Abstract
The idea that birds are maniraptoran theropod dinosaurs is now considered an evolutionary consensus. An “open” (i.e., completely or substantially perforate) acetabulum is considered an important synapomorphy verifying the bird–dinosaur nexus. Here, I present anatomical evidence from the acetabulum and its important appurtenances, the supracetabular crest and the antitrochanter, that hip anatomy differs substantially between dinosaurs and birds. Given the thin bone of the acetabular walls and the varied tissue, both hard and soft, in the acetabular region and especially the lower part of the basin, it is apparent that many avian skeletons exhibit some anatomical loss of soft tissue and thin bone, some perhaps related to changes in gait, but also in part related to the dramatic trend in bone reduction associated with flight, especially in more advanced crown taxa. Many basal birds and early diverging neornithines tend to have a nearly closed or partially closed acetabula, thus rendering the current terms “open” or “closed” acetabula inaccurate; they should be modified or replaced. Given new evidence presented here, the relationship of “dinosaurs” and birds must be re-evaluated.
1. Introduction
As early as Huxley []—and in a modern context, Ostrom [,], Padian [], and Gauthier []—the anatomy of the acetabulum and associated pelvic structures has been considered an important character linking dinosaurs and birds (Aves sensu traditum [,,]; the “Avialae” of [,]). It remains so today [,], and many studies have attempted to chart the transition from the putatively ancestral theropod locomotory system to that observed in extant birds (Neornithes) [,,,,,,,,].
The acetabulum is a concave pelvic surface formed by the ilium, ischium, and pubis, which accommodates the head of the femur in tetrapods, providing pivotal ambulatory movement. The femoral head and acetabulum are each lined by an articular surface lubricated with a film of synovial fluid. Given the putative descent of birds from theropod dinosaurs, the morphology of the acetabulum took on new importance, but the connectivity of acetabular morphology and function is still not fully understood. Triassic archosaurs such as Lagosuchus (synonymized with Marasuchus []), Ticinosuchus, and others exhibit a closed acetabulum with a ventral foramen, and Late Triassic “rauisuchians” like Postosuchus exhibit an entirely theropodan pelvic anatomy, except for the acetabulum, which is largely closed. Crocodilians, like other ancient archosaurs, have a closed acetabulum with an acetabular foramen, likewise seen in pterosaurs and humans. An open acetabulum is considered one of the most unchallengeable synapomorphies of dinosaurs and birds as opposed to stem dinosauromorphs and dinosauriforms that had not yet achieved fully upright posture and still exhibit a closed or partially closed acetabulum [,,]. Many modern birds have a mainly open acetabulum, but in others, the walls of the basin are partially ringed by thin bone covered by a fibrous sheet. The pit of the acetabulum is perforated by the foramen acetabuli at the confluence of the ilium, ischium, and pubis, located near the center of the basin. The rim of the acetabulum, the fibrocartilaginous labrum, is a white ring of cartilage lined by hyaline cartilage that provides an articulating surface for the femoral head, which has a pit (fovea) that receives the acetabular ligament. The deep floor is referred to as the acetabular fossa.
Despite this schematic characterization, it is more difficult to adequately assess acetabular morphology in extant birds than is generally realized. Preparatory methods used to produce skeletal collections in museums (e.g., defleshing with dermestid beetles, oxidation methods involving H2O2 and ammonia, chemical bleach, and maceration) can damage or destroy fragile anatomical features. Fossils present even greater challenges to faithful preservation: the quality of the preserving sediment, microbial or micro-faunal destruction of soft tissue and thin bone, compaction and water-rock interaction, differential destruction from preservation diagenesis, and weathering of the surviving fossilized material are all factors, too easily forgotten. Taphonomic factors can obscure not only anatomical interpretation, as is clear from disputes on the composition and homology of fibrous or filament-like structures in various theropod dinosaurs [,,,,,,,,,,,,] but can also obscure phylogenetic signals in analyses that are wholly reliant on morphological (particularly paleontological) data or which attempt to integrate data types, as illustrated by the problem of “stemward slippage” in attempts to understand the origin of major phyla [,,,,,,,,,,]. Given the evolutionary tendency of birds toward reduction and thinning of bone, the avian skeleton (and especially the acetabulum) is particularly susceptible to taphonomic alteration or distortion during preparation for collections. It would, therefore, be unsurprising if important data might be overlooked in the examination of fossil and museum material.
Spurred by these considerations, I here provide a new look at the argument that the hip joints of birds and dinosaurs are as similar as has been claimed for the past several decades. In addition to the perforation of the acetabulum with which we have been concerned thus far, there are two additional anatomical components that are of particular significance in this regard: (1) the formation and function of a supracetabular crest and (2) the homology and function of the antitrochanter. Three sets of tax must be evaluated to assess the distribution of these character states and thus critically reexamine the familiar claim that birds have the hips (and legs) of theropod dinosaurs: first, basal archosaurs and dinosaurs, to establish the putatively ancestral condition for Aves; second, extant birds (Neornithes), to establish the derived avian condition; and finally, those basal avian forms that according to the best knowledge currently available from the fossil record have temporally and morphologically diverged the least from the primitive avian bauplan. This last group—basal birds—will be compared with unambiguously feathered “maniraptorans”, i.e., Oviraptorosauria, Troodontidae, and Dromaeosauridae (together, with birds constituting the Pennaraptora of recent authors). These taxa are universally considered to possess numerous derived avian character states [,,,,,,,,], but significant disagreement has arisen regarding the phylogenetic interpretation of these data. Whereas the pennaraptorans are typically considered to be primarily flightless nonavian theropods [], others [,,,,,,,,] have argued that some are secondarily flightless (“neoflightless”) theropods derived from volant lineages, that they represent independent, parallel acquisitions of avian morphotypes in a general process of “ornithization” across some archosaur lineages []; that some pennaraptoran lineages independently acquired flight while remaining outside of Aves [,]; or that they are secondarily flightless birds that have been misidentified as theropods due to systemic homoplasy associated with flight loss and its developmental mechanisms [,,,,,,,,].
Comparative morphological study is the primary basis for homology determination in all applications of phylogenetic analysis to paleontological data: obviously, analyses are only as sound as the primary data upon which they are based. Accordingly, this paper will focus on the morphological and biofunctional level of investigation rather than the data-analytical. The reader is referred to other works [,,,,] for a review of problems with phylogenetic analyses of paleontological data in the dispute on the origin of birds.
Given the range of taxa discussed in this review, the anatomical nomenclature adopted in the Nomina Anatomica Avium [], although appropriate for Neornithes and Mesozoic birds, has not been used. The Nomina Anatomica Avium, like most modern avian anatomical references, portrays Gallus (domestic chicken) and Anas (domestic duck) with fully open, dinosaurian acetabula, unlike older images (Figure 1), which show substantial medial acetabular walls.
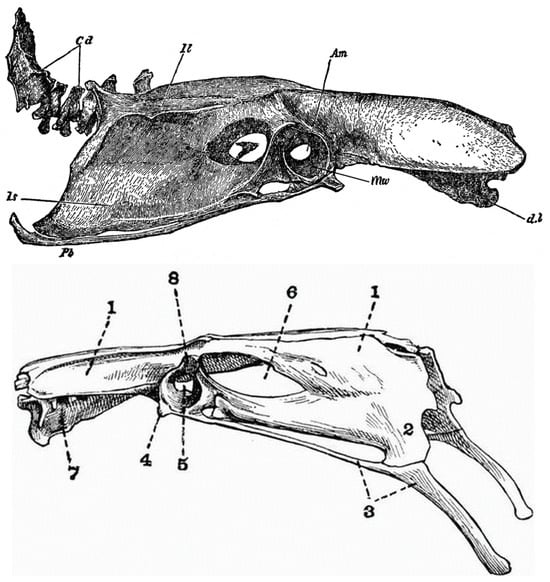
Figure 1.
Pelves (Os coxae + Synsacrum, or innominate bone) of (upper) right lateral view of the domestic fowl Gallus gallus; below, Mallard Anas platyrhynchos in left lateral view. (See lower image for anatomical terms). ote considerable medial “acetabular” walls (mw) surrounding the acetabular foramen (foramen acetabuli). Abbreviations: 1, pre- and postacetabular ischium; 2, ilium; 3, pubis; 4, preacetabular tuberculum; 5, acetabulum with acetabular foramen, surrounded by medial “acetabular wall” (see mw in Gallus above; 6, ischiadic foramen; 7, intervertebral foramen; 8, antitrochanter). Gallus, Encyclopaedia Brittanica, 1911; Anas, The Vertebrate Skeleton, Cambridge Press, 1897; public domain.
The continued illogical application of “phylogenetic nomenclature” [], “phylonyms” [], and the arbitrary redefinition of established taxon names necessitates the following nomenclatural clarifications. “Aves”, as noted above, is used sensu traditum. “Archosauria” is also used sensu traditum [,] and is therefore equivalent to the Archosauriformes of Nesbitt [], Ezcurra [], and de Queiroz et al. []. Chiappe [,,] restricted “Ornithurae” from its historical usage [,,] to a clade consisting only of “the common ancestor of Hesperornithiformes and Neornithes plus all taxa descended from it” [] (p. 205) (see also [,,]); here the term is used in a more inclusive sense [,,,,,,,] in which it is equivalent to the “Ornithuromorpha” of Chiappe [] and many recent phylogenetic analyses. For ease of reference, Table 1 provides brief (and taxonomically informal) characterizations of the major subdivisions of Archosauria that are relevant to this review.

Table 1.
Archosauria and some of its major taxonomic subdivisions. Taxa are informally characterized for readers unfamiliar with archosaur taxonomy. For detailed discussions, readers are invited to consult the References. Abbreviations: mya, million years ago.
2. Hip Joint Morphology in Basal Archosauria and in Dinosauria
The acetabulum in Triassic archosaurs is a mostly closed or imperforate structure that is structurally primitive, with the ilium, ischium, and pubis adjoining each other at the lower part of the articular cavity [,,,,,]. The acetabular foramen arises at the junction between the pelvic bones and provides passage for the ligament that attaches to the femoral head. The surface of the acetabulum is somewhat uneven and exhibits a thick and strong rim for the attachment of the prominent white fibrocartilaginous collar, the labrum, bounded by an articular region, lined by hyaline cartilage. The transverse acetabular ligament, articulating with the head of the femur, parallels nutrient vessels to the hip joint. Most sauropsid (i.e., reptiles—including birds—and their related amniote stem forms) acetabula have a small perforation in the pit of the eponymous basin. Basal archosaurs have an S-shaped femur with no in-turned head and without a well-developed upper rim (supracetabular crest), whereas dinosaurs have a relatively straight, vertically oriented femur with a fully in-turned head; the acetabulum is completely open with an upper bony rim over the hip socket known as the supracetabular crest [] that overhangs the acetabulum and distributes transmitted stresses and prevents unwanted excursions of the femur (Figure 2).
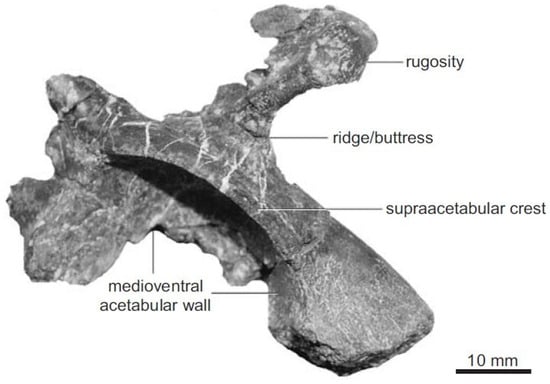
Figure 2.
Late Triassic herrerasaurian dinosauriform Caseosaurus crosbyensis from New Mexico, illustrating the well-developed supracetabular crest characteristic of later dinosaurs []; use, CC by 4.0.
Among other modifications to the dinosaurian pelvis, femur, and axial skeleton, the combination of a vertically oriented femur with fully in-turned head articulating within a fully perforate acetabulum buttressed by a supracetabular crest allowed smooth pivoting of the hind limb about the hip joint, directly under the body, during the caudofemoralis-dominated excursion of the femur through the power and retracting strokes. It is a key morphofunctionally-integrated character complex in the “hip-driven” system of terrestrial locomotion, ancestral to archosaurs, whose biomechanical apogee is achieved in the striding, obligate cursorial bipedalism of theropod dinosaurs [,,,,,,,]. This character complex is already evident in Lagosuchus (Figure 3) and is still more pronounced in silesaurids [,,] and herrerasaurians (Figure 2) (note that there is some character discordance in Triassic dinosaurs, e.g., contrast the condition in Pampadromaeus [] and Eoraptor []), and is fully established in primitive theropods. The condition in the large Jurassic tetanurine Allosaurus (Figure 3) may be taken as stereotypical of its development in Theropoda.
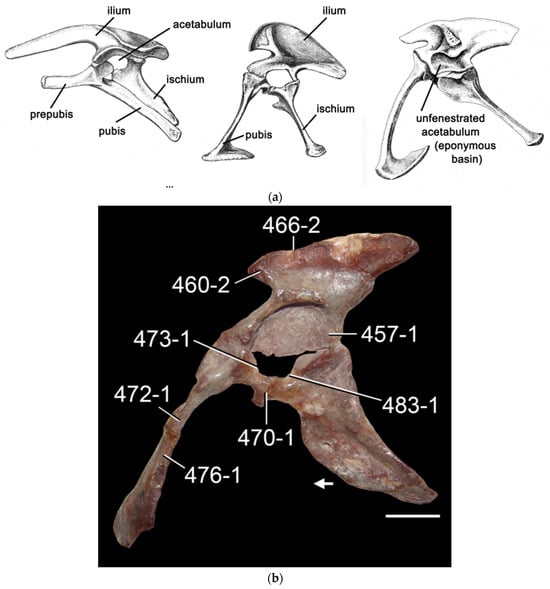
Figure 3.
Mesozoic pelves: (a) compare, from left to right, pelves of Stegosaurus, an ornithischian; Allosaurus, a saurischian theropod (both from varied sources); and Postosuchus a Triassic “rauisuchian” archosaur (note that aside from the open acetabulum in dinosaurs and the closure of the acetabulum in Postosuchus the pelves are otherwise quite similar) (modified from []); (b) pelvis of the dinosaurmorph Lagosuchus (modified from [], used under CC by 4.0 license). Abbreviations: 457-1: Acetabular antitrochanter present; 460-2: Preacetabular process of the ilium present, longer than 2/3 of its height, and not extending beyond the front edge of the pubic peduncle; 466-2: Concave dorsal margin of the iliac blade; 470-1: Pubis-ischium contact present and reduced to a thin proximal contact; 472-1: Total length of the pubis 3.94–4.87 times longer than the anteroposterior length of the acetabulum; 473-1: Recessed anterior and posterior portions of the acetabular margin of the pubis; 476-1: Rod-like, posteriorly curved pubic shaft; 483-1: The ischium’s articular surfaces with the ilium and pubis are separated by a fossa. (Postosuchus, courtesy S. Chatterjee).
A structure frequently homologized with the avian antitrochanter has been reported in many archosaurs [,], including basal dinosaurs [,,], basal theropods and ceratosaurs [,,], and a miscellany of coelurosaurs [,,]. There is no osteological evidence that these structures are homologous: the avian antitrochanter forms primarily [] or almost exclusively [] from the ischium, whereas the so-called “antitrochanters” of these nonavian taxa are almost exclusively iliac in composition (see Section 3 for further discussion on the development and function of the antitrochanter). Moreover, while continuity of function is not a requirement of homology, it should be noted that putative “antitrochanters” of so great a range of taxa cannot possibly have performed the specialized mechanical role that the true antitrochanter does in extant birds as part of their peculiar hindlimb locomotory system [,]. Taxonomic distribution further argues against homology and, thus, against the soundness of applying avian anatomical terms to disparate structures that bear only superficial resemblance to one another.
Among Maniraptora, an antitrochanter has been reported in alvarezsaurs [,,,], therizinosauroids [,,], oviraptorosaurs [,], troodontids [], and dromaeosaurids [,,,,,]. The putative “antitrochanters” of alvarezsaurs and therizinosauroids are iliac in composition and in the case of alvarezsaurs it is not even clear that they are articular structures []. The zeal for indiscriminate homologizing of osteological features sometimes extends to absurd levels: in the dromaeosaurid Mahakala, for example, the femur is proposed to possess a “fossa articularis antitrochanterica” [sic] [] (p. 42) (the authors presumably meant “Facies articularis antitrochanterica”)—a character state otherwise observed only in enantiornithines and ornithurines—even though Mahakala lacks an antitrochanter []. With these preliminary clarifications made, pennaraptoran taxa in which an antitrochanter has been reported will be discussed further in Section 4.
3. Hip Joint Morphology in Extant Birds (Neornithes)
In Paleognathae, the acetabula are somewhat occluded, but some of the structural medial walls may be lost in preparation or handling. Generally, one can see considerable occlusion in specimens of Struthio (Figure 4a), Aepyornis, and Dromaius (Figure 5); it is more open in Apteryx. In the volant lithornithids and tinamous (Tinamiformes), the acetabulum appears partially closed (Figure 5b), conforming closely with Gallus and Anas (Figure 1), with bony walls of the basin but open at the bottom (in some fossils, the bony interior has been lost). Lithornithids and tinamous have somewhat smaller antitrochanters and very limited supracetabular crests (Figure 5) if indeed the term is used correctly because the structure of the so-called crest is about the same below and above the acetabulum; it is minimally a misnomer. Antitrochanters are also evident in struthionids, rheas, kiwis, and other “ratites” (Figure 4a and Figure 5f). Interestingly, the acetabulum is partially or mainly closed in most ground-dwellers, unlike any putative dinosaurian relatives despite the presumed similarity in gait (Figure 5).
Figure 4.
Acetabulum of (a) juvenile ostrich Struthio camelus (24-day old wet specimen, normal incubation 36–45 days; the specimen is fragile and preservative renders image with poor resolution), and (b) adult Scaled Quail Callipepla squamata showing acetabular closure and substantial medial wall, in left lateral view. The approximate diameter of the acetabulum in the Struthio and Callipepla specimens is approximately 3.5 mm and 4.5 mm, respectively. Note the fully developed antitrochanter (at the upper right of the acetabulum) in the adult quail. (Author’s specimens).
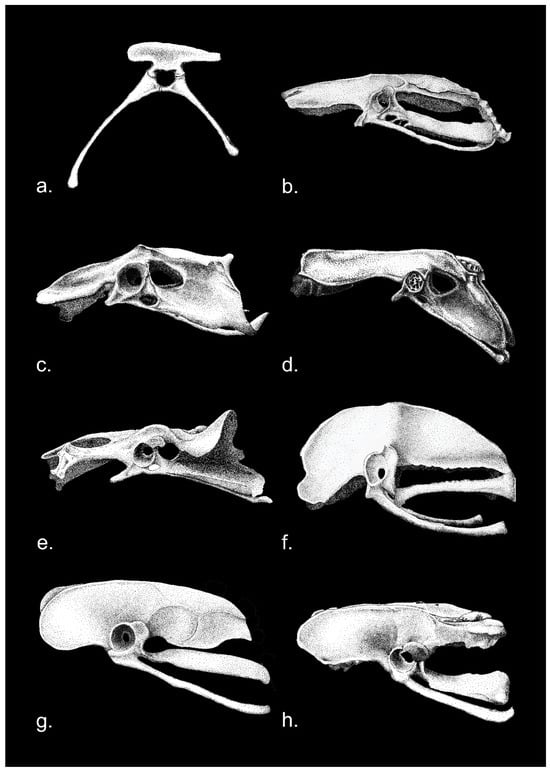
Figure 5.
Pelvis of the basal theropod Coelophysis (a) compared to an array of primarily ground-dwelling birds: (b) Solitary Tinamou (Tinamus solitarius), (c) Wild Turkey (Meleagris gallopavo), (d) Japanese Quail (Coturnix japonica), (e) Greater Roadrunner (Geococcyx californianus), (f) Emu (Dromaius novaehollandiae), (g) Bush Moa (Anomalopteryx didiformis), and (h) Elephant Bird (Aepyornis hildebrandti). The avian acetabula show a range of closure from partial to nearly complete occlusion. Note the contrast between the condition in these cursorial birds, which presumably should most closely approach the putatively ancestral theropod condition with respect to their acetabular morphology, and the condition in Coelophysis. Sources: drawing of Coelophysis provided by the late N. Colbert; tinamou drawn from photos provided by P. Houde; Coturnix japonica drawn from an original photo from S. Mehta, interpreted by the author; Dromaius drawn from the pelvis in the author’s collection; Meleagris drawn from photos in the author’s collection and photos; Geococcyx drawn from images and checked with specimen from the author’s collection. Art by K. Grow.
In Neognathae, the acetabulum is typically considered to be open, based usually on observation of skeletal preparations. The problem with this interpretation is illustrated by phasianid galliforms and particularly by the Japanese Quail (Coturnix japonica), a common model species for embryological study. Here, the acetabulum is almost closed by lattice-like endochondral bone that is easily shattered in skeletal specimens [pers. obs.] (Figure 5d). There is some variation between taxa, and in two Scaled Quail (Callipepla squamata) carcass dissections and oxidation preparations, the acetabulum is largely closed by substantial medial bony walls about 2/3 the depth of the basin, with only a central foramen remaining [] (compare with Figures 4b and 5c,d). Preparation by oxidation method using 15 to 17% HO might be sufficient to disintegrate some of these interior bony walls in small specimens, and they can be revealed only by careful dissection []. In examining chickens for biological studies, one sees that much of the anatomy within the acetabulum may have vanished in preparation, and many older specimens were processed with clorax that can easily disintegrate bone.
Surprisingly, chickens bred and prepared for human consumption provide further insight. Dissection of a common rotisserie-prepared chicken demonstrates that commercial chickens are going to market long before normal maturity in the wild. Such post-hatching chickens appear partially paedomorphic from post-hatching morphological characters. They are stuffed with food for about 4 to 6 weeks when they reach market size, without the skeleton fully formed, as evidenced by still developing epiphyses and considerable cartilage. On average, the growth timing for such chickens is about 6 weeks or 42 days. In contrast, wild chickens, depending on the species, take 16 to 24 weeks or 112 to 168 days to full maturity. One can see that this processing creates a form of induced paedomorphosis, which explains the large amounts of cartilage all over and incompletely fused and cartilaginous epiphyses (Figure 6). Studying these specimens provides a glimpse into their post-hatching ontogeny, the epigenetic landscape at a stage of near completion. It is surprising, given the frequent characterization of birds as having—like dinosaurs—completely open acetabula to discover that rotisserie specimens have partially closed acetabula with a perforation covered by soft tissue (Figure 6a). To make certain this observation was not anomalous, I studied and dissected 14 individuals of rotisserie chickens and two fully mature Poulet Rouge fowl (mature chickens that were farm-raised with no additives, including antibiotics). These acetabula had closed basin walls lined with bone and fibrocartilage with an acetabular membrane, and the acetabular ligament, which attaches to the femoral head, can be seen arising from a dimple covered by a strong fascia membrane in the lower upper part of the basin from the center and connecting to the head of the femur (Figure 6b).
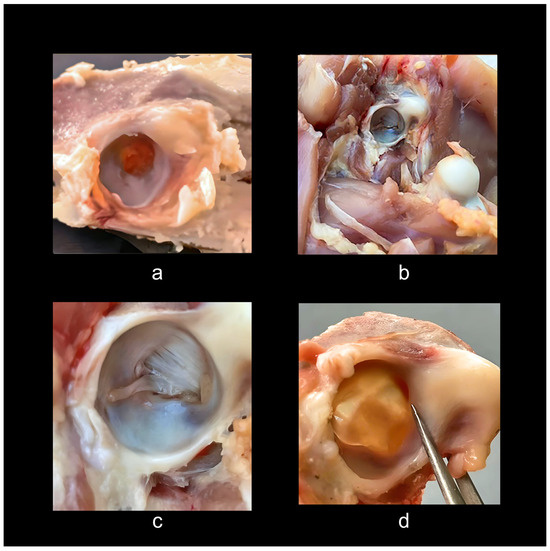
Figure 6.
Acetabula of rotisserie chickens. These images show post-hatching additions, notably considerable cartilage, and the antitrochanter: (a) rotisserie chicken post-hatching, the acetabulum foramen near the opening, showing considerable cartilage and an almost calcified antitrochanter; (b) Poulet Rouge fully mature chicken showing a mature ossified antitrochanter and femoral head; (c) mature Poulet Rouge showing white labrum surrounding the acetabular edge, ossified antitrochanter and femoral head (note the remnant of Ligamentum capitis femoris affixes to the femoral head, helping to stabilize the femur); (d) photo light shone from the base of the acetabulum shows the extent of medial bony walls and the basin of a fibrous sheet devoid of bone. Epiphyses of rotisserie specimens are entirely cartilaginous or in the process of transforming into endochondral bone. (Rotisserie chicken acetabula, approximately 10 mm; Poulet Rouge, 12 mm). Photos and dissections by the author.
Egawa et al. [] argue that many anatomical landmarks in birds show developmental timing that parallels their phylogenetic order of acquisition, a Haeckelian interpretation, implying that the process of terminal addition may play a major role in ontogeny. Yet, so-called “terminal addition” is nothing more than the end of the epigenetic landscape, whether in or out of the eggshell, and most vertebrates have considerable additions following hatching in birds or birth in mammals (note especially marsupials where the premature fetus will finish much of its ontogeny within the marsupium with a mouth attached to the nipples). In the case of quail, not only were the acetabula imperforate throughout embryogenesis, but even the adult acetabulum may be almost closed (Figure 5d). Egawa et al. [] further suggested that a dinosaur-type perforated acetabulum arises from acetabular cartilage loss in chicken embryos. They linked acetabular perforation in “non-avian” dinosaurs and birds (represented in their study by Struthio and Gallus) with high susceptibility to bone morphogenetic protein (BMP) antagonists and Wnt ligands []. They point out that in non-archosauromorph sauropsids with unperforated adult acetabula, the pelvic anlagen are not susceptible to the joint-secreted molecules, whereas in the transition to non-dinosaurian archosauromorphs with unperforated acetabula in the adult, the pelvic anlagen became susceptible to BMP antagonists but less susceptible to Wnt ligands, leading to no cartilage loss. Tsai and colleagues studied joint function and anatomy in the two extant archosaur clades (birds and crocodilians). Unsurprisingly, they demonstrated topologically and histologically similar articular soft tissues in the hip joints of both groups [,]. In all extant archosaurs, the proximal femur has a hyaline cartilage core attached to its metaphysis via a fibrocartilaginous sleeve, so epiphyseal cartilage in extinct forms would have been substantial [,]. The loss of this epiphyseal cartilage in fossilization, therefore, indicates that the preserved ends of the bone in crown group archosaurs are almost certainly not joint surfaces [] and this complicates functional inferences [,]. In a later study by Griffin et al. [], the situation becomes more confusing as the authors note that “The hip socket in Coturnix quail remained imperforate throughout embryonic development” [] (p. 347) (my emphasis). On the same page, they write that “…two derived avian states…resembled early ancestral/developmental morphologies (that is, instances of localized paedomorphosis): the broad, flat avian ischium resembled the early embryonic state and that of ancestral archosaurian [my emphasis] condition more than it did the intermediate dinosaurian conditions.” It should be noted in passing that although studies like those of Egawa et al. [], Griffin et al. [], and many others [,,,] exhibit a surprisingly Haeckelian recapitulationism, the data they provide are more reasonably interpreted in accordance with von Baer’s principles of simple conservativism of early embryos, without the orthogenetic implications of Haeckel’s recapitulation of past ancestors. In the words of Paul Ehrlich and colleagues: “The resemblance of early vertebrate embryos is readily explained without resort to mysterious forces compelling each individual to reclimb its phylogenetic tree.” [] (p. 66).
The avian antitrochanter is probably a neomorphic structure located lateral to the posterodorsal rim of the acetabulum of the pelvis (if so, the term “avian antitrochanter” would be a pleonasm). It is a striking feature in living birds but evolved only relatively late, appearing as the center of gravity in birds shifted forward due to the enlargement of the flight musculature. This phylogenetically late addition comports with the fact that it appears late in embryology and, upon hatching, is still cartilaginous. The avian antitrochanter is unique among vertebrates, and a significant portion of the femoral-pelvic articulation is located outside of the acetabulum. Hertel and Campbell [] demonstrated that the antitrochanter-femur articulation transfers long-axis rotational movement of the femur to the pelvis []. This ridge, which is easily seen, lies above the acetabulum and is thickest, just above the mid-portion of the depression. They concluded that this derived avian feature evolved as an aid in maintaining balance during bipedal locomotion []. It is a brace to prevent unwanted abduction of the hindlimb and to absorb stresses on the femur in locomotion. The term “antitrochanter” is a misnomer as its facet is not in opposition to the femur [], and although it is believed to serve as a brace to control excessive abduction of the femur to pelvis, in specialized foot-propelled divers (Cretaceous hesperornithiforms, modern loons, and grebes), the antitrochanters are quite large compared to other birds (Figure 7), but the femora may project straight out from the sides of the pelvis at a 90° angle, fully abducted. Antitrochanter function in derived birds, therefore, clearly requires further investigation. Indeed, one would have to explain functionally why the antitrochanter should be basically similar in “ratites” and hesperornithiforms.
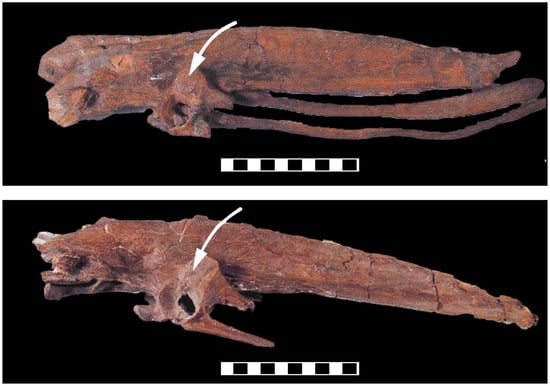
Figure 7.
Sacra of Hesperornis (above) and Parahesperornis (below), large divers of the Late Cretaceous, in left lateral view showing nearly closed acetabula. Often called diving dinosaurs, the acetabula show that they are definitively not allied with dinosaurs. Note also the prominent antitrochanters (arrows). Hesperornithiforms were capable of full lateral abduction of the hindlimbs during foot-propelled swimming (like loons and grebes), so the function of the antitrochanter in birds must extend beyond preventing excessive abduction of the femur. Hesperornithiforms are often termed diving dinosaurs, but given their acetabula anatomy this designation is erroneous. Images courtesy M. Everhart and the University of Kansas, Museum of Natural History, and University of Nebraska State Museum. Scale cm.
The newly described taxon Telluraves (arboreal land birds or core land birds), erected from whole genome comparisons, includes small arboreal birds that are all strong fliers and whose close relationships have long been recognized (like woodpeckers and passerines), but also (and more controversially) parrots, the diurnal birds of prey (“raptors”), and seriemas [,]. Most of these taxa exhibit fully open acetabula with greatly reduced medial walls, which might well be interpreted as a trend in modern birds to jettison all but essential “baggage”, including bone, i.e., to reduce all anatomical structures that might hinder maximal flight efficiency. This possibility is further suggested by comparison with the condition in more primitive land birds, e.g., paleognaths, galliforms, and some cuculiforms (like the Greater Roadrunner, Geococcyx californianus). In all these taxa, there is still significant medial occlusion of the acetabulum (Figure 5), presumably both because the antitrochanter had developed (permitting the form of bipedal locomotion peculiar to birds) and because there was no additional selective benefit for further reduction of the bony acetabular walls. In turkey (Meleagris), there is major occlusion and some chachalacas (Ortalis) may have nearly complete acetabular occlusion. Interestingly, among those Telluraves which were or are primarily cursorial, cariamiforms like the extinct phorusrhacids (e.g., Llallawavis []) and extant seriemas (relicts of an older Neogene radiation [,]) exhibit considerable medial occlusion of the acetabulum.
4. The Hip Joint Morphology in Basal Birds and in Pennaraptora
The basal avian assemblage, ranging from the Late Jurassic to the Early Cretaceous, includes the archaeopterygids, Chongmingia, Jinguofortis, Fukuipteryx, Cratonavis, omnivoropterygids (=“sapeornithids”), jeholornithids, and confucuisornithids [,,,,,,,,,]. Included in this group are taxa whose position is labile in phylogenetic analyses [,] but which are generally recovered near archaeopterygids, the most notable of which are the anchiornithids Anchiornis, Xiaotingia, Eosinopteryx, “Aurornis” (likely junior synonyms of Anchiornis) [], and Fujianvenator, the oldest. Rahonavis [] has variously been considered a basal bird or a dromaeosaurid [,,]; it will here be discussed under the same heading as other basal birds. The phylogenetic interpretation of Balaur, described as either an aberrant dromaeosaurid [,,,,] or a flightless basal bird [,,], is unclear, and its morphology is both bizarre and specialized; it will not be considered further here. Zhongornis was initially interpreted as a basal non-pygostylian bird [], whereas others argue that it is a scansoriopterygid []. Scansoriopterygids were first described as tree dwellers (e.g., Epidendrosaurus and Scansoriopteryx) but henceforth have often been interpreted in reconstructions as ground-dwelling cursors, which is clearly incorrect; they were almost certainly trunk-climbers and gliders and they have closed acetabula. The anatomy and phylogenetic interpretation of the enigmatic scansoriopterygids is too fraught a subject involving too many complexities to be addressed here, and the reader is referred to previous work [,,,] that showed a definitive absence of diagnostic dinosaurian characters. Zhongornis will not be discussed further here. Zhongjianornis was initially described as a basal bird [], but further study suggests that it is a basal ornithurine [].
The maniraptoran taxa most important to the dispute on the origin of birds are the pennaraptorans (as noted earlier). Accordingly, and for the sake of simplicity, it is the pelvic morphology of these taxa—the oviraptorosaurs, troodontids, and dromaeosaurids—that in the present paper will be compared with that of basal birds.
(a). Basal birds.—In archaeopterygids the acetabulum is medially occluded, as is clearest from the London specimen [] (see also []), and neither an antitrochanter nor a supracetabular crest are developed, as is most clearly shown by the London, Berlin, Eichstätt and Munich specimens ([,,]).
Agnolin and Novas [] (Figure 3B) interpreted a slight swelling on the posteroventral rim of the acetabulum in the Berlin specimen as an antitrochanter, but this interpretation seems incorrect because (1) it is positioned on the posteroventral rim of the acetabulum, whereas the avian antitrochanter is posterodorsal to the acetabulum [,,]; it is composed solely of the ilium, whereas the avian antitrochanter is composed exclusively or principally from the ischium [,,] and (2) a comparable structure is not seen in the pelvis of other specimens. Note that in those archaeopterygid specimens that are preserved on their backs (or partially rotated onto their backs), as in the London “Thermopolis” [] and 11th skeletal exemplars [], the hind limbs splay markedly (although the ilia are barely preserved in the “Thermpolis” specimen, the femora are not disarticulated, and are within their natural range of motion; the same is true of the 11th specimen, where the ilia are also poorly preserved). Death posture in an articulated, or largely articulated, skeleton corresponds to a range of motion in life, and in theropods, a sprawling orientation, even postmortem, is mechanically prevented so long as the femora, with their strongly inturned heads, remain in articulation [,]. It is therefore unsurprising that the femoral head of archaeopterygids is not fully inturned (despite repeated claims to the contrary) as was already clear from the Eichstätt specimen [] (see also []). Wellnhofer [] stated that in the “Thermopolis” specimen, the femoral head is fully inturned, but this is incorrect: the putative head of the right femur in this specimen is a calcite deposit or other obscuring piece of matrix, an interpretation confirmed by the fact that it does not fluoresce under UV light []. Similarly, in the Daiting specimen, the femoral head is not fully inturned []. Although Paul (2002) claims that a supracetabular crest is present in archaeopterygids, it is absent in the London, Berlin, Eichstätt and Munich specimens (Figure 8d) (compare with [] (Figure 1B–E) and see also []); the only particular indication of such a structure is in the large Solnhofen specimen (with the most robust hindlimbs among the archaeopterygid material), which may in fact belong to a separate genus Wellnhoferia [,,,].
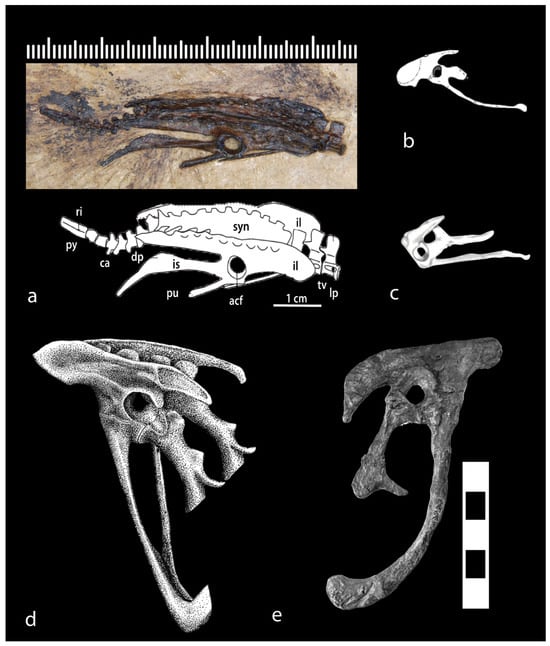
Figure 8.
Pelves of Mesozoic birds showing medial acetabular walls: (a) photograph and interpretive drawing of Early Cretaceous ornithurine Gansus []; (b,c) Early Cretaceous sparrow-size enantiornithines, (b) Qiliania []; (c) Pterygornis []; (d) Late Jurassic Archaeopteryx (image composites of varied sizes, primarily after P. Wellnhofer, based primarily on the Munich specimen, with details from the London, Berlin, and Eichstätt specimens; (e) Microraptor, the Early Cretaceous four-winged bird-like glider (dromaeosaur), considered by many an early bird. (Microraptor photo courtesy D. Burnham); scale cm for e Art by K. Grow.
Anchiornithids are closely allied with archaeopterygids: “Anchiornis huxleyi shared derived features with avialans […] Morphological comparisons strongly suggest Anchiornis is more closely related to avialans than to deinonychosaurians or troodontids.” [] (p. 4). The acetabulum in Anchiornis is partially occluded by substantial medial bony walls ([] (Figure 4c); []; D. Burnham, pers. comm.) (Figure 9). A weak though clearly demarcated crest is present above the acetabulum ([] (Figure 4c); []), but it is not broad and is more like a reinforcement rim, as it proceeds down the sides of the acetabular circle. As in basal birds, there is no antitrochanter.

Figure 9.
Femora and right pubis of Anchiornis, with the hip joint partially visible due to taphonomic displacement of the proximal femur, showing part of the expansive bony medial acetabular wall (arrow). Abbreviations: lf; left femur; pb, pubic boot; rf, right femur; rpu, right pubis. Image courtesy R. Pei.
There appears to be neither a well-developed supracetabular crest nor an antitrochanter in Xiaotingia [] (Figure 2c) or “Aurornis” [] (Figure 2c,d) (if “Aurornis” is a valid taxon and not a junior synonym of Anchiornis), but these taxa are likely all anchiornithids. The situation in the recently described Fujianvenator is also uncertain, but the specimen is very close anatomically to Anchiornis. There is little reason to believe that Anchiornis and Fujianvenator could not have been volant or near-volant, and in most aspects, they morphologically qualify as basal birds. Note that seriemas (Cariamidae), terrestrial birds with reduced wings and long legs (South American counterparts of African secretarybirds), are still able fliers, and both roost and build their nests in trees.
The ilia and ischia are not preserved in Chongmingia []. The condition in Jingufortis is unclear: the acetabulum is obscured by the synsacrum, but the ilium appears to lack a supracetabular crest or antitrochanter []. The acetabulum of Fukuipteryx is open, but there does not appear to be either a supracetabular crest or antitrochanter [] (Figure 5d). The acetabulum of Cratonavis appears to be mostly perforate (with perhaps slight medial occlusion), but there is neither a supracetabular crest nor antitrochanter [] (extended data Figure 3a,b). Rahonavis has a fully perforate acetabulum and poorly developed supracetabular crest; the purported antitrochanter, however, is iliac in origin [].
The incongruent distribution and parallel development of character states among basal birds is a serious obstacle to evolutionary interpretation and phylogenetic reconstruction at all levels of the basal avian radiation crownward of the archaeopterygids and those taxa closely related to the archaeopterygids [,,]. Omnivoropterygids (e.g., Sapeornis, Kompsornis), jeholornithids (e.g., Jeholornis), and confuciusornithids (e.g., Confuciusornis, Eoconfuciusornis) are usually compressed into their slabs and the hip joints in these taxa are often not fully visible or well preserved. In other cases, specimens seem to provide conflicting data: for example, some specimens of Sapeornis seem to show at least partial medial occlusion of the acetabulum, whereas others appear to show substantial perforation [] (Figure 8a,b) and the actetabulum of “Shenshiornis”, probably a junior synonym of Sapeornis, is completely perforate [] (Figure 4c). A small antitrochanter may be present in confuciusornithids ([], which incorrectly characterized confuciusornithids as terrestrial birds; contrast with [,]). Confuciusornithids lack a supracetabular crest [,,], and both omnivoropterygids and jeholornithids lack supracetabular crests and antitrochanters ([,]; [] (Figure 4c); [] (Figure 4D)).
The situation in basal birds is perhaps reciprocally illuminated by consideration of some more derived Cretaceous taxa (Figure 8a–c). Thus, in both the amphibious ornithurine Gansus (Figure 8a) and enantiornithines like Qiliania and Pterygornis (Figure 8a–c), the acetabula are mostly perforate but nevertheless exhibit bony walls extending slightly into the acetabular basin (in the more archaic enantiornithines there is substantially greater closure of the acetabulum than in ornithurines contra []; in some, like Sinornis, the closure seems extensive [] (Figure 8.4A,B). Interestingly, the poorly known Amazonian herbivorous river bird, the Hoatzin (Opisthocomus hoazin), has a similar acetabulum with reduced medial walls [pers. obs.]. Significant medial occlusion—and evidence of taphonomic destruction—is also evident in the flightless ornithurine Patagopteryx [], which is often (though perhaps spuriously) recovered as a basal ornithurine (or “ornithuromorph”) [,,,,,]. This medial occlusion persists in the hesperornithids Hesperornis and Parahesperornis (Figure 7), highly adapted flightless foot-propelled divers with pachyostotic bones like penguins (the same condition is observed in Baptornis [] (Figure 5a); it is interesting in this regard to note that Natovenator, an at least semiaquatic dromaeosaur that appears to have been adapted for efficient swimming, is reported to have a “small” acetabulum []. Partial medial occlusion of the acetabulum continues to be observed in taxa close to Neornithes (e.g., Iaceornis, the pelvis of which was assigned to Apatornis by Marsh []) [] (pp. 46–52), though not in Ichthyornis []. In both enantiornithines and ornithurines, the maintenance of balance in obligately bipedal locomotion is linked to the presence of an antitrochanter []. The phylogenetically late acquisition of this character state (in addition to persisting medial occlusion of the acetabulum and the absence of a supracetabular shelf in basal birds) is consistent with the argument that the acquisition of obligate bipedalism in birds (with the forelimbs fully decoupled from the hindlimbs during locomotory excursion) occurred only after they had first passed through a “tetrapteryx” stage [,,,].
(b) Pennaraptora.—Character conflict indicative of both parallelism and mosaicism is even more significant a source of interpretive difficulties when considering the morphology of the pennaraptorans: it is responsible for significant disagreements about the relationships of paravian taxa [,,,,,,]. Foremost among these is disagreement on the monophyly of both Deinonychosauria and Dromaeosauridae: both taxa have recently been recovered as paraphyletic. Some analyses recover troodontids either as more closely related to Aves than to dromaeosaurids or farther removed from Aves than a clade (“Eumanirpatora”) of dromaeosaurids sensu stricto and “averaptorans” (unenlagiids, microraptorines, and anchiornithids); likewise, some analyses recover microraptorines and unenlagiids as successive outgroups to Aves rather than clustering with more “typical” dromaeosaurids like the familiar Deinonychus and Velociraptor [,,,]. For the determination of homology and character state polarity, special reference must be given to those pennaraptoran taxa that are basal to their respective clades, but character conflict alone interferes with the reliable reconstruction of the phylogenetic relationships of these taxa, as does the possibility of systemic interference with phylogenetic signal from the profound morphological transformations induced by loss of flight. Reliance on simple tabulations of step count difference between constrained and unconstrained trees [] to determine whether the most basal members of pennaraptoran clades have been correctly resolved by parsimony analysis of current data matrices is naïve and takes no account of the statistical significance of differences in tree populations []. These difficulties preclude obvious answers and will be reflected in the brief comparative survey below.
Oviraptorosaurs uniformly display fully perforate acetabula, but the distribution of the supracetabular crest and antitrochanter is unclear, and their interpretation is difficult. Caudipterygids lack supracetabular crests and antitrochanters ([] (pl. VI); [] (pl. III); [] (Figure 5)), as is also the case in caenagnathoids (i.e., caenagnathids and oviraptorids) ([] (Figure 6a); [] (Figure 4L); []; [] (Figure 2k); [] (Figure 7)). Avimimus portensosus, however, possesses an ischial antitrochanter that, therefore, appears homologous with that in birds ([]; [] (Figure 4c)). Funston et al. [], nevertheless, report a new species, Avimimus nemegtensis, that lacks an antitrochanter. Since it is not clear why a single genus should be polymorphic for this character state, it is difficult to interpret this character conflict. It should perhaps be noted that embryonic oviraptorosaur material preserved close to hatching, as well as neonate oviraptorosaurs, do not reveal an antitrochanter ([] (Figures 3 and 10); [] (Figure 7); [] (Figure 1)), suggesting that, as in enantiornithines and ornithurines, the antitrochanter in Avimimus portentosus appeared only late in ontogeny. Yet, as the material from Avimimus nemegtensis appears to be equivalent in ontogenetic age to Avimimus portentosus, it remains difficult to explain why this latter taxon—if it has been correctly referred to Avimimus—lacks an antitrochanter. It is possible that despite its topological similarity to the condition in birds, the structure in Avimimus portentosus was independently acquired and is nonhomologous.
Troodontids have fully perforated acetabula, although the condition in crucial taxa, like Jianianhualong and Jinfengopteryx, is unclear [,]. They lack supracetabular crests []. Makovicky and Norell [] reported an iliac “antitrochanter” in troodontids. If correct, this structure would not be homologous with the avian condition, but as Daliansaurus [] (Figure 10a), Gobivenator [] (Figure 2n), and Sinovenator [] (Figure 1f) all lack an antitrochanter, there is reason to doubt the attribution of the structure to troodontids in the first instance.
Dromaeosaurids, in the comparative context of this review, must be considered in three discrete phenotypic clusters until the monophyly or paraphyly of the group has been more concretely determined: microraptorines, unenlagiids, and the “typical” taxa like Deinonychus and Velociraptor (including those forms which exhibit trends toward larger body size). The halszkaraptorines are apparently specialized semiaquatic forms and can, therefore, be bracketed from the present overview. The microraptorine pelvis is a very close match with that of Archaeopteryx, including medial occlusion of the acetabulum (Figure 8d,e) and absence of either a strong supracetabular crest or antitrochanter (Figure 8e) ([] (Figure 31); [] (Figure 2); [] (Figure 24); [] (Figure 1c)). Interestingly, the acetabulum of Hesperonychus opens dorsolaterally, which would enable it to abduct the hindlimbs in a splayed fashion as in other gliders like Microraptor, which no doubt abducted feathered hindlimbs to provide airfoils []. Yet, Microraptor is often depicted flying with the legs drooping beneath the animal [], which is contrary to the biomechanics of gliding animals, all of which splay their hindlimbs out in flight. The Microraptor hindlimb could not have evolved asymmetric, aerodynamic flight feathers if positioned beneath the animal. It is, therefore, unsurprising that the femoral head exhibits medial offset in the Microraptor [] (Figure 8). There is significant medial occlusion of the acetabulum in unenlagiids, no supracetabular crest (or it is at best weakly developed), and no antitrochanter [] (Figure 2c); []. In Bambiraptor, the acetabulum shows partial medial occlusion, the supracetabular crest is only weakly developed, and a clearly demarcated antitrochanter (as opposed to some posterodorsal thickening of the iliac acetabular margin associated with the ischiadic peduncle) is not evident []. In Deinonychus [] (Figure 64) and Velociraptor [] (Figures 11 and 12); [] (Figures 17a and 18), the supracetabular crest remains weak, a clearly demarcated antitrochanter is absent, and in Velociraptor the acetabulum continues to exhibit medial occlusion.
5. Discussion
Partial closure of the acetabula and absence of an antitrochanter in early birds is surprising, for it is inconsistent with the persistent assertion that these taxa had developed obligate bipedalism like their presumed theropod ancestors. That some pennaraptorans (notably the microraptorines and troodontids) also exhibit partial closure of the acetabulum and lack an antitrochanter is a further incongruity in that these taxa should exhibit “typical” theropod pelvic girdle modifications for terrestrial cursoriality. Of course, partial or even full closure of the acetabulum does not of itself entail that an archosaur was incapable of bipedalism (at least facultatively) or of maintaining a parasagittal gait: poposauroids [,,,,] and rauisuchids like Postosuchus [] were fully parasagittal and obligately bipedal, and obligately quadrupedal ornithischians in which the acetabulum secondarily exhibits either partial (e.g., ceratopsids) or full occlusion (e.g., ankylosaurs) still held their femora erect rather than splayed [,]. Compsognathids are “prototypical” small theropods [], yet they lack a supracetabular crest or antitrochanter [,,] and still appear to have been obligately bipedal cursors (for an alternative view, see [,,]). To give only one more example, although they appear to have been ideally suited for arboreal trunk climbing and their pelvic osteology (as reviewed above) suggests that they were not obligately bipedal (let alone cursorial), nevertheless microraptorines may have been opportunistically piscivorous and capable of hunting lakeshore environments [,]. In many cases, there is not a simple correspondence between gross anatomy and behavior (yet we also know that early birds were experimenting with a range of trophic specializations, as indicated by evidence for granivory, folivory, and frugivory in omnivoropterygids and jeholornithids [,,,,]). The cumulative anatomical picture for a given range of taxa and its integration with supplementary data is necessary for sound behavioral and ecological inferences. It is in this context that the evidence presented here on the pelvic osteology of early birds and pennaraptorans reinforces the conclusion that, particularly when the abductive ability permitted to the hindlimb is considered, the first birds—and at least taxa like the microraptorines—were not terrestrial cursorial bipeds, like theropods, but trunk-climbing gliders [,,]. This would be consistent with arguments that (at least some of) these taxa are “neoflightless” theropods, whose immediate ancestors underwent transformations in the theropod locomotory system (perhaps because of a shift to arboreality) [,,,,,,,,]. Yet these data are also consistent with the argument that they are misidentified birds and, like them, are instead descended from more basal, arboreal archosaurs that had yet to acquire the theropod system of obligately bipedal locomotion [,,,,,,,,]. Homoplasy-driven character conflict confounds morphological interpretations and necessarily precludes facile assessment of alternative hypotheses or overreliance on the results of phylogenetic analyses.
Nevertheless, the data reviewed here are a further indication, supplementing the discovery of a “tetrapteryx” stage in early aviation evolution, that obligate bipedality in birds is a secondary, phylogenetically later acquisition, only fully refined in the Cretaceous. This is fundamentally incompatible with the argument—advanced since Huxley, championed by Ostrom, repeated ad infinitum—that the obvious reason birds are obligate bipeds and that they never passed through an evolutionary phase in which all four limbs were integrated into the flight mechanism, is that birds are descended from obligate bipeds, viz. theropods. This neat, tidy, almost irresistible line of argumentation (buttressed, so it has seemed to many, by phylogenetic analyses) has motivated and continues to motivate fruitless efforts to provide a biologically (or even biophysically) coherent, epistemologically sound explanation for the origin of avian flight in a terrestrial, gravity-resisted context (of which WAIR—wing-assisted incline running [,]—is but the latest forlorn example [,,,]). None of this is now consistent with available fossil evidence, or, as this paper has labored to show, even by a closer consideration of the morphology of neornithine birds. These data instead suggest that the reason bipedalism in birds differs fundamentally from bipedalism in all other archosaurs (belated recognition that modern birds are not quite the well-behaved “ordinary” parasagittal or planar bipeds that they have long been considered continues to grow [,]) is that birds started their evolutionary trajectory toward obligate bipedalism from a different starting point than the system employed to such success by dinosaurs. It is just as significant that the pennnaraptorans (oviraptorosaurs, troodontids, and dromaeosaurs)—whose (apparently) basal exemplars exhibit a remarkable number of derived avian characters—also show evidence of derivation from forms not unlike basal birds in their pelvic osteology and the locomotory habits that can be inferred from this osteology. This is a logical explanation for the surprising persistence of medial occlusion of the acetabulum in many pennaraptorans and the absence, in most, of either well-defined supracetabular crests or antitrochanters. These data are also consistent with the extensive hindlimb plumage observed in microraptorines [,,,]; if Jinfengopteryx is, in fact, a basal troodontid [,] (but see discussions in [,,]) then troodontids also primitively exhibited a “tetrapteryx” bauplan. Taken together, these data suggest that ancestral pennaraptorans fundamentally differed from theropods in their locomotory strategies. As is so often the case upon critical comparative examination of pennaraptoran morphology, the pelvic data that have been reviewed in this paper underscore the question: are these “theropods” actually “hidden birds” [,,,].
6. Conclusions
The hypothesis that birds are maniraptoran theropod dinosaurs, despite the certitude with which it is proclaimed, continues to suffer from unaddressed difficulties [,,,,]. This paper has reviewed the evidence for yet another such difficulty and presented evidence consistent with alternatives to that hypothesis. It is believed that careful study of neglected areas of avian anatomy, or reexamination of areas long thought to be understood, will yield further such difficulties, and it is hoped that this paper will be an encouragement toward such efforts. Until problems like those discussed here—and many others that continue to be dismissed either by appeal to “consensus” or through overconfidence in the results of phylogenetic analysis of morphological data—have satisfactorily been resolved, skepticism toward the current consensus and continued investigation of alternative hypotheses are needful for the promotion of critical discourse in vertebrate phylogenetics and evolutionary biology.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
I thank Rui Pei for permission to use photographs of Anchiornis; David Burnham for sharing first-hand knowledge of the pelves of Microraptor and Anchiornis from specimens observed at the Shandong Museum and for providing 18 photos; Peter Houde for providing photos of pelves of 3 species of Lithornis (lithornithids) and four genera of tinamous; Zhiheng Li for providing information on Jeholornis and Sapeornis; and Lindsey Fortnash for sharing her osteological collection and photos of pelves. For comments on earlier drafts of this paper, I thank Frances C. James and John A. Pourtless IV; the latter also granted permission to incorporate unpublished manuscript material in the current paper. Kaysia Grow skillfully prepared the figures, and especially the art for Figure 5. Lastly, I thank Emma Li for encouraging the submission of this paper and John A. Pourtless IV for editorial assistance.
Conflicts of Interest
The author declares no conflict of interest.
References
- Huxley, T.H. On the animals which are most nearly intermediate between birds and reptiles. Ann. Mag. Nat. Hist. 1868, 2, 66–75. [Google Scholar]
- Ostrom, J.H. Archaeopteryx and the origin of birds. Biol. J. Linn. Soc. 1976, 8, 91–182. [Google Scholar] [CrossRef]
- Ostrom, J.H. The Question of the Origin of Birds. In Origins of the Higher Groups of Tetrapods: Controversy and Consensus; Schultze, H.-P., Trueb, L., Eds.; Cornell University Press: Ithaca, NY, USA, 1991; pp. 467–484. ISBN 0-8014-2497-6. [Google Scholar]
- Padian, K. Macroevolution and the origin of major adaptations: Vertebrate flight as a paradigm for the analysis of patterns. In Proceedings of the Third North American Paleontological Convention, Montreal, QC, Canada, 5–7 August 1982; Mamet, B.L., Copeland, M.J., Eds.; Business and Economic Service: Toronto, CA, USA, 1982; Volume 2, pp. 485–489. [Google Scholar]
- Gauthier, J.A. Saurischian monophyly and the origin of birds. Mem. Calif. Acad. Sci. 1986, 8, 1–55. [Google Scholar]
- Chiappe, L.M. Enantiornithine (Aves) tarsometatarsi and the avian affinities of the Late Cretaceous Avisauridae. J. Vertebr. Paleontol. 1992, 12, 344–350. [Google Scholar] [CrossRef]
- Feduccia, A. The Origin and Evolution of Birds, 2nd ed.; Yale University Press: New Haven, CT, USA, 1999; ISBN 0-300-07861-7. [Google Scholar]
- Mayr, G. Avian Evolution: The Fossil Record of Birds and Its Paleobiological Significance; Wiley: Chichester, UK, 2017; ISBN 9781119020769. [Google Scholar]
- De Queiroz, K.; Cantino, P.D.; Gauthier, J.A. (Eds.) Phylonyms: A Companion to the PhyloCode; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-138-33293-5. [Google Scholar]
- Paul, G.S. Dinosaurs of the Air: The Evolution and Loss of Flight in Dinosaurs and Birds; Johns Hopkins University Press: Baltimore, MD, USA, 2002; ISBN 0-8018-6763-0. [Google Scholar]
- Makovicky, P.J.; Zanno, L.E. Theropod Diversity and the Refinement of Avian Characteristics. In Living Dinosaurs: The Evolutionary History of Birds; Dyke, G., Kaiser, G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 9–29. ISBN 978-0-470-65666-2. [Google Scholar]
- Gatesy, S.M. Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology 1990, 16, 170–186. [Google Scholar] [CrossRef]
- Gatesy, S.M. Functional evolution of the hind limb and tail from basal birds to theropods. In Functional Morphology in Vertebrate Paleontology; Thomason, J.J., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 219–234. ISBN 9780521629218. [Google Scholar]
- Gatesy, S.M. Locomotory Evolution on the Line to Modern Birds. In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 432–447. ISBN 0-520-20094-2. [Google Scholar]
- Carrano, M.T. Locomotion in non-avian dinosaurs: Integrating data from hindlimb kinematics, in vivo strains, and bone morphology. Paleobiology 1998, 24, 450–469. [Google Scholar] [CrossRef]
- Farlow, J.O.; Gatesy, S.M.; Holtz, T.R., Jr.; Hutchinson, J.R.; Robinson, J.M. Theropod locomotion. Amer. Zool. 2000, 40, 640–663. [Google Scholar] [CrossRef]
- Hutchinson, J.R.; Gatesy, S.M. Adductors, abductors, and the evolution of archosaur locomotion. Paleobiology 2000, 26, 734–751. [Google Scholar] [CrossRef]
- Hutchinson, J.R. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes). Zool. J. Linn. Soc. 2001, 131, 123–168. [Google Scholar] [CrossRef]
- Hutchinson, J.R.; Allen, V. The evolutionary continuum of limb function from early theropods to birds. Naturwissenschaften 2009, 96, 423–448. [Google Scholar] [CrossRef]
- Griffin, C.T.; Botelho, J.F.; Hanson, M.; Fabbri, M.; Smith-Paredes, D.; Carney, R.M.; Norell, M.A.; Egawa, S.; Gatesy, S.M.; Rowe, T.B.; et al. The developing bird pelvis passes through ancestral dinosaur conditions. Nature 2022, 608, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Agnolin, F.L.; Ezcurra, M.D. The validity of Lagosuchus talampayensis Romer, 1971 (Archosauria, Dinosauriformes), from the Late Triassic of Argentina. Breviora 2019, 565, 1–21. [Google Scholar] [CrossRef]
- Charig, A.J. The evolution of the archosaur pelvis and hindlimb: An explanation in functional terms. In Studies in Vertebrate Evolution: Essays Presented to F. R. Parrington; Joysey, K.A., Kemp, T.S., Eds.; Oliver and Boyd: Edinburgh, UK, 1972; pp. 121–155. ISBN 9780050021311. [Google Scholar]
- Sereno, P.C.; Arcucci, A.B. Dinosaurian precursors from the Middle Triassic of Argentina: Lagerpeton chanarensis. J. Vertebr. Paleontol. 1994, 13, 385–399. [Google Scholar] [CrossRef]
- Sereno, P.C.; Arcucci, A.B. Dinosaurian precursors from the Middle Triassic of Argentina: Marasuchus lilloensis, gen. nov. J. Vertebr. Paleontol. 1994, 14, 53–73. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. Evolution of birds: Ichthyosaur integumental fibers conform to dromaeosaur protofeathers. Naturwissenschaften 2003, 90, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Lingham-Soliar, T. The dinosaurian origin of feathers: Perspectives from dolphin (Cetacea) collagen fibers. Naturwissenschaften 2003, 90, 563–567. [Google Scholar] [CrossRef]
- Feduccia, A.; Lingham-Soliar, T.; Hinchliffe, J.R. Do feathered dinosaurs exist? Testing the hypothesis on neontological and paleontological evidence. J. Morphol. 2005, 266, 125–166. [Google Scholar] [CrossRef]
- Lingham-Soliar, T.; Feduccia, A.; Wang, X. A new Chinese specimen indicates that “protofeathers” in the Early Cretaceous theropod dinosaur Sinosauropteryx are degraded collagen fibers. Proc. R. Soc. B 2007, 274, 1823–1829. [Google Scholar] [CrossRef]
- Feduccia, A. The Riddle of the Feathered Dragons: Hidden Birds of China; Yale University Press: New Haven, CT, USA, 2012; ISBN 978-0-300-16435-0. [Google Scholar]
- Feduccia, A. Bird origins anew. Auk 2013, 130, 1–12. [Google Scholar] [CrossRef]
- Feduccia, A. Romancing the Birds and Dinosaurs: Forays in Postmodern Paleontology; BrownWalker: Irvine, CA, USA, 2020; ISBN 978-1-59942-606-8. [Google Scholar]
- Foth, C. On the identification of feather structures in stem-line representatives of birds: Evidence from fossils and actuopalaeontology. Paläontol. Z. 2012, 86, 91–102. [Google Scholar] [CrossRef]
- Rauhut, O.W.M.; Foth, C.; Tischlinger, H.; Norell, M.A. Exceptionally preserved juvenile megalosauroid theropod dinosaur with filamentous integument from the Late Jurassic of Germany. Proc. Natl. Acad. Sci. USA 2012, 109, 11746–11751. [Google Scholar] [CrossRef] [PubMed]
- Godefroit, P.; Sinitsa, S.M.; Dhouailly, D.; Bolotsky, Y.L.; Sizov, A.V.; McNamara, M.E.; Benton, M.J.; Spagna, P. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 2014, 345, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Godefroit, P.; Sinitsa, S.M.; Cincotta, A.; McNamara, M.E.; Reshetova, S.A.; Dhouailly, D. Integumentary Structures in Kulindadromeus zabaikalicus, a Basal Neornithischian Dinosaur from the Jurassic of Siberia. In The Evolution of Feathers: From Their Origin to the Present; Foth, C., Rauhut, O.W.M., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 47–65. ISBN 978-3-030-27223-4. [Google Scholar]
- Smith, N.A.; Chiappe, L.M.; Clarke, J.A.; Edwards, S.V.; Nesbitt, S.J.; Norell, M.A.; Stidham, T.A.; Turner, A.; van Tuinen, M.; Vinther, J.; et al. Rhetoric vs. reality: A commentary on “Bird Origins Anew” by A. Feduccia. Auk Ornithol. Adv. 2015, 132, 467–480. [Google Scholar] [CrossRef]
- Xu, X. Filamentous Integuments in Novavialan Theropods and Their Kin: Advances and Future perspectives for Understanding the Evolution of Feathers. In The Evolution of Feathers: From Their Origin to the Present; Foth, C., Rauhut, O.W.M., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 67–78. ISBN 978-3-030-27223-4. [Google Scholar]
- Sansom, R.S.; Gabbott, S.E.; Purnell, M.A. Non-random decay of chordate characters causes bias in fossil interpretation. Nature 2010, 463, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Sansom, R.S.; Gabbott, S.E.; Purnell, M.A. Decay of vertebrate characters in hagfish and lamprey (Cyclostomata) and the implications for the vertebrate fossil record. Proc. R. Soc. B 2011, 278, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Sansom, R.S.; Willis, M.A. Fossilization causes organisms to appear erroneously primitive by distorting evolutionary trees. Sci. Rep. 2013, 3, 2458. [Google Scholar] [CrossRef] [PubMed]
- Murdock, D.J.E.; Gabbott, S.E.; Mayer, G.; Purnell, M.A. Decay of velvet worms (Onychophora), and bias in the fossil record of lobophodians. BMC Evol. Biol. 2014, 14, 222. [Google Scholar] [CrossRef]
- Nanglu, K.; Caron, J.-B.; Cameron, C.B. Using experimental decay of modern forms to reconstruct the early evolution and morphology of fossil enteropneusts. Paleobiology 2015, 41, 460–478. [Google Scholar] [CrossRef]
- Sansom, R.S. Bias and sensitivity in the placement of fossil taa resulting from interpretations of missing data. Syst. Biol. 2015, 64, 256–266. [Google Scholar] [CrossRef]
- Sansom, R.S.; Gabbott, S.E.; Purnell, M.A. Atlas of vertebrate decay: A visual and taphonomic guide to fossil interpretation. Palaeontology 2013, 56, 457–474. [Google Scholar] [CrossRef]
- Sansom, R.S. Preservation and phylogeny of Cambrian ecdysozoans tested by experimental decay of Priapulus. Sci. Rep. 2016, 6, 32817. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.E.G.; McMahon, S. The role of experiments in investigating the taphonomy of exceptional preservation. Palaeontology 2016, 59, 1–11. [Google Scholar] [CrossRef]
- Asher, R.J.; Smith, M.R. Phylogenetic signal and bias in paleontology. Syst. Biol. 2022, 71, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.C.; Sansom, R.S. Multivariate mapping of ontogeny, taphonomy and phylogeny to reconstruct problematic fossil taxa. Proc. R. Soc. B 2023, 290, 20230333. [Google Scholar] [CrossRef] [PubMed]
- Padian, K.; Chiappe, L.M. The origin and early evolution of birds. Biol. Rev. 1998, 73, 1–42. [Google Scholar] [CrossRef]
- James, F.C.; Pourtless, J.A., IV. Cladistics and the Origin of Birds: A Review and Two New Analyses; Ornithological Monographs; The American Ornithologists’ Union: Washington, DC, USA, 2009; Volume 66, pp. 1–78. [Google Scholar] [CrossRef]
- Chatterjee, S. The Rise of Birds: 225 Million Years of Evolution, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2015; ISBN 978-1-4214-1590-1. [Google Scholar]
- Hartman, S.; Mortimer, M.; Wahl, W.R.; Lomax, D.R.; Lippincott, J.; Lovelace, D.M. A new paravian dinosaur from the Late Jurassic of North America supports a late acquisition of avian flight. PeerJ 2019, 7, e7247. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Dong, Z.; Azuma, Y.; Barsbold, R.; Tomida, Y. Oviraptorosaurs compared to birds. In Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution, Beijing, China, 1–4 June 2000; Zhou, Z., Zhang, Z., Eds.; Science Press: Beijing, China, 2002; pp. 175–189, ISBN 9787030105516. [Google Scholar]
- Maryańska, T.; Omsólska, H.; Wolsan, M. Avialan status for Oviraptorosauria. Acta Paleontol. Pol. 2002, 47, 97–116. [Google Scholar]
- Persons, W.S., IV; Currie, P.J. Dragon tails: Convergent caudal morphology in winged archosaurs. Acta Geol. Sin.-Engl. 2012, 86, 1402–1412. [Google Scholar] [CrossRef]
- Sorkin, B. Aerial ability in basal Deinonychosauria. Bull. Gunma Mus. Nat. Hist. 2014, 18, 21–34. [Google Scholar]
- Sorkin, B. Scansorial and aeriability in Scansoriopterygidae and basal Oviraptorosauria. Hist. Biol. 2021, 31, 3202–3214. [Google Scholar] [CrossRef]
- Hutson, J.D.; Hutson, K.N. An examination of forearm bone mobility in Alligator mississippiensis (Daudin, 1802) and Struthio camelus Linnaeus, 1758 reveals that Archaeopteryx and dromaeosaurs shared an adaptation for gliding and/or flapping. Geodiversitas 2015, 37, 325–344. [Google Scholar] [CrossRef]
- Hutson, J.D.; Hutson, K.N. Retention of the flight-adapted avian finger-joint complex in the Ostrich helps identify when wings began evolving in dinosaurs. Ostrich 2018, 89, 173–186. [Google Scholar] [CrossRef]
- Kurochkin, E.N. Parallel evolution of theropod dinosaurs and birds. Entomol. Rev. 2006, 86, S45–S58. [Google Scholar] [CrossRef]
- Dececchi, T.A.; Larsson, H.C.E.; Pittman, M.; Habib, M.B. High flyer or high fashion? A comparison of flight potential among small bodied paravians. Bull. Am. Mus. Nat. Hist. 2020, 440, 295–320. [Google Scholar]
- Pei, R.; Pittman, M.; Goloboff, P.A.; Dececchi, T.A.; Habib, M.B.; Kaye, T.G.; Larsson, H.C.; Norell, M.A.; Brusatte, S.L.; Xu, X. Potential for powered flight neared by most close avialan relatives, but few crossed its thresholds. Curr. Biol. 2020, 30, 4033–4046.e8. [Google Scholar] [CrossRef]
- Czerkas, S.A.; Zhang, D.; Li, J.; Li, Y. Flying dromaeosaurs. In Feathered Dinsoaurs and the Origin of Flight; Czerkas, S.J., Ed.; Dinosaur Museum: Blanding, UT, USA, 2002; pp. 127–135. ISBN 1-932075-01-1. [Google Scholar]
- Feduccia, A. Birds are dinosaurs: Simple answer to a complex problem. Auk 2002, 119, 1187–1201. [Google Scholar] [CrossRef][Green Version]
- Martin, L.D. A basal archosaurian origin for birds. Acta Zoolog. Sin. 2004, 50, 978–990. [Google Scholar][Green Version]
- Martin, L.D. Origins of avian flight—A new perspective. Oryctos 2008, 7, 45–54. [Google Scholar][Green Version]
- Feduccia, A.; Czerkas, S.A. Testing the neoflightless hypothesis: Propatagium reveals flying ancestry of oviraptorosaurs. J. Ornithol. 2015, 156, 1067–1074. [Google Scholar] [CrossRef]
- Pourtless, J.A., IV. Skepticism, the critical standpoint, and the origin of birds: A partial critique of Havstad and Smith (2019). Biol. Philos. 2022, 37, 57. [Google Scholar] [CrossRef]
- Baumel, J.J.; King, A.S.; Breazile, J.S.; Evans, H.E.; Vanden Berge, J.C. (Eds.) Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd ed.; Nuttal Ornithological Club: Cambridge, MA, USA, 1993; ISBN 9781877973345. [Google Scholar]
- Cantino, P.D.; de Queiroz, K. International Code of Phylogenetic Nomenclature (PhyloCode) Version 6; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-138-332829. [Google Scholar]
- Benton, M.J.; Clark, J.M. Archosaur phylogeny and the relationships of the Crocodylia. In The Phylogeny and Classification of the Tetrapods, Volume 1: Amphibians, Reptiles, Birds; Benton, M.J., Ed.; Clarendon Press: Oxford, UK, 1988; pp. 295–338. ISBN 9780198577058. [Google Scholar]
- Benton, M.J. Scleromochlus taylori and the origin of dinosaurs and pterosaurs. Philos. Trans. R. Soc. B 1999, 354, 1423–1446. [Google Scholar] [CrossRef]
- Nesbitt, S.J. The early evolution of archosaurs: Relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 2011, 352, 1–292. [Google Scholar] [CrossRef] [PubMed]
- Ezcura, M.D. The phylogenetic relationships of basal archosauromorphs, with an emphasis on the systematics of proterosuchian archosauriforms. PeerJ 2016, 4, e1778. [Google Scholar] [CrossRef]
- Chiappe, L.M. Cretaceous avian remains from Patagonia shed new light on the early radiation of birds. Alcheringa 1991, 15, 333–338. [Google Scholar] [CrossRef]
- Chiappe, L.M. The first 85 million years of avian evolution. Nature 1995, 378, 349–355. [Google Scholar] [CrossRef]
- Chiappe, L.M. Late Cretaceous birds of southern South America: Anatomy and systematics of Enantiornithines and Patagopteryx deferrariisi. Münchner Geowiss. Abh. A 1996, 30, 203–244. [Google Scholar]
- Martin, L.D. The origin and early radiation of birds. In Perspectives in Ornithology; Brush, A.H., Clark, G.A., Jr., Eds.; Cambridge University Press: New York, NY, USA, 1983; pp. 291–338. ISBN 978-0-521-24857-0. [Google Scholar]
- Martin, L.D. The beginning of the modern avian radiation. Trav. Doc. Lab. Géol. Lyon 1987, 99, 9–19. [Google Scholar]
- Sereno, P.C.; Rao, C.; Li, J. Sinornis santensis (Aves: Enantiornithes) from the Early Cretaceous of Northeastern China. In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 184–208. ISBN 0-520-20094-2. [Google Scholar]
- Padian, K.; Hutchinson, J.R.; Holtz, T.R., Jr. Phylogenetic definitions and nomenclature of the major taxonomic categories of the carnivorous Dinosauria (Theropoda). J. Vertebr. Paleontol. 1999, 19, 69–80. [Google Scholar] [CrossRef]
- Clarke, J.A.; Norell, M.A. The Morphology and Phylogenetic Position of Apsaravis ukhaana from the Late Cretaceous of Mongolia. Am. Mus. Novit. 2002, 3387, 1–46. [Google Scholar] [CrossRef]
- Clarke, J.A. Morphology, phylogenetic taxonomy, and systematics of Ichythyornis and Apatornis (Avialae: Ornithurae). Bull. Am. Mus. Nat. Hist. 2004, 286, 1–179. [Google Scholar] [CrossRef]
- Feduccia, A. Mesozoic aviary takes form. Proc. Natl. Acad. Sci. USA 2006, 103, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Hou Hou, L.; Martin, L.D.; Zhou, Z.; Feduccia, A.; Zhang, F. A diapsid skull in a new species of the primitive bird Confuciusornis. Nature 1999, 399, 679–682. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, F. Discovery of an ornithurine bird and its implications for Early Cretaceous avian radiation. Proc. Natl. Acad. Sci. USA 2005, 102, 18998–19002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, F. Mesozoic birds of China—A synoptic review. Front. Biol. China 2007, 2, 74–98. [Google Scholar] [CrossRef]
- Chiappe, L.M. Basal Bird Phylogeny: Problems and Solutions. In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 448–472. ISBN 0-520-20094-2. [Google Scholar]
- Romer, A. The Osteology of the Reptiles; University of Chicago Press: Chicago, IL, USA, 1956; ISBN 9780226724911. [Google Scholar]
- Naples, V.L.; Martin, L.D.; Simmons, J. The pelvis in early birds and dinosaurs. In Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution, Beijing, China, 1–4 June 2000; Zhou, Z., Zhang, Z., Eds.; Science Press: Beijing, China, 2002; pp. 203–210. [Google Scholar]
- Baron, M.G.; Williams, M.E. A re-evaluation of the enigmatic dinosauriform Caseosaurus crosbyensis from the Late Triassic of Texas, USA and its implications for early dinosaur evolution. Acta Palaeontol. Pol. 2018, 63, 129–145. [Google Scholar] [CrossRef]
- Dzik, J. A beaked herbivorous archosaur with dinosaur affinities from the early Late Triassic of Poland. J. Vertebr. Paleontol. 2003, 23, 556–574. [Google Scholar] [CrossRef]
- Peecook, B.R.; Sidor, C.A.; Nesbitt, S.J.; Smith, R.M.H.; Steyer, J.S.; Angielczyk, K.D. A new silesaurid from the upper Ntawere Formation of Zambia (Middle Triassic) demonstrates the rapid diversification of Silesauridae (Avemetatarsalia, Dinosauriformes). J. Vertebr. Paleontol. 2013, 33, 1127–1137. [Google Scholar] [CrossRef]
- Martz, J.W.; Small, B.J. Non-dinosaurian dinosauromorphs from the Chinle Formation (Upper Triassic) of the Eagle Basin, northern Colorado: Dromomeron romeri (Lagerpetidae) and a new taxon, Kwanasaurus williamparkeri (Silesauridae). PeerJ 2019, 7, e7551. [Google Scholar] [CrossRef]
- Langer, M.C.; McPhee, B.W.; Marsola, J.C.dA.; Roberto-da-Silva, L.; Cabreira, S.F. Anatomy of the dinosaur Pampadromaeus barberenai (Saurischia—Sauropodomorpha) from the Late Triassic Santa Maria Formation of southern Brazil. PLoS ONE 2019, 14, e0212543. [Google Scholar] [CrossRef]
- Sereno, P.C.; Martínez, R.N.; Alcober, O.A. Osteology of Eoraptor lunensis (Dinosauria, Sauropodomorpha). J. Vertebr. Paleontol. 2013, 32 (Suppl. S1), 83–179. [Google Scholar] [CrossRef]
- Langer, M.C. Basal Saurischia. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 25–46. ISBN 978-0-520-25408-4. [Google Scholar]
- Pacheco, C.; Müller, R.T.; Langer, M.; Pretto, F.A.; Kerber, L.; Dias da Silva, S. Gnathovorax cabreirai: A new early dinosaur and the origin and initial radiation of predatory dinosaurs. PeerJ 2019, 7, e7963. [Google Scholar] [CrossRef] [PubMed]
- Tykoski, R.S.; Rowe, T. Ceratosauria. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 47–70. ISBN 978-0-520-25408-4. [Google Scholar]
- Pol, D.; Rauhut, O.W.M. A Middle Jurassic abelisaurid from Patagonia and the early diversification of theropod dinosaurs. Proc. Roy. Soc. B 2012, 279, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Marsola, J.C.A.; Bittencourt, J.S.; Butler, R.J.; Da Rosa, A.A.S.; Sayão, J.M.; Langer, M.C. A new dinosaur with theropod affinities from the Santa Maria Formation, south Brazil. J. Vertebr. Paleontol. 2018, 38, e1531878. [Google Scholar] [CrossRef]
- Turner, A.H.; Makovicky, P.J.; Norell, M.A. A review of dromaeosaurid systematics and paravian phylogeny. Bull. Am. Mus. Nat. Hist. 2012, 371, 1–206. [Google Scholar] [CrossRef]
- Brusatte, S.L.; Lloyd, G.T.; Wang, S.C.; Norell, M.A. Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Curr. Biol. 2014, 24, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Hertel, F.; Campbell, K.E., Jr. The antitrochanter of birds: Form and function in balance. Auk 2007, 124, 789–805. [Google Scholar] [CrossRef][Green Version]
- Perle, A.; Chiappe, L.M.; Barsbold, R.; Clark, J.M.; Norell, M.A. Skeletal morphology of Mononykus olecranus (Theropoda: Avialae) from the Late Cretaceous of Mongolia. Am. Mus. Novit. 1994, 3105, 1–29. [Google Scholar][Green Version]
- Novas, F.E. Anatomy of Patagonykus puertai (Theropoda, Avialae, Alvarezsauridae), from the Late Cretaceous of Patagonia. J. Vertebr. Paleontol. 1997, 17, 137–166. [Google Scholar] [CrossRef]
- Chiappe, L.M.; Norell, M.A.; Clark, J.M. The Cretaceous, Short-Armed Alvarezsauridae Mononykus and Its Kin. In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 87–120. ISBN 0-520-20094-2. [Google Scholar]
- Lee, S.; Park, J.-Y.; Lee, Y.-N.; Kim, S.-H.; Lü, J.; Barsbold, R.; Tsogtbaatar, K. A new alvarezsaurid dinosaur from the Nemegt Formation of Mongolia. Sci. Rep. 2019, 9, 15494. [Google Scholar] [CrossRef]
- Clark, J.M.; Maryańska, T.; Barsbold, R. Therizinosauroidea. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 151–164. ISBN 978-0-520-25408-4. [Google Scholar]
- Zanno, L.E. Osteolgy of Falcarius utahensis (Dinosauria: Theropoda): Characterizing the anatomy of basal therizinosaurs. Zool. J. Linn. Soc. 2010, 158, 196–230. [Google Scholar] [CrossRef]
- Liao, C.-C.; Zanno, L.E.; Wang, S.; Xu, X. Postcranial osteology of Beipiaosaurus inexpectus (Theropoda: Therizinosauria). PLoS ONE 2021, 16, e0257913. [Google Scholar] [CrossRef] [PubMed]
- Kurzanov, S.M. New data on the pelvic structure of Avimimus. Paleontol. J. 1983, 4, 115–116. [Google Scholar]
- Osmólska, H.; Currie, P.J.; Barsbold, R. Oviraptorosauria. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 165–183. ISBN 978-0-520-25408-4. [Google Scholar]
- Makovicky, P.J.; Norell, M.A. Troodontidae. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 184–195. ISBN 978-0-520-25408-4. [Google Scholar]
- Agnolin, F.L.; Novas, F.E. Unenlagiid theropods: Are they members of the Dromaeosauridae (Theropoda, Maniraptora)? An. Acad. Bras. Cienc. 2011, 83, 117–162. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Brusatte, S.L. A large, short-armed, winged dromaeosaurid (Dinosauria: Theropoda) from the Early Cretaceous of China and its implications for feather evolution. Sci. Rep. 2015, 5, 11775. [Google Scholar] [CrossRef] [PubMed]
- Gianechini, F.A.; Makovicky, P.J.; Apesteguía, S.; Cerda, I. Postcrainal skeletal anatomy of the holotype and referred specimens of Buitreraptor gonzalezorum Makovicky, Apesteguía and Agnolín 2005 (Theropoda, Dromaeosauridae), from the Late Cretaceous of Patagonia. PeerJ 2018, 6, e4558. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.A.; O’Connor, P.M.; Chiappe, L.M.; Turner, A.H. The osteology of the Late Cretaceous paravian Rahonavis ostromi from Madagascar. Palaeontol. Electron. 2020, 23, a29. [Google Scholar] [CrossRef] [PubMed]
- Novas, F.E.; Agnolín, F.L.; Motta, M.J.; Egli, F.B. Osteology of Unenlagia comaheunsis (Theropoda, Paraves, Unenlagiidae) from the Late Cretaceous of Patagonia. Anat. Rec. 2021, 304, 2741–2788. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.H.; Pol, D.; Norell, M.A. Anatomy of Mahakala omnogovae (Theropoda: Dromaeosauridae), Tögrögiin Shiree, Mongolia. Am. Mus. Novit. 2011, 3722, 1–66. [Google Scholar] [CrossRef]
- Mehta, S.; Guha, K.; Shalini, S.; Kumar, C. Gross anatomical studies on the os-coxae and synsacrum of Japanese quail (Coturnix coturnix japonica). Indian J. Vet. Anat. 2014, 26, 126–127. [Google Scholar]
- Egawa, S.; Saito, D.; Abe, G.; Koji, T. Morphogenetic mechanism of the acquisition of the dinosaur-type acetabulum. R. Soc. Open Sci. 2018, 5, 180604. [Google Scholar] [CrossRef]
- Tsai, H.P.; Holliday, C.M. Articular soft tissue anatomy of the archosaur hip joint: Structural homology and functional implications. J. Morphol. 2015, 276, 601–630. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.P.; Middleton, K.M.; Hutchinson, J.R.; Holliday, C.M. Hip joint articular soft tissues of non-dinosaurian Dinosauromorpha and early Dinosauria: Evolutionary and biomechanical implications for Saurischia. J. Vertebr. Paleontol. 2018, 38, e1427583. [Google Scholar] [CrossRef]
- Benton, M.J. Dinosaurs Rediscovered: How a Scientific Revolution is Rewriting History; Thames and Hudson: New York, NY, USA, 2020; ISBN 9780500052006. [Google Scholar]
- Holliday, C.M.; Ridgely, R.C.; Sedlmayr, J.C.; Witmer, L.M. Cartilaginous epiphyses in extant archosaurs and their implications for reconstructing limb function in dinosaurs. PLoS ONE 2010, 5, e13120. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, B.-A.S.; Hanson, M.; Fabbri, M.; Pritchard, A.; Bever, G.S.; Hoffman, E. How to Make a Bird Skull: Major Transitions in the Evolution of the Avian Cranium, Paedomorphosis, and the Beak as a Surrogate Hand. Integr. Comp. Biol. 2016, 56, 389–403. [Google Scholar] [CrossRef]
- Botelho, J.F.; Smith-Paredes, D.; Soto-Acuña, S.; O’Connor, J.; Palma, V.; Vargas, A.O. Molecular development of fibular reduction in birds and its evolution from dinosaurs. Evolution 2016, 70, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Smith-Paredes, D.; Núñez-León, D.; Soto-Acuña, S.; O’Connor, J.; Botelho, J.F.; Vargas, A.O. Dinosaur ossification centres in embryonic birds uncover developmental evolution of the skull. Nat. Ecol. Evol. 2018, 2, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Decoupling the skull and skeleton in a Cretaceous bird with unique appendicular morphologies. Nat. Ecol. Evol. 2023, 7, 20–31. [CrossRef] [PubMed]
- Ehrlich, P.R.; Holm, R.W.; Parnell, D. The Process of Evolution; McGraw-Hill: New York, NY, USA, 1963; ISBN 0070191336. [Google Scholar]
- Yuri, T.; Kimball, R.T.; Harshman, J.; Bowie, R.C.; Braun, M.J.; Chojnowski, J.L.; Han, K.L.; Hackett, S.J.; Huddleston, C.J.; Moore, W.S.; et al. Parsimony and model-based analyses of indels in avian nuclear genes reveal congruent and incongruent phylogenetic signals. Biology 2013, 2, 419–444. [Google Scholar] [CrossRef]
- A new Mesembriornithinae (Aves, Phorusrhacidae) provides new insights in the phylogeny and sensory capabilities of terror birds. J. Vertebr. Paleontol. 2015, 35, e912656. [CrossRef]
- A Well-Preserved Partial Skeleton of the Poorly Known Early Miocene Serieam Noriegavis santacruensis. Acta Paleontol. Pol. 2013, 60, 589–598. [CrossRef]
- O’Connor, J.; Chiappe, L.M.; Bell, A. Pre-modern Birds: Avian Divergences in the Mesozoic. In Living Dinosaurs: The Evolutionary History of Birds; Dyke, G., Kaiser, G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 39–114. ISBN 978-0-470-65666-2. [Google Scholar]
- Wang, M.; Wang, X.; Wang, Y.; Zhou, Z. A new basal bird from China with implications for morphological diversity in early birds. Sci. Rep. 2016, 6, 19700. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Stidham, T.A.; Zhou, Z. A new clade of basal Early Cretaceous pygostylian birds and developmental plasticity of the avian shoulder girdle. Proc. Natl. Acad. Sci. USA 2018, 115, 10708–10713. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Azuma, Y.; Kawabe, S.; Shibata, M.; Miyata, K.; Wang, M.; Zhou, Z. An unusual bird (Theropoda, Avialae) from the Early Cretaceous of Japan suggests complex evolutionary history of basal birds. Commun. Biol. 2019, 2, 399. [Google Scholar] [CrossRef] [PubMed]
- Agnolin, F.L.; Motta, M.J.; Egli, F.B.; Lo Coco, G.; Novas, F.E. Paravian Phylogeny and the Dinosaur-Bird Transition: An Overview. Front. Earth Sci. 2019, 6, 252. [Google Scholar] [CrossRef]
- Pittman, M.; O’Connor, J.; Field, D.J.; Turner, A.H.; Ma, W.; Makovicky, P.J.; Xu, X. Pennaraptoran Systematics. Bull. Am. Mus. Nat. Hist. 2020, 440, 7–36. [Google Scholar]
- Pei, R.; Li, Q.; Meng, Q.; Norell, M.A.; Gao, K.-Q. New specimens of Anchiornis huxleyi (Theropoda: Paraves) from the Late Jurassic of northeastern China. Bull. Am. Mus. Nat. Hist. 2017, 411, 1–66. [Google Scholar] [CrossRef]
- Forster, C.A.; Sampson, S.D.; Chiappe, L.M.; Krause, D.W. The theropod ancestry of birds: New evidence from the Late Cretaceous of Madagascar. Science 1998, 279, 1915–1919. [Google Scholar] [CrossRef]
- Pittman, M.; Xu, X. Pennaraptoran Theropod Dinosaurs Past Progress and New Frontiers. Bull. Am. Mus. Nat. Hist. 2020, 440, 1–353. [Google Scholar] [CrossRef]
- Csiki, Z.; Vremir, M.; Brusatte, S.L.; Norell, M.A. An aberrant island-dwelling theropod dinosaur from the Late Cretaceous of Romania. Proc. Natl. Acad. Sci. USA 2010, 107, 15357–15361. [Google Scholar] [CrossRef]
- Brusatte, S.L.; Vremir, M.; Csiki-Sava, Z.; Turner, A.H.; Watanabe, A.; Erickson, G.M.; Norell, M.A. The osteology of Balaur bondoc, an island-dwelling dromaeosaurid (Dinosauria, Theropoda) from the Late Cretaceous of Romania. Bull. Am. Mus. Nat. Hist. 2013, 374, 1–100. [Google Scholar] [CrossRef]
- Godefroit, P.; Cau, A.; Dong-Yu, H.; Escuillié, F.; Wenhao, W.; Dyke, G. A Jurassic avialan dinosaur from China resolves the early phylogenetic history of birds. Nature 2013, 498, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Foth, C.; Tischlinger, H.; Rauhut, O.W.M. New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 2014, 511, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Cau, A.; Brougham, T.; Naish, D. The phylogenetic affinities of the Late Cretaceous Romanian theropod Balaur bondoc (Dinosauria, Maniraptoran): Dromaeosaurid or flightless bird? PeerJ 2015, 3, e1032. [Google Scholar] [CrossRef]
- Gao, C.; Chiappe, L.M.; Meng, Q.; O’Connor, J.K.; Wang, X.; Cheng, X.; Liu, J. A new basal lineage of Early Cretaceous birds from China and its implications on the evolution of the avian tail. Palaeontology 2008, 51, 775–791. [Google Scholar] [CrossRef]
- O’Connor, J.K.; Sullivan, C. Reinterpretation of the Early Cretaceous maniraptoran (Dinosauria, Theropoda) Zhongornis haoae as a scansoriopterygid-like non-avian, and morphological resemblances between Scansoriopterygids and basal oviraptorosaurs. Vertebr. PalAsiat. 2014, 52, 3–30. [Google Scholar]
- Czerkas, S.A.; Yuan, C. An arboreal maniraptoran from Northeast China. In Feathered Dinosaurs and the Origin of Flight; Czerkas, S.J., Ed.; Dinosaur Museum: Blanding, UT, USA, 2002; pp. 63–95. ISBN 1-932075-01-1. [Google Scholar]
- Czerkas, S.A.; Feduccia, A. Jurassic archosaur is a non-dinosaurian bird. J. Ornithol. 2014, 155, 841–851. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, F.; Li, Z. A new Lower Cretaceous bird from China and tooth reduction in early avian evolution. Proc. R. Soc. B 2010, 277, 219–227. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.K.; Zhou, Z. A redescription of Chaoyangia beishanensis (Aves) and a comprehensive phylogeny of Mesozoic birds. J. Syst. Palaeontol. 2013, 11, 889–906. [Google Scholar] [CrossRef]
- Martin, L.D. Mesozoic Birds and the Origin of Birds. In Origins of the Higher Groups of Tetrapods: Controversy and Consensus; Schultze, H.-P., Trueb, L., Eds.; Cornell University Press: Ithaca, NY, USA, 1991; pp. 485–540. ISBN 0-8014-2497-6. [Google Scholar]
- Wellnhofer, P. Archaeoptery: The Icon of Evolution; Verlag Dr. Friedrich Pfeil: München, Germany, 2009; ISBN 978-3-89937-108-6. [Google Scholar]
- Schwarz, D.; Kundrát, M.; Tischlinger, H.; Dyke, G.; Carney, R.M. Ultraviolet light illuminates the avian nature of the Berlin Archaeopteryx skeleton. Sci. Rep. 2019, 9, 6518. [Google Scholar] [CrossRef]
- Mayr, G.; Pohl, B.; Hartman, S.; Peters, D.S. The teneth skeletal specimen of Archaeopteryx. Zool. J. Linn. Soc. 2007, 149, 97–116. [Google Scholar] [CrossRef]
- Burnham, D. Archaeopteryx—A re-evaluation suggesting an arboreal habitat and an intermediate stage in the trees down origin of flight. Neues Jahrb. Geol. Paläontol.-Abh. 2007, 245, 33–44. [Google Scholar] [CrossRef]
- Wellnhofer, P. Das fünfte Skelettexemplar von Archaeopteryx. The fifth skeletal example of Archaeopteryx. Palaeontogr. A 1974, 147, 169–216. [Google Scholar]
- Kundrát, M.; Nudds, J.; Kear, B.P.; Lü, J.; Ahlberg, P. The first specimen of Archaeopteryx from the Upper Jurasic Mörnsheim Formation of Germany. Hist. Biol. 2019, 31, 3–63. [Google Scholar] [CrossRef]
- Agnolin, F.; Novas, F.E. Avian Ancestors: A Review of the Phylogenetic Relationships of the Theropods Unenlagiidae, Microraptoria, Anchiornis, and Scansoriopterygidae; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-5636-6. [Google Scholar]
- Elzanowski, A. A new genus and species for the largest specimen of Archaeopteryx. Acta Paleontol. Pol. 2001, 46, 519–532. [Google Scholar]
- Elzanowski, A. Archaeopterygidae (Upper Jurassic of Germany). In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 129–159. ISBN 0-520-20094-2. [Google Scholar]
- Wang, Y.-A.; O’connor, J.K.; Li, D.Q.; You, H.-L. New information on postcranial skeleton of the Early Cretaceous Gansus yumenensis (Aves: Ornithuromorpha). Hist. Biol. 2016, 28, 666–679. [Google Scholar] [CrossRef]
- Ji, S.A.; Atterholt, J.; O’Connor, J.K.; Lamanna, M.C.; Harris, J.D.; Li, D.-Q.; You, H.-L.; Dodson, P. A new, three-dimensionally preserved enantiornithine bird (Aves: Ornithothoraces) from Gansu Province, north-western China. Zool. J. Linn. Soc. 2011, 162, 201–219. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Zhou, Z. Insight into the growth pattern and bone fusion of basal birds from an Early Cretaceous enantiornithine bird. Proc. Natl. Acad. Sci. USA 2017, 114, 11470–11475. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.; Norell, M.; Sullivan, C.; Hone, D.; Erickson, G.; Wang, X.; Han, F.; Yu, G. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin. Sci. Bull. 2009, 54, 430–435. [Google Scholar] [CrossRef]
- Xu, X.; You, H.; Han, F. An Archaeopteryx-like theropod from China and the origin of Avialae. Nature 2011, 475, 465–470. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, F. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Can. J. Earth Sci. 2003, 40, 731–747. [Google Scholar] [CrossRef]
- Hu, D.; Li, L.; Hou, L.; Xu, X. A New Sapeornithid Bird from China and Its Implications for Early Avian Evolution. Acta Geol. Sin.-Engl. 2010, 84, 472–482. [Google Scholar] [CrossRef]
- Chiappe, L.M.; Ji, S.; Ji, Q.; Norell, M.A. Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of northeastern China. Bull. Am. Mus. Nat. Hist. 1999, 242, 1–89. [Google Scholar]
- Martin, L.D.; Zhou, Z.; Hou, L.; Feduccia, A. Confuciusornis sanctus compared to Archaeopteryx lithographica. Naturwissenschaften 1998, 85, 286–289. [Google Scholar] [CrossRef]
- Zhou, Z.; Hou, L. The Discovery and Study of Mesozoic Birds in China. In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 160–183. ISBN 0-520-20094-2. [Google Scholar]
- Zhou, Z.; Zhang, F. A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature 2002, 418, 405–409. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.K.; Sun, C.; Xu, X.; Wang, X.; Zhou, Z. A new species of Jeholornis with complete caudal integument. Hist. Biol. 2012, 24, 29–41. [Google Scholar] [CrossRef]
- Chiappe, L.M.; Walker, C.A. Skeletal Morphology and Systematics of the Cretaceous Euenantiornithes (Ornithothoraces: Enantiornithes). In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 240–267. ISBN 0-520-20094-2. [Google Scholar]
- Chiappe, L.M. Osteology of the Flightless Patagopteryx deferrariisi from the Late Cretaceous of Patagonia (Argentina). In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 281–316. ISBN 0-520-20094-2. [Google Scholar]
- You, H.-L.; Lamanna, M.C.; Harris, J.D.; Chiappe, L.M.; O’connor, J.; Ji, S.A.; Lu, J.C.; Yuan, C.X.; Li, D.Q.; Zhang, X.; et al. A Nearly Modern Amphibious Bird from the Early Cretaceous of Northwestern China. Science 2006, 312, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, X.; O’Connor, J.K.; Lloyd, G.T.; Wang, X.; Wang, Y.; Zhang, X.; Zhou, Z. The oldest record of ornithuromorpha from the early cretaceous of China. Nat. Commun. 2015, 6, 6987. [Google Scholar] [CrossRef]
- O’Connor, J.K.; Zelenkov, N.V. The Phylogenetic Position of Ambiortus: Comparison with Other Mesozoic Birds from Asia. Paleontol. J. 2013, 47, 1270–1281. [Google Scholar] [CrossRef]
- Martin, L.D.; Tate, J., Jr. The Skeleton of Baptornis advenus (Aves: Hesperornithiformes). Smithson. Contrib. Paleobiol. 1976, 27, 35–66. [Google Scholar]
- Lee, S.; Lee, Y.-N.; Currie, P.J.; Sissons, R.; Park, J.-Y.; Kim, S.-H.; Barsbold, R.; Tsogtbaatar, K. A non-avian dinosaur with a streamlined body exhibits potential adaptations for swimming. Commun. Biol. 2022, 5, 1185. [Google Scholar] [CrossRef]
- Marsh, O.C. Odontornithes, a Monograph of the Extinct Toothed Birds of North America; Report of the United States Geological Exploration of the Fortieth Parallel; US Government Printing Office: Washington, DC, USA, 1880; Volume 7, pp. 1–201. [Google Scholar]
- Benito, J.; Chen, A.; Wilson, L.E.; Bhullar, B.-A.S.; Burnham, D.; Field, D.J. Forty new specimens of Ichthyornis provide unprecedented insight into the postcranial morphology of crownward stem group birds. PeerJ 2022, 10, e13919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Wang, X.-L.; Zhang, F.-C.; Xu, X. Important features of Caudipteryx—Evidence from two nearly complete new specimens. Vertebr. PalAsiat. 2000, 38, 241–254. [Google Scholar]
- Zhou, Z.-H.; Wang, X.-L. A new species of Caudipteryx from the Yixian Formation of Liaoning, northeast China. Vertebr. PalAsiat. 2000, 38, 111–127. [Google Scholar]
- Qiu, R.; Wang, X.; Wang, Q.; Li, N.; Zhang, J.; Ma, Y. A new caudipterid from the Lower Cretaceous of China with information on the evolution of the manus of Oviraptorosauria. Sci. Rep. 2019, 9, 6431. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.J.; Russell, D.A. Osteology and relationships of Chirostenotes pergracilis (Saurischia, Theropoda) from the Judith River (Oldman) Formation of Alberta, Canada. Can. J. Earth Sci. 1988, 25, 972–986. [Google Scholar] [CrossRef]
- Smith, D. The Type Specimen of Oviraptor philoceratops, a Theropod Dinosaur from the Upper Cretaceous of Mongolia. Neues Jahrb. Geol. Paläontol. Abh. 1992, 186, 365–388. [Google Scholar]
- Balanoff, A.M.; Norell, M.A. Osteology of Khaan meckennai (Oviraptorosauria: Theropoda). Bull. Am. Mus. Nat. Hist. 2012, 372, 1–77. [Google Scholar] [CrossRef]
- Funston, G.F.; Tsogtbaatar, C.; Tsogtbaatar, K.; Kobayashi, Y.; Sullivan, C.; Currie, P.J. A new twi-fingered dinosaur sheds light on the radiation of Oviraptorosauria. R. Soc. Open Sci. 2020, 7, 201184. [Google Scholar] [CrossRef]
- Rhodes, M.W.; Funston, G.F.; Currie, P.J. New material reveals the pelvic morphology of Caenagnathidae (Theropoda, Oviraptorosauria). Cretac. Res. 2020, 114, 104521. [Google Scholar] [CrossRef]
- Funston, G.F.; Mendonca, S.E.; Currie, P.J.; Barsbold, R. Oviraptorosaur anatomy, diversity and ecology in the Nemegt Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 494, 101–120. [Google Scholar] [CrossRef]
- Norell, M.A.; Clark, J.M.; Chiappe, L.M. An Embryonic Oviraptorid (Dinosauria: Theropoda) from the Upper Cretaceous of Mongolia. Am. Mus. Novit. 2001, 3315, 1–20. [Google Scholar] [CrossRef]
- Weishampel, D.B.; Fastovsky, D.E.; Watabe, M.; Varricchio, D.; Jackson, F.; Tsogtbaatar, K.; Barsbold, R. New oviraptorid embryos from Bugin-Tsav, Nemegt Formation (Upper Cretaceous), Mongolia, with insights into their habitat and growth. J. Vertebr. Paleonol. 2008, 28, 1110–1119. [Google Scholar] [CrossRef]
- Lü, J.; Currie, P.J.; Xu, L.; Zhang, X.; Pu, H.; Jia, S. Chicken-sized oviraptorid dinosaurs from central China and their ontogenetic implications. Naturwissenschaften 2013, 100, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Currie, P.; Pittman, M.; Xing, L.; Meng, Q.; Lü, J.; Hu, D.; Yu, C. Mosaic evolution in an asymmetrically feathered troodontid dinosaur with transitional features. Nat. Commun. 2017, 8, 14972. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, S.; Lü, J.; You, H.; Chen, W.; Liu, Y.; Liu, Y. First avialian bird from China. Geol. Bull. China 2005, 24, 197–210. [Google Scholar]
- Shen, C.; Lü, J.; Liu, S.; Kundrát, M.; Brusatte, S.L.; Gao, H. A New Troodontid from the Lower Cretaceous Yixian Formation of Liaoning Province, China. Acta Geol. Sin.-Engl. 2017, 91, 763–780. [Google Scholar] [CrossRef]
- Tsuihiji, T.; Barsbold, R.; Watabe, M.; Tsogtbaatar, K.; Tsogtbaatar, C.; Fujiyama, Y.; Suzuki, S. An exquisitely preserved troodontid theropod with new information on the palatal structure from the Upper Cretaceous of Mongolia. Naturwissenschaften 2014, 101, 131–142. [Google Scholar] [CrossRef]
- Xu, X.; Norell, M.A.; Wang, X.-L.; Makovicky, P.J.; Wu, X.-C. A basal troodontid from the Early Cretaceous of China. Nature 2002, 415, 780–784. [Google Scholar] [CrossRef]
- Gong, E.-P.; Martin, L.D.; Burnham, D.A.; Falk, A.R.; Hou, L.-H. A new species of Microraptor from the Jehol Biota of northeastern China. Palaeoworld 2012, 21, 81–91. [Google Scholar] [CrossRef]
- Hwang, S.H.; Norell, M.A.; Ji, Q.; Gao, K. New Specimens of Microraptor zhaoianus (Theropoda: Dromaeosauridae) from Northeastern China. Am. Mus. Novit. 2002, 3381, 1–44. [Google Scholar] [CrossRef]
- Longrich, N.R.; Currie, P.J. A microraptorine (Dinosauria—Dromaeosauridae) from the Late Cretaceous of North America. Proc. Natl. Acad. Sci. USA 2009, 106, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Brusatte, S.L. A Mesozoic aviary. Science 2017, 335, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Novas, F.E.; Puerta, P.F. New evidence concerning avian origins from the Late Cretaceous of Patagonia. Nature 1997, 387, 390–392. [Google Scholar] [CrossRef]
- Novas, F.E. Avian Traits in the Ilium of Unenlagia comahuensis (Maniraptora, Avialae). In Feathered Dragons: Studies on the Transition from Dinosaurs to Birds; Currie, P.J., Koppelhus, E.B., Shugar, M.A., Wright, J.L., Eds.; Indiana University Press: Bloomington, IN, USA, 2004; pp. 150–166. ISBN 0-253-34373-9. [Google Scholar]
- Burnham, D. New Information on Bambiraptor feinbergi (Theropoda: Dromaeosauridae) from the Late Cretaceous of Montana. In Feathered Dragons: Studies on the Transition from Dinosaurs to Birds; Currie, P.J., Koppelhus, E.B., Shugar, M.A., Wright, J.L., Eds.; Indiana University Press: Bloomington, IN, USA, 2004; pp. 67–111. ISBN 0-253-34373-9. [Google Scholar]
- Ostrom, J.H. Osteology of Deinonychus antirrhopus, an Unusual Theropod from the Lower Cretaceous of Montana. Bull. Peabody Mus. Nat. Hist. 1969, 30, 1–165. [Google Scholar]
- Norell, M.A.; Makovicky, P.J. Important Features of the Dromaeosaurid Skeleton: Information from a New Specimen. Am. Mus. Novit. 1997, 3215, 1–28. [Google Scholar]
- Norell, M.A.; Makovicky, P.J. Important Features of the Dromaeosaurid Skeleton II: Information from Newly Collected Specimens of Velociraptor mongoliensis. Am. Mus. Novit. 1999, 3282, 1–45. [Google Scholar]
- Nesbitt, S. The anatomy of Effigia okeeffeae (Archosauria, Suchia), theropod-like convergence, and the distribution of related taxa. Bull. Am. Mus. Nat. Hist. 2007, 302, 1–84. [Google Scholar] [CrossRef]
- Bates, K.T.; Schachner, E.R. Disparity and convergence in bipedal archosaur locomotion. J. R. Soc. Interface 2012, 9, 1339–1353. [Google Scholar] [CrossRef]
- Gauthier, J.A.; Nesbitt, S.J.; Schachner, E.R.; Bever, G.S.; Joyce, W.G. The Bipedal Stem Crocodilian Poposaurus gracilis: Inferring Function in Fossils and Innovation in Archosaur Locomotion. Bull. Peabody Mus. Nat. Hist. 2011, 52, 107–126. [Google Scholar] [CrossRef]
- Farlow, J.O.; Schachner, E.R.; Sarrazin, J.C.; Klein, H.; Currie, P.J. Pedal Proportions of Poposaurus: Convergence and Divergence in the Feet of Archosaurs. Anat. Rec. 2014, 297, 1022–1046. [Google Scholar] [CrossRef]
- Schachner, E.R.; Irmis, R.B.; Huttenlocker, A.K.; Sanders, K.; Cieri, R.L.; Nesbitt, S.J. Osteology of the Late Triassic Bipedal Archosaur Poposaurus gracilis (Archosauria: Pseudosuchia) from Western North America. Anat. Rec. 2020, 303, 874–917. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, J.C. Postcranial skeleton of Postosuchus kirkpatricki (Archosauria: Paracrocodylomorpha), from the Upper Triassic of the United States. Geol. Soc. Spec. Publ. 2013, 379, 525–553. [Google Scholar] [CrossRef]
- Vickaryous, M.K.; Maryańska, T.; Weishampel, D.B. Ankylosauria. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 363–392. ISBN 978-0-520-25408-4. [Google Scholar]
- Dodson, P.; Forster, C.A.; Sampson, S.D. Ceratopsidae. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 494–513. ISBN 978-0-520-25408-4. [Google Scholar]
- Xing, L.; Miyashita, T.; Wang, D.; Niu, K.; Currie, P.J. A new compsognathid theropod dinosaur from the oldest assemblage of the Jehold Biota in the Lower Cretaceous Huajiying Formation, northeastern China. Cretac. Res. 2020, 107, 104285. [Google Scholar] [CrossRef]
- Ostrom, J. The Osteology of Compsognathus longipes Wagner. Zitteliana 1978, 4, 73–118. [Google Scholar]
- Currie, P.J.; Chen, P.-J. Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can. J. Earth Sci. 2001, 38, 1705–1727. [Google Scholar] [CrossRef]
- Chatterjee, S.; Templin, R.J. Feathered Coelurosaurs from China: New Light on the Arboreal Origin of Avian Flight. In Feathered Dragons: Studies on the Transition from Dinosaurs to Birds; Currie, P.J., Koppelhus, E.B., Shugar, M.A., Wright, J.L., Eds.; Indiana University Press: Bloomington, IN, USA, 2004; pp. 251–281. ISBN 0-253-34373-9. [Google Scholar]
- Chatterjee, S.; Templin, R.J. Palaeoecology, Aerodynamics, and the Origin of Avian Flight. In Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Perturbations through Time; Talent, J.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 585–612. ISBN 978-94-024-0491-3. [Google Scholar]
- Xing, L.; Persons, W.S., IV; Bell, P.R.; Xu, X.; Zhang, J.; Miyashita, T.; Wang, F.; Currie, P.J. Piscivory in the feathered dinosaur Microraptor. Evolution 2013, 67, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lim, J.D.; Lockley, M.G.; Xing, L.; Kim, D.H.; Piñuela, L.; Romilio, A.; Yoo, J.S.; Kim, J.H.; Ahn, J. Smallest known raptor tracks suggest microraptorine activity in lakeshore setting. Sci. Rep. 2018, 8, 16908. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Y.; McDonald, P.G.; Wroe, S.; O’Connor, J.K.; Bjarnason, A.; Bevitt, J.J.; Yin, X.; Zheng, X.; Zhou, Z.; et al. Earliest evidence for fruit consumption and potential seed dispersal by birds. eLife 2022, 11, e74751. [Google Scholar] [CrossRef]
- Wu, Y.; Ge, Y.; Hu, H.; Stidham, T.A.; Li, Z.; Bailleul, A.M.; Zhou, Z. Intra-gastric phytoliths provide evidence for folivory in basal avialans of the Early Cretaceous Jehol Biota. Nat. Commun. 2023, 14, 4558. [Google Scholar] [CrossRef]
- Burnham, D.A.; Feduccia, A.; Martin, L.D.; Falk, A.R. Tree climbing—A fundamental avian adaptation. J. Syst. Palaeontol. 2011, 9, 103–107. [Google Scholar] [CrossRef]
- Padian, K. Evolutionary insights from an ancient bird. Nature 2018, 557, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Dial, K.P. Wing-Assisted Incline Running and the Evolution of Flight. Science 2003, 299, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.N.; Panyutina, A.A. Where was WAIR in avian flight evolution? Biol. J. Linn. Soc. 2022, 137, 145–156. [Google Scholar] [CrossRef]
- Kambic, R.E.; Roberts, T.J.; Gatesy, S.M. Long-axis rotation: A missing degree of freedom in avian bipedal locomotion. J. Exp. Biol. 2014, 217, 2770–2782. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Z.; Wang, X.; Kuang, X.; Zhang, F.; Du, X. Four-winged dinosaurs from China. Nature 2003, 421, 335–340. [Google Scholar] [CrossRef]
- Pol, D.; Goloboff, P.A. The Impact of Unstable Taxa in Coelurosaurian Phylogeny and Resampling Support Measures for Parsimony Analyses. Bull. Am. Mus. Nat. Hist. 2020, 440, 97–115. [Google Scholar]
- James, F.C. How many dinosaurs are birds? BioScience 2021, 71, 991–994. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).