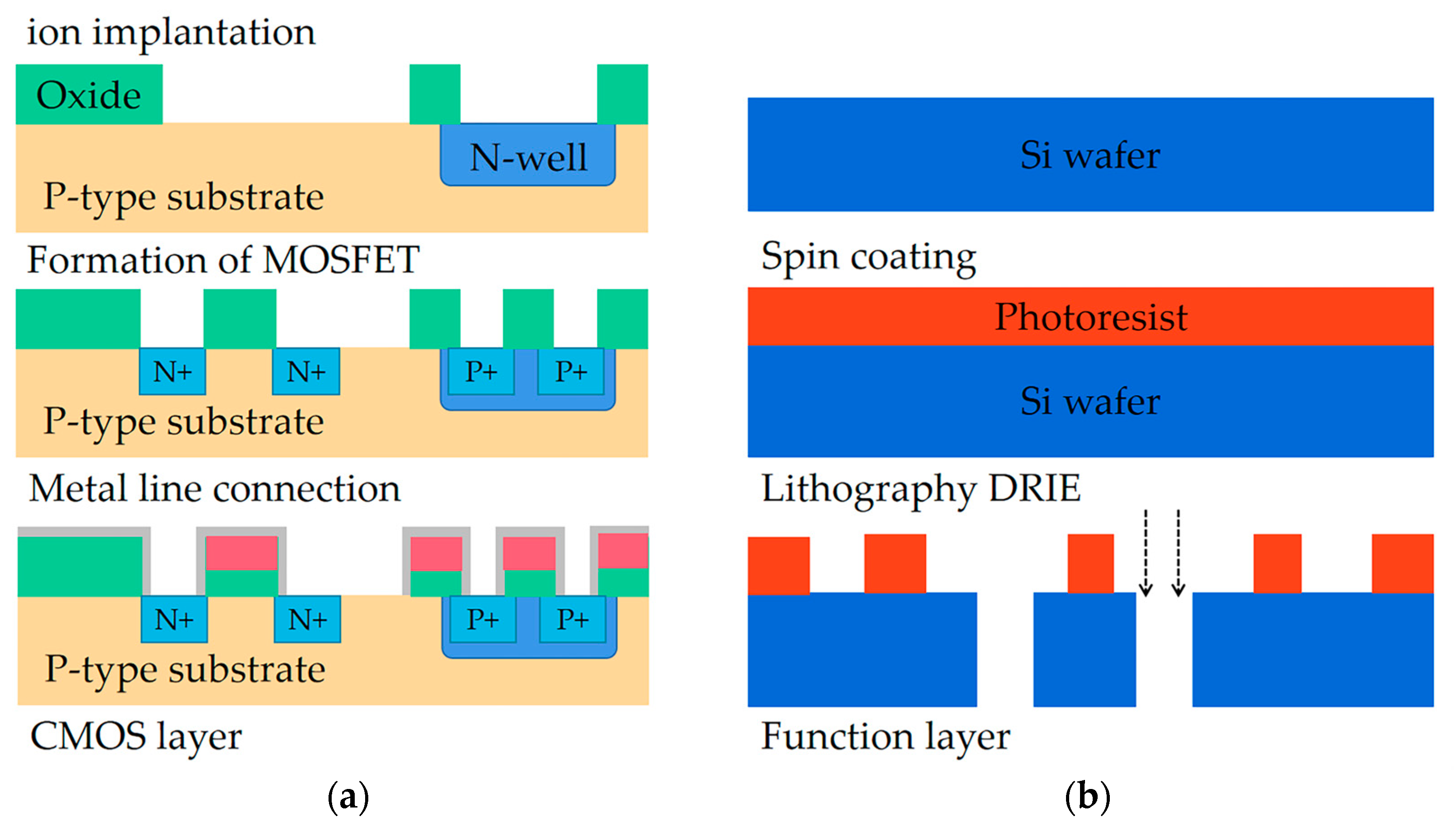
2.4. Microfluidic Chip Fabrication
2.5. Microchannel Pretreatment
2.6. Cell Treatment
3. Results and Discussion
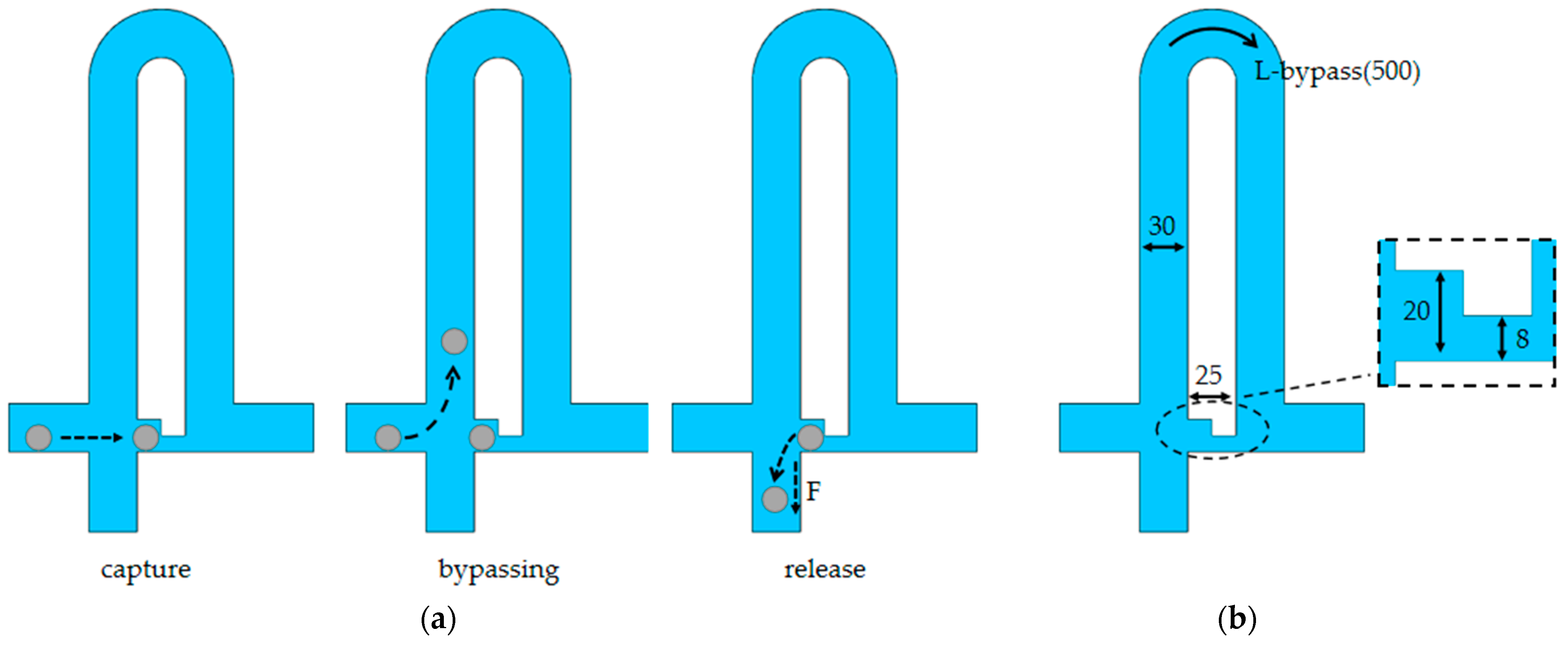
3.1. Mechanism of Single-Cell Functional Chip
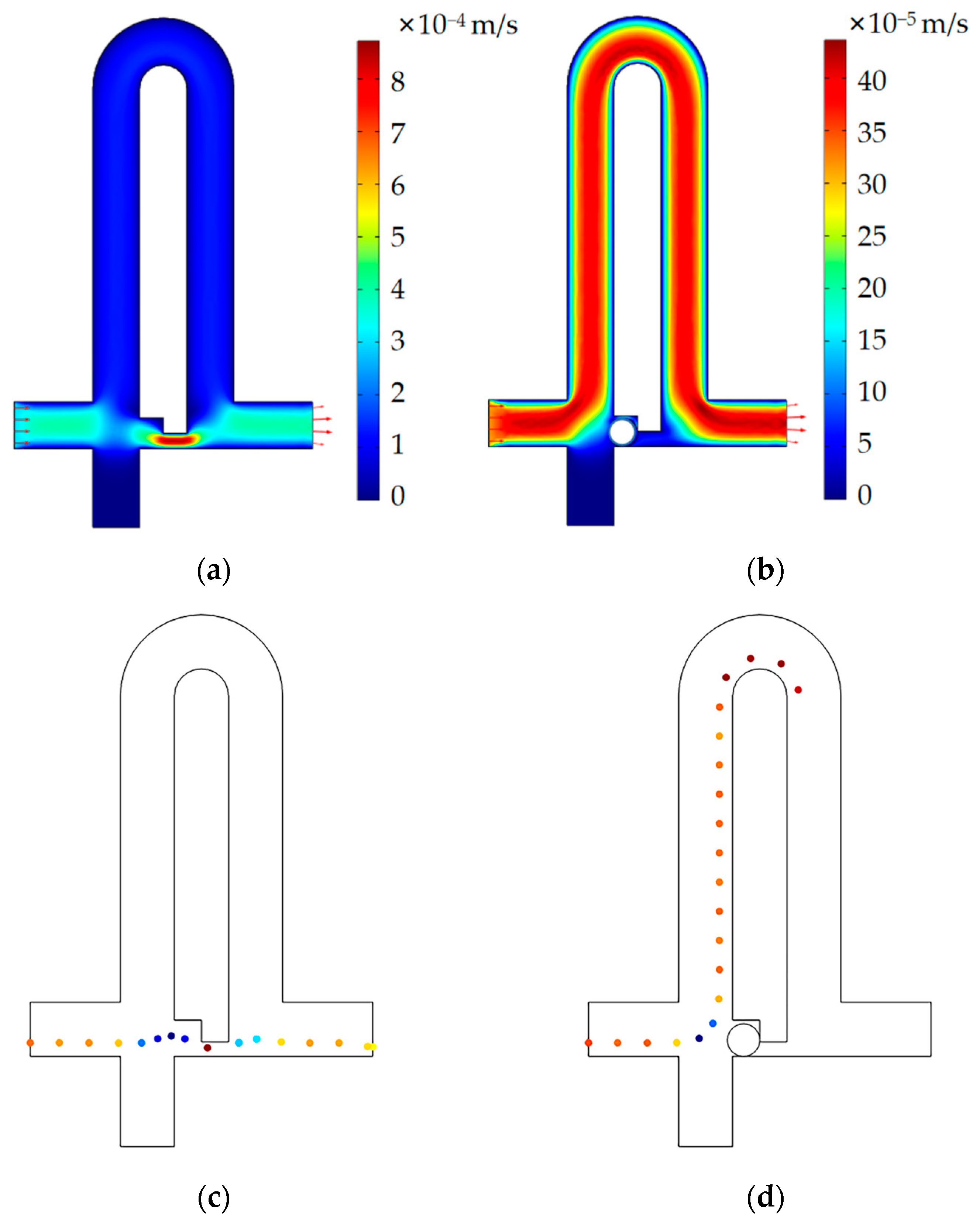
3.2. Numerical Simulation of the Single-Cell Functional Module
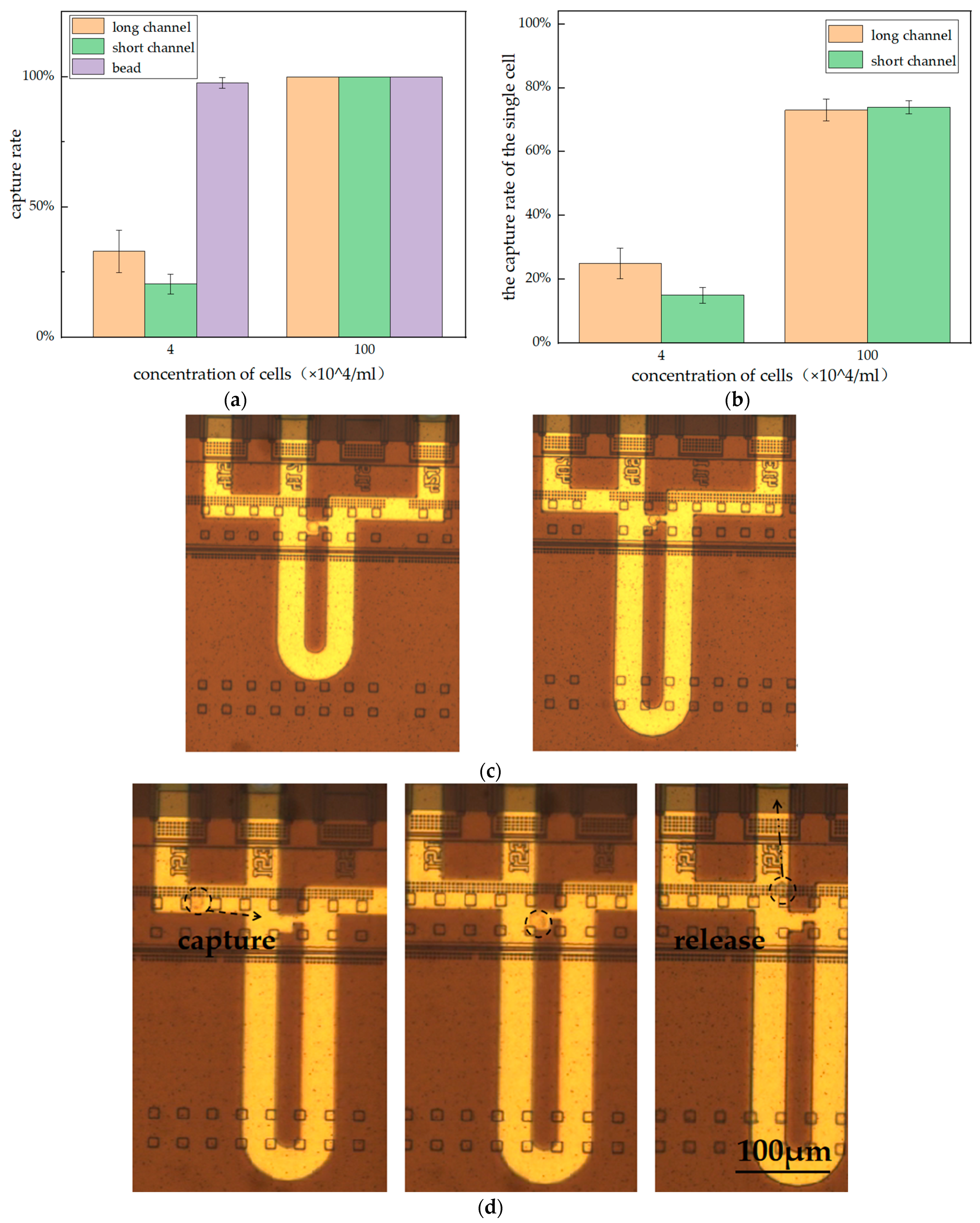
3.3. Optimization of Capture Conditions
3.4. Comparison of the Capture Rate of the Single Bead with Two Structures
3.5. Cell Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoendube, J.; Wright, D.; Zengerle, R.; Koltay, P. Single-Cell Printing Based on Impedance Detection. Biomicrofluidics 2015, 9, 014117. [Google Scholar] [CrossRef]
- Schatz, D.G.; Swanson, P.C. V(D)J Recombination: Mechanisms of Initiation. Annu. Rev. Genet. 2011, 45, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Kortmann, H.; Blank, L.M.; Schmid, A. Single Cell Analysis Reveals Unexpected Growth Phenotype of S. Cerevisiae. Cytometry A 2009, 75, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ranzoni, A.M.; Strzelecka, P.M.; Cvejic, A. Application of Single-Cell RNA Sequencing Methodologies in Understanding Haematopoiesis and Immunology. Essays Biochem. 2019, 63, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Blainey, P.C.; Quake, S.R. Dissecting Genomic Diversity, One Cell at a Time. Nat. Methods 2014, 11, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Klepárník, K.; Foret, F. Recent Advances in the Development of Single Cell Analysis—A Review. Anal. Chim. Acta 2013, 800, 12–21. [Google Scholar] [CrossRef]
- Picelli, S. Single-Cell RNA-Sequencing: The Future of Genome Biology Is Now. RNA Biol. 2017, 14, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, W.; Xin, H.; Deng, G. Single Cell Isolation and Analysis. Front. Cell Dev. Biol. 2016, 4, 116. [Google Scholar] [CrossRef]
- Valihrach, L.; Androvic, P.; Kubista, M. Platforms for Single-Cell Collection and Analysis. Int. J. Mol. Sci. 2018, 19, 807. [Google Scholar] [CrossRef]
- Bonner, R.F.; Emmert-Buck, M.; Cole, K.; Pohida, T.; Chuaqui, R.; Goldstein, S.; Liotta, L.A. Laser Capture Microdissection: Molecular Analysis of Tissue. Science 1997, 278, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J. New Techniques for Isolation of Single Prokaryotic Cells. FEMS Microbiol. Rev. 2000, 24, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for Single-Cell Isolation. Int. J. Mol. Sci. 2015, 16, 16897–16919. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.A.; Shimono, Y.; Qian, D.; Clarke, M.F. The Biology of Cancer Stem Cells. Annu. Rev. Cell Dev. Biol. 2007, 23, 675–699. [Google Scholar] [CrossRef]
- Alamgeer, M.; Peacock, C.D.; Matsui, W.; Ganju, V.; Watkins, D.N. Cancer Stem Cells in Lung Cancer: Evidence and Controversies: Lung Cancer Stem Cells. Respirology 2013, 18, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Xu, Y.; Tanaka, Y.; Sato, K.; Mawatari, K.; Konno, T.; Ishihara, K.; Kitamori, T. Single-Cell Attachment and Culture Method Using a Photochemical Reaction in a Closed Microfluidic System. Biomicrofluidics 2010, 4, 032208. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Li, Q. Microfluidic Techniques for Dynamic Single-Cell Analysis. Microchim. Acta 2010, 168, 177–195. [Google Scholar] [CrossRef]
- Zare, R.N.; Kim, S. Microfluidic Platforms for Single-Cell Analysis. Annu. Rev. Biomed. Eng. 2010, 12, 187–201. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on Chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Single-Cell Microbiology: Tools, Technologies, and Applications. Microbiol. Mol. Biol. Rev. 2004, 68, 538–559. [Google Scholar] [CrossRef]
- Hellmich, W.; Pelargus, C.; Leffhalm, K.; Ros, A.; Anselmetti, D. Single Cell Manipulation, Analytics, and Label-Free Protein Detection in Microfluidic Devices for Systems Nanobiology. Electrophoresis 2005, 26, 3689–3696. [Google Scholar] [CrossRef]
- Tsai, H., Jr.; Lin, L. A Thermal-Bubble-Actuated Micronozzle-Diffuser Pump. J. Microelectromech. Syst. 2002, 11, 665–671. [Google Scholar] [CrossRef]
- Torniainen, E.D.; Govyadinov, A.N.; Markel, D.P.; Kornilovitch, P.E. Bubble-Driven Inertial Micropump. Phys. Fluids 2012, 24, 122003. [Google Scholar] [CrossRef]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by Size of Epithelial Tumor Cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Kahn, H.J.; Presta, A.; Yang, L.-Y.; Blondal, J.; Trudeau, M.; Lickley, L.; Holloway, C.; McCready, D.R.; Maclean, D.; Marks, A. Enumeration of Circulating Tumor Cells in the Blood of Breast Cancer Patients After Filtration Enrichment: Correlation with Disease Stage. Breast Cancer Res. Treat. 2004, 86, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Rivet, C.A.; Kemp, M.L.; Lu, H. Imaging Single-Cell Signaling Dynamics with a Deterministic High-Density Single-Cell Trap Array. Anal. Chem. 2011, 83, 7044–7052. [Google Scholar] [CrossRef] [PubMed]



| Properties | Micromanipulation | FACS | LCM | Other Microfluidics | Our Technology |
|---|---|---|---|---|---|
| Throughput | Low | High | Low | High | High |
| Starting number of cells | Low | High | Low | Low | Low |
| Operational complexity | High | Normal | High | Low | Low |
| Portability | Low | Normal | Low | Normal | High |
| Others | Low efficiency and long collection time | Expensive equipment and cell dissociation | May compromise RNA quality | Relies heavily on external drive pumps | No external drive pumps are required |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Wang, K.; Huang, P.; Liu, D.; Guan, Y. Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology. Sensors 2023, 23, 3623. https://doi.org/10.3390/s23073623
Xu C, Wang K, Huang P, Liu D, Guan Y. Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology. Sensors. 2023; 23(7):3623. https://doi.org/10.3390/s23073623
Chicago/Turabian StyleXu, Chao, Kun Wang, Peng Huang, Demeng Liu, and Yimin Guan. 2023. "Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology" Sensors 23, no. 7: 3623. https://doi.org/10.3390/s23073623
APA StyleXu, C., Wang, K., Huang, P., Liu, D., & Guan, Y. (2023). Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology. Sensors, 23(7), 3623. https://doi.org/10.3390/s23073623





