Living Algal Community Voucher and Taxonomy in Dickinson County Iowa: A Case for Collection-Based Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site Descriptions
2.2. Taxonomic Evaluations
3. Results
3.1. Common Taxa
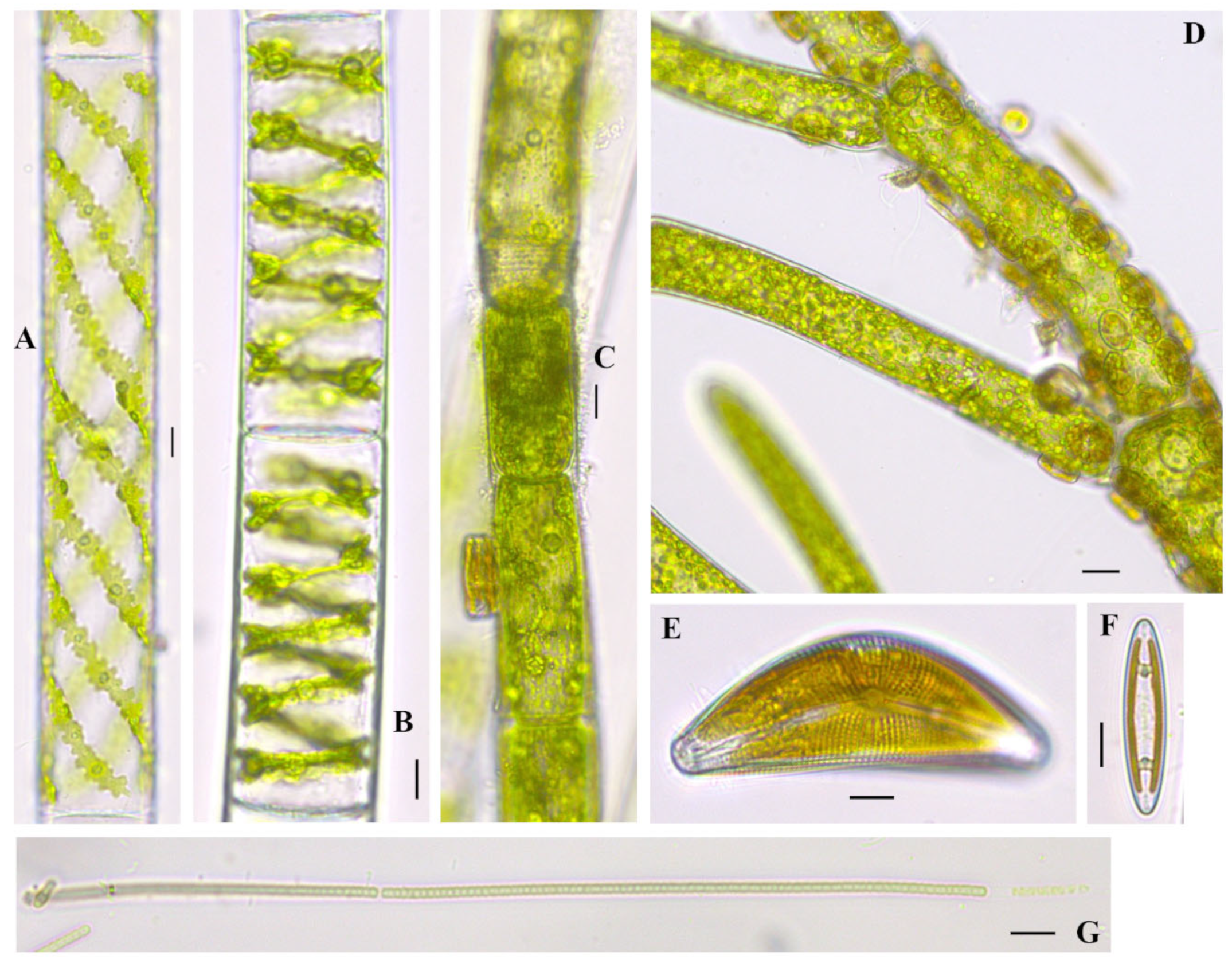
Cladophora glomerata (Linnaeus) Kützing 1843
Figure 3A–D; Figure 5D–F; Figure 6C; Figure 7D;
References: [40] (p. 138), Pl. 20, Figures 8 and 9; Pl. 21, Figures 1 and 2.
Documented from: Millers Bay, Emerson Bay, East and West Okoboji Lakes.
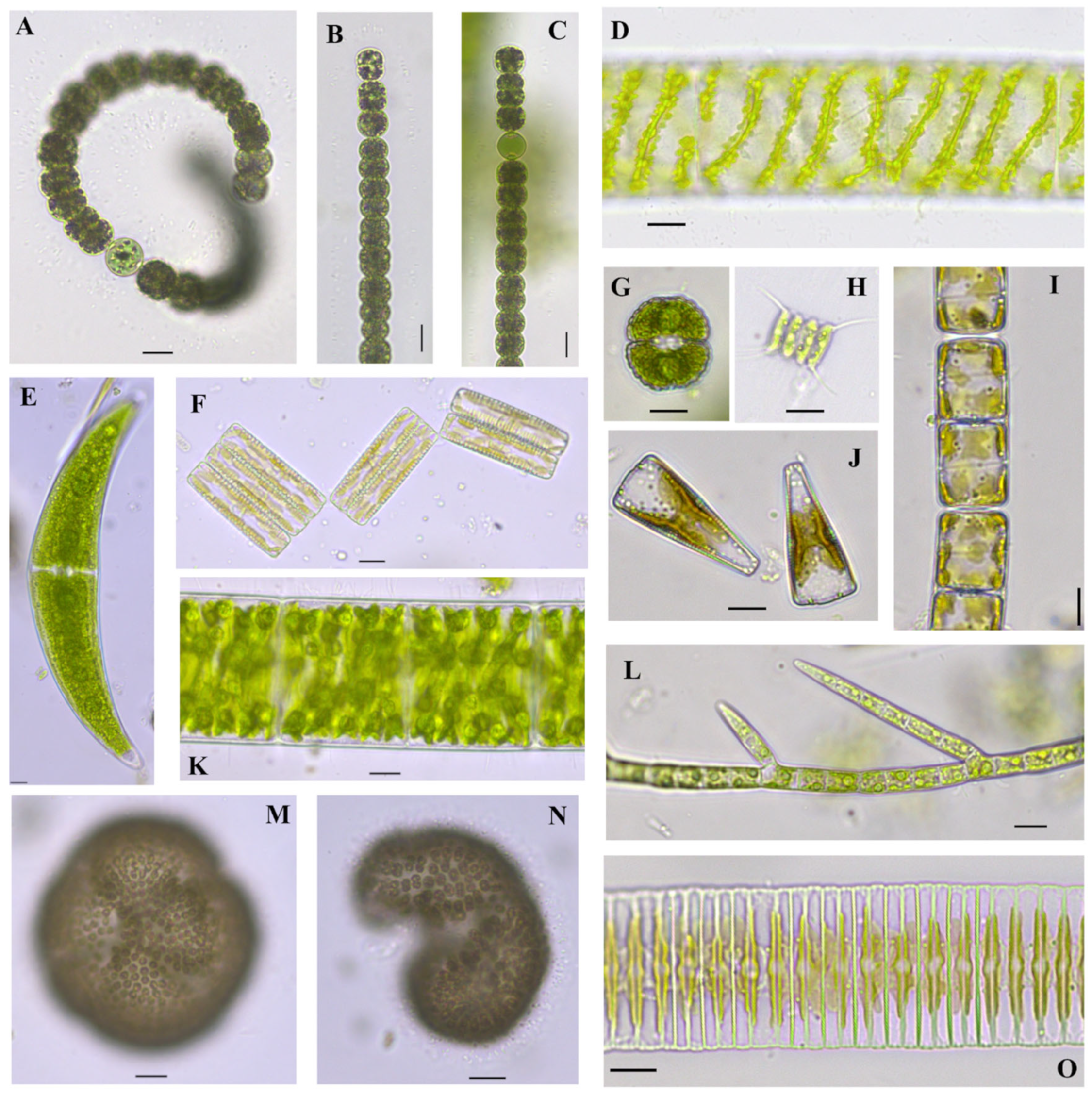
Closterium parvulum Nägeli 1849
Figure 4I
References: [42] (p. 38), Pl. 6, Figure H.
Documented from: Freda Haffner Kettlehole State Preserve, Dickinson County.
Closterium cf. dianae var. minus Hieronymus 1895
Figure 4J
References: [35] (p. 623), Pl. 153, Figure L.
Documented from: Freda Haffner Kettlehole State Preserve, Dickinson County.
Cocconeis pediculus Ehrenberg 1838
Figure 6C
References: [43] (p. 240), Pl. 15, Figures 3 and 4.
Documented from: East Okoboji Lake
Cymbella mexicana (Ehrenberg) Cleve 1894
Figure 3J; Figure 7E
References: [37], vol. 2., Pl. 12, Figures 1 and 2
Documented from: Emerson Bay and West Okoboji Lake
Dolichospermum circinale (Rabenhorst ex Bornet & Flahault) Wacklin, Hoffmann & Komárek 2009
Figure 2A
References: [34] (p. 703), Figure 867;
Documented from: Center Lake
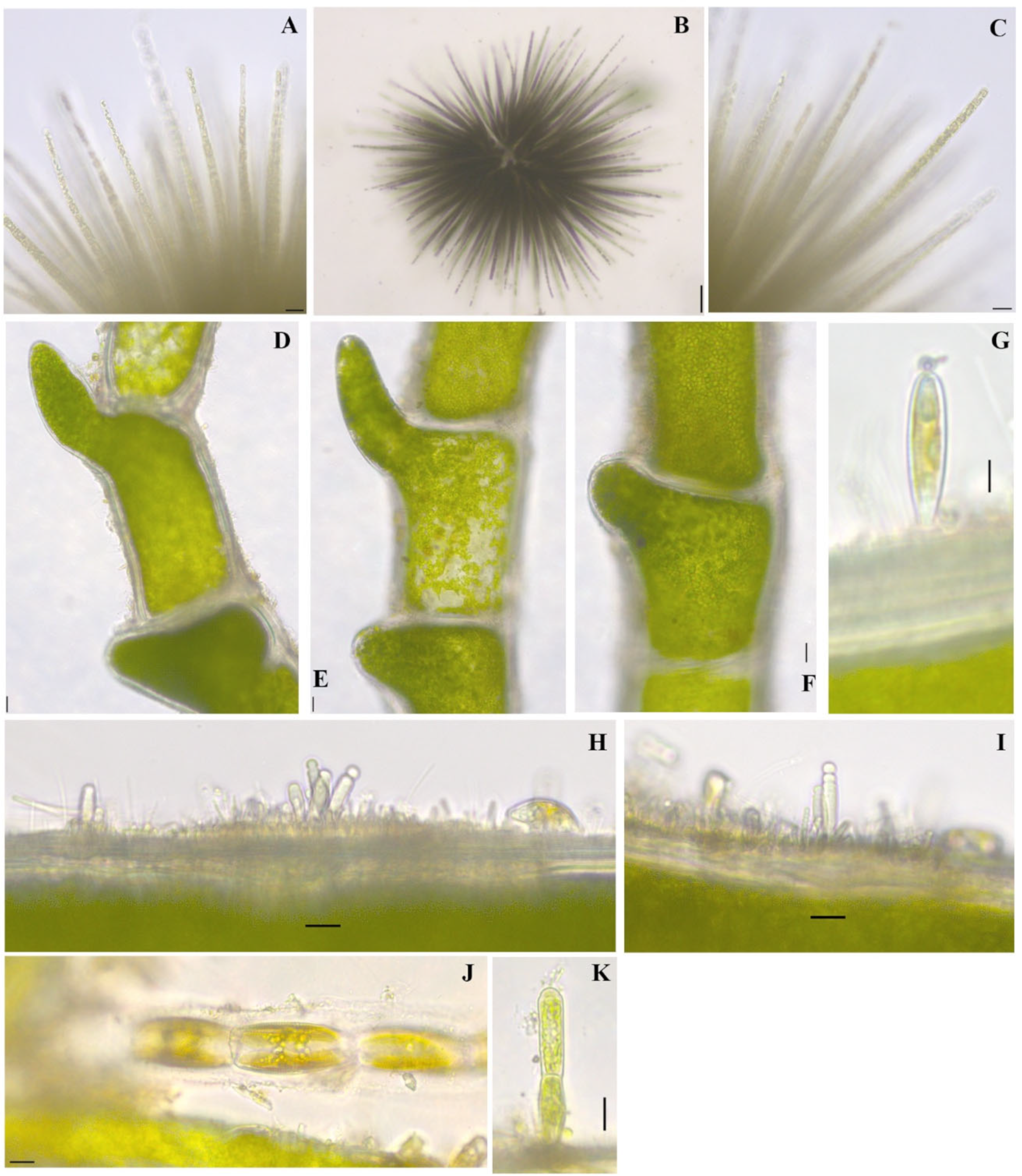
Gloeotrichia echinulata P.G.Richter 1894
Figure 3L; Figure 5A–C
References: [40] (pp. 557–558) Pl. 134, Figures 1 and 2
Documented from: Emerson Bay and Millers Bay
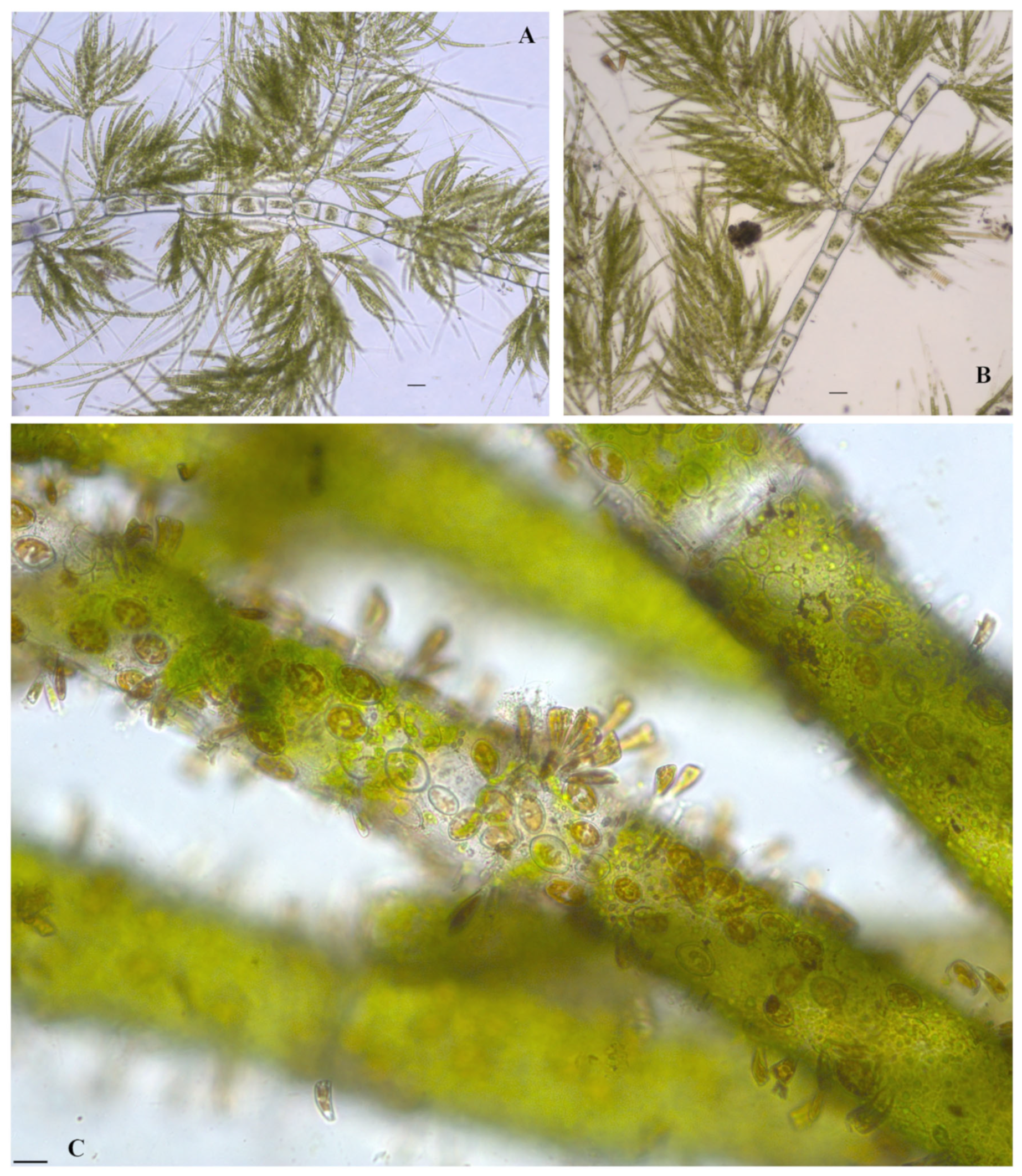
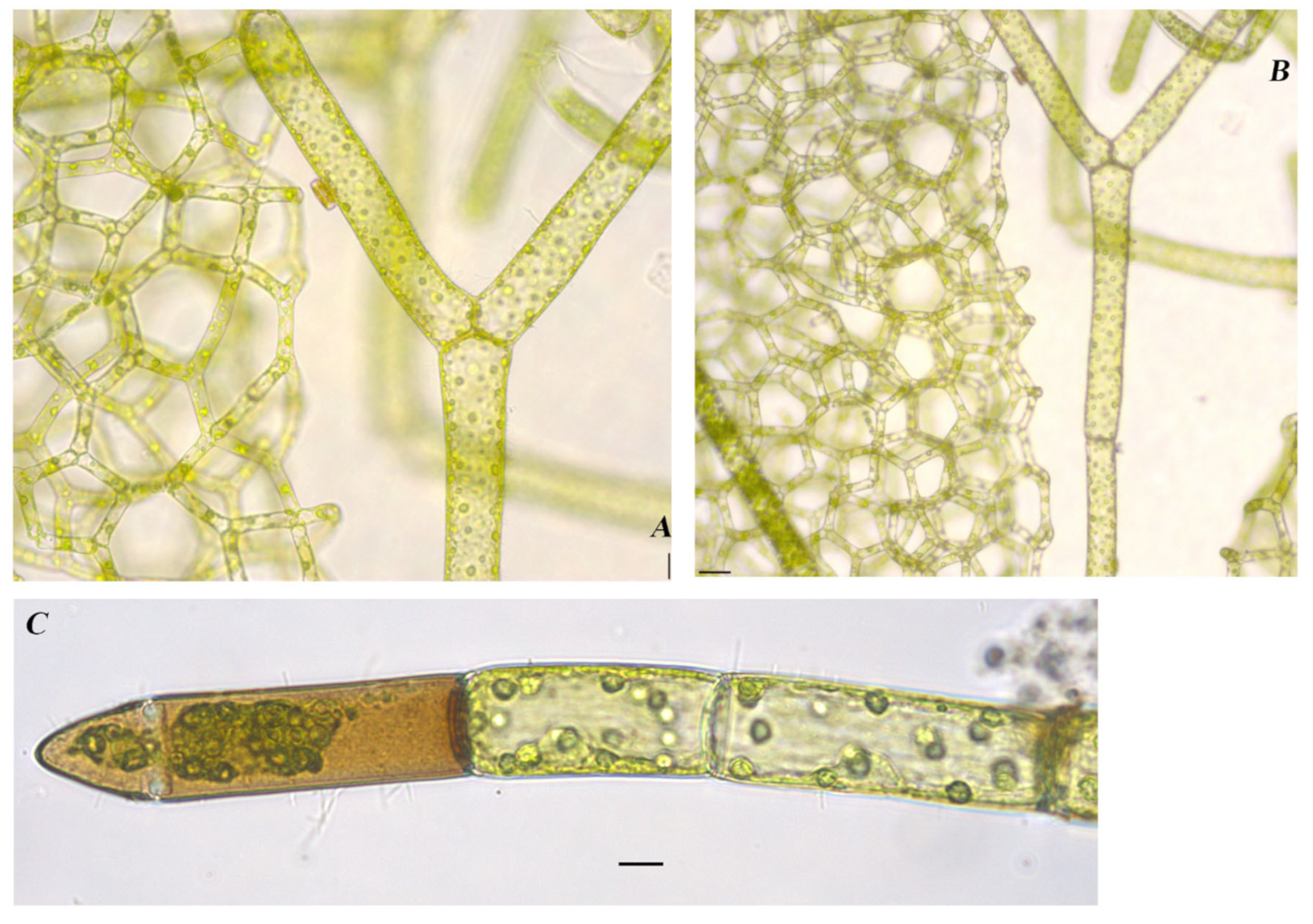
Hydrodictyon reticulatum (Linnaeus) Bory 1824
Figure 9A,B
References: [40] (p. 219), Pl. 47, Figure 1
Documented from: Upper Gar Lake
Klebsormidium cf. mucosum (J.B. Petersen) Lokhorst 1985
Figure 8J–N
References: [35] (p. 556), Pl. 138E.
Documented from: Big Spirit Lake
Microcystis aeruginosa (Kützing) Kützing 1846
Figure 3H,I
References: [35] (p. 69), Pls. 4C, 11K.
Documented from: Emerson Bay
Phacus acuminatus A. Stokes 1885
Figure 4B
References: [35] (p. 206), Pl. 50S.
Documented from: Freda Haffner Kettlehole State Preserve
Spirogyra cf. borgeana Transeau 1916
Figure 7B
Documented from: West Okoboji Lake
Woronichinia naegeliana (Unger) Elenkin 1933
Figure 2M,N
References: [35] (p. 77), Pls. 6B, 13 C, D.
Documented from: Center Lake
3.2. Unique Ttaxa
Chaetophoropsis elegans (Roth) B.Wen Liu, Qian Xiong, X.Dong Liu, Z.Yu Hu & G.Xiang Liu 2019
Taxonomic synonym: Chaetophora elegans (Roth) C. Agardh 1812
Figure 8D,E
Documented from: Big Spirit Lake
Draparnaldia acuta (C.Agardh) Kützing 1845
Figure 6A,B
References: [40] (pp. 119–120), Pl. 15, Figure 5
Documented from: East Okoboji Lake
Glaucocystis nostochinearum Itzigsohn 1866
Figure 4A,C
Documented from: Freda Haffner Kettlehole State Preserve
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J. Challenges for taxonomy. Nature 2002, 417, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, M.; Ács, É.; Almeida, S.F.; Blanco, S.; Dreßler, M.; Ector, L.; Karjalainen, S.M.; Liess, A.; Mertens, A.; Van der Wal, J.; et al. Quality assurance of diatom counts in Europe: Towards harmonized datasets. Hydrobiologia 2016, 772, 1–14. [Google Scholar] [CrossRef]
- Conix, S.; Cuypers, V.; Zachos, F.E.; De Block, A. Taxonomic disagreement about ranks in gray-area taxa: A vignette study. BioScience 2023, 73, 728–737. [Google Scholar] [CrossRef]
- De Luca, D.; Piredda, R.; Sarno, D.; Kooistra, W. Resolving cryptic species complexes in marine protists: Phylogenetic haplotype networks meet global DNA metabarcoding datasets. ISME J. 2021, 15, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Zieritz, A.; Lee, P.S.; Eng, W.W.H.; Lim, S.Y.; Sing, K.W.; Chan, W.N.; Loo, J.S.; Mahadzir, F.N.; Ng, T.H.; Yeo, D.C.; et al. DNA metabarcoding unravels unknown diversity and distribution patterns of tropical freshwater invertebrates. Freshw. Biol. 2022, 67, 1411–1427. [Google Scholar] [CrossRef]
- Lai, J.; Liang, Q.; Zhang, X.; Liu, Y.; Wang, M.; Yang, W.; Sun, T.; Li, Y.; Jin, H.; Liu, Y.; et al. FWAlgaeDB, an integrated genome database of freshwater algae. Front. Environ. Sci. 2023, 11, 1178097. [Google Scholar] [CrossRef]
- Noble, P.J.; Seitz, C.; Lee, S.S.; Manoylov, K.M.; Chandra, S. Characterization of algal community composition and structure from the nearshore environment, Lake Tahoe (United States). Front. Ecol. Evol. 2023, 10, 1053499. [Google Scholar] [CrossRef]
- Alers-García, J.; Lee, S.S.; Spaulding, S.A. Resources and practices to improve diatom data quality. Limnol. Oceanogr. Bull. 2021, 30, 48–53. [Google Scholar] [CrossRef]
- Johnson, K.M.; Gaiser, E.; Manoylov, K.M. Diatom Voucher Flora and Comparison of Collection and Taxonomic Methods for Biodiversity Hotspot Upper Three Runs Creek. Water 2023, 15, 2578. [Google Scholar] [CrossRef]
- Brown, S.; Manoylov, K.M. Assessing Diatom Community Dynamics in a Recovering Agricultural Stream in Middle Georgia, USA. Phycology 2023, 3, 294–304. [Google Scholar] [CrossRef]
- Bishop, I.W.; Esposito, R.M.; Tyree, M.; Spaulding, S.A. A diatom voucher flora from selected southeast rivers (USA). Phytotaxa 2017, 332, 101–140. [Google Scholar] [CrossRef]
- Spaulding, S.A.; Potapova, M.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.org: Supporting taxonomists, connecting communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Barinova, S.; Smith, T. Flora of Algae and Cyanobacteria of Continental Waters of Israel in the XXI Century: Taxonomy, Autecology and Water Quality Indicators. Diversity 2022, 14, 328. [Google Scholar] [CrossRef]
- Löbl, I.; Klausnitzer, B.; Hartmann, M.; Krell, F.T. The silent extinction of species and taxonomists—An appeal to science policymakers and legislators. Diversity 2023, 15, 1053. [Google Scholar] [CrossRef]
- Wheeler, Q.D.; Raven, P.H.; Wilson, E.O. Taxonomy: Impediment or expedient? Science 2004, 303, 285. [Google Scholar] [CrossRef]
- Heathcote, A.J.; Downing, J.A. Impacts of eutrophication on carbon burial in freshwater lakes in an intensively agricultural landscape. Ecosystems 2012, 15, 60–70. [Google Scholar] [CrossRef]
- Volker, R.; Smith, S.G. Changes in the aquatic vascular flora of Lake East Okoboji in historic times. Proc. Iowa Acad. Sci. 1965, 72, 65–72. [Google Scholar]
- Egertson, C.J.; Kopaska, J.A.; Downing, J.A. A century of change in macrophyte abundance and composition in response to agricultural eutrophication. Hydrobiologia 2004, 524, 145–156. [Google Scholar] [CrossRef]
- Braun, L.E. Deciduous Forests of Eastern North America; Hafner Publishing Company: New York, NY, USA, 1964. [Google Scholar]
- Smith, D.D. Iowa prairie: Original extent and loss, preservation and recovery attempts. J. Iowa Acad. Sci. JIAS 1998, 105, 94–108. [Google Scholar]
- Rose, E.T. Toxic algae in Iowa lakes. Proc. Iowa Acad. Sci. 1953, 60, 738–746. [Google Scholar]
- Greene, S.B.D.; LeFevre, G.H.; Markfort, C.D. Improving the spatial and temporal monitoring of cyanotoxins in Iowa lakes using a multiscale and multi-modal monitoring approach. Sci. Total Environ. 2021, 760, 143327. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Manassaram-Baptiste, D.; LePrell, R.; Bolton, B. Cyanobacteria and algae blooms: Review of health and environmental data from the harmful algal bloom-related illness surveillance system (HABISS) 2007–2011. Toxins 2015, 7, 1048–1064. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, A.J.; Ramstack Hobbs, J.M.; Anderson, N.J.; Frings, P.; Engstrom, D.R.; Downing, J.A. Diatom floristic change and lake paleoproduction as evidence of recent eutrophication in shallow lakes of the Midwestern USA. J. Paleolimnol. 2014, 53, 17–34. [Google Scholar] [CrossRef]
- Wehr, J.D.; Sheath, R.G.; Kociolek, J.P. Freshwater Algae of North America, 2nd Edition: Ecology and Classification; Academic Press: Cambridge, MA, USA, 2015; p. 1066. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. In World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023; Available online: http://www.algaebase.org (accessed on 22 November 2023).
- Iowa DNR Ambient Lake Monitoring. Available online: https://www.iowadnr.gov/Environmental-Protection/Water-Quality/Water-Monitoring/Ambient-Lake-Monitoring (accessed on 22 November 2023).
- American Public Health Association; American Water Works Association; Water Pollution Control Federation; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater Centennial Edition; American Public Health Association: Washington, DC, USA, 2005; pp. 10–32. [Google Scholar]
- Stevenson, R.J.; Bahls, L.L. Periphyton protocols. In Rapid Bioassessment Protocols for Use in Wadeable Streams and Rivers; Barbour, M.T., Gerritsen, J., Snyder, B.D., Stribling, J.B., Eds.; EPA 841-B-99-002; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999; pp. 6.1–6.22. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999; Volume 339.
- Iowa DNR Lakes Ponds & Reservoirs. Available online: https://www.iowadnr.gov/Fishing/Where-to-Fish/Lakes-Ponds-Reservoirs (accessed on 22 November 2023).
- Iowa DNR Iowa’s Ecoregions. Available online: https://programs.iowadnr.gov/bionet/Docs/Ecoregions/ (accessed on 22 November 2023).
- Prescott, G.W. Iowa Algae; The University of Iowa Press: Iowa City, IA, USA, 1931. [Google Scholar]
- Komárek, J. Süsswasserflora von Mitteleuropa. Cyanoprokaryota: 3rd Part: Heterocystous Genera; Springer Spektrum: Heidelberg, Germany, 2013; Volume 19, pp. 1–1130. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algae Flora of the British Isles; Press Syndicate of the University of Cambridge: Cambridge, UK, 2002. [Google Scholar]
- Potapova, M. Diatoma vulgaris. In Diatoms of North America. 2009. Available online: https://diatoms.org/species/diatoma_vulgaris (accessed on 22 November 2023).
- Patrick, R.; Reimer, C.W. The Diatoms of the United States, Exclusive of Alaska and Hawaii: Entomoneidaceae, Cymbellaceae, Gomphonemaceae, Epithemiaceae; Monographs of the Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1975; Volume 2. [Google Scholar]
- Potapova, M. Melosira varians. In Diatoms of North America. 2009. Available online: https://diatoms.org/species/melosira_varians (accessed on 22 November 2023).
- Prescott, G.W. Algae of the Western Great Lakes Area. With an Illustrated Key to the Genera of Desmids and Freshwater Diatoms, 2nd ed.; Brown Company Publishers: Dubuque, IA, USA, 1962; pp. 1–977. [Google Scholar]
- Prescott, G.W. Algae of the Western Great Lakes Area Exclusive of Desmids and Diatoms; Bulletin of the Cranbrook Institute of Science: Bloomfield Hills, MI, USA, 1951; Volume 31, pp. 1–946. [Google Scholar]
- Brook, A.J.; Williamson, D.B.; John, D.M. Phylum Chlorophyta. Suborder Desmidiineae. In The Freshwater Algal Flora of the British Isles. An Identification Guide to Freshwater and Terrestrial Algae, 2nd ed.; John, D.M., Whitton, B.A., Brook, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 636–741. [Google Scholar]
- John, D.M.; Williamson, D.B. A Practical Guide to the Desmids of the West of Ireland; Martin Ryan Institute, National University of Ireland: Galway, Ireland, 2009; p. 38. [Google Scholar]
- Patrick, R.; Reimer, C.W. The Diatoms of the United States, Exclusive of Alaska and Hawaii: Fragilariaceae, Eunotiaceae, Achnanthaceae, Naviculacae; Monographs of the Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1966. [Google Scholar]
- Transeau, E.N. Notes on the Zygnemales. Ohio J. Sci. 1916, 16, 17–31. [Google Scholar]
- Woods, P. Stephanodiscus Reimeri. In Diatoms of North America. 2011. Available online: https://diatoms.org/species/stephanodiscus_reimeri (accessed on 22 November 2023).
- Komárek, J. Studies on the Cyanophytes of Cuba 4-6. Folia Geobot. 1989, 24, 57–97. [Google Scholar] [CrossRef]
- Smith, G.M. The plankton algae of the Okoboji Region. Trans. Am. Microsc. Soc. 1926, 45, 156–233. [Google Scholar] [CrossRef]
- Takahashi, T.; Nishida, T.; Tuji, A.; Saito, C.; Matsuzaki, R.; Sato, M.; Toyooka, K.; Yasuda, H.; Nozaki, H. Delineation of six species of the primitive algal genus Glaucocystis based on in situ ultrastructural characteristics. Sci. Rep. 2016, 6, 29209. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.E.; Graham, J.M.; Wilcox, L.W. Algae, 2nd ed.; Benjamin Cummings: San Francisco, CA, USA, 2009; p. 616. [Google Scholar]
- Li, X.; Dreher, T.W.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom-forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef]
- Dayton, P.K.; Tegner, M.J.; Edwards, P.B.; Riser, K.L. Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol. Appl. 1998, 8, 309–322. [Google Scholar] [CrossRef]
- Iowa State University Iowa Environmental Mesonet. Available online: https://mesonet.agron.iastate.edu/ASOS/reports/mon_prec.php?year=2017 (accessed on 22 November 2023).
- Graham, J.M.; Lembi, C.A.; Adrian, H.L.; Spencer, D.E. Physiological responses to temperature and irradiance in Spirogyra (Zygnematales, Charophyceae). J. Phycol. 1995, 31, 531–540. [Google Scholar] [CrossRef]
- Tiffany, L.H. The filamentous algae of Northwestern Iowa with special reference to the Oedogoniaceae. Trans. Am. Microsc. Soc. 1926, 45, 69–132. [Google Scholar] [CrossRef]
- Munawar, M. Ecological studies of Euglenineae in certain polluted and unpolluted environments. Hydrobiologia 1972, 39, 307–320. [Google Scholar] [CrossRef]
- Prescott, G.W. Desmids. Bot. Rev. 1948, 14, 644–676. [Google Scholar] [CrossRef]
- Griffiths, B.M. On Glaucocystis Nostochinearum, Itzigsohn. Ann. Bot. 1915, 3, 423–432. [Google Scholar] [CrossRef]
- Moreira, D.; Le Guyader, H.; Philippe, H. The origin of red algae and the evolution of chloroplasts. Nature 2000, 405, 69–72. [Google Scholar] [CrossRef] [PubMed]
- McClenachan, L.; Ferretti, F.; Baum, J.K. From archives to conservation: Why historical data are needed to set baselines for marine animals and ecosystems. Conserv. Lett. 2012, 5, 349–359. [Google Scholar] [CrossRef]
- Zu Ermgassen, P.S.; Spalding, M.D.; Blake, B.; Coen, L.D.; Dumbauld, B.; Geiger, S.; Grabowski, J.H.; Grizzle, R.; Luckenbach, M.; McGraw, K.; et al. Historical ecology with real numbers: Past and present extent and biomass of an imperilled estuarine habitat. Procedings R. Soc. B Biol. Sci. 2012, 279, 3393–3400. [Google Scholar] [CrossRef]
- Thurstan, R.H.; Campbell, A.B.; Pandolfi, J.M. Nineteenth century narratives reveal historic catch rates for Australian snapper (Pagrus auratus). Fish Fish. 2016, 17, 210–225. [Google Scholar] [CrossRef]
- Cardinale, B.J. Biodiversity improves water quality through niche partitioning. Nature 2011, 472, 86. [Google Scholar] [CrossRef]
- Whitton, B.A. Biology of Cladophora in freshwaters. Water Res. 1970, 4, 457–476. [Google Scholar] [CrossRef]
- Higgins, S.N.; Malkin, S.Y.; Todd Howell, E.; Guildford, S.J.; Campbell, L.; Hiriart-Baer, V.; Hecky, R.E. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. J. Phycol. 2008, 44, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Whitton, B.A.; Kelly, M.G. Use of algae and other plants for monitoring rivers. Aust. J. Ecol. 1995, 20, 45–56. [Google Scholar] [CrossRef]
- Andrade, A.D.; Rollemberg, M.C.E.; Nóbrega, J.A. Proton and metal binding capacity of the green freshwater alga Chaetophora elegans. Process Biochem. 2005, 40, 1931–1936. [Google Scholar] [CrossRef]
- El Jamal, M.M.; Ncibi, M.C. Biosorption of methylene blue by Chaetophora elegans algae: Kinetics, equilibrium and thermodynamic studies. Acta Chim. Slov. 2012, 59, 24–31. [Google Scholar] [PubMed]
- Gibson, M.T.; Whitton, B.A. Hairs, phosphatase activity and environmental chemistry in Stigeoclonium, Chaetophora and Draparnaldia (Chaetophorales). Br. Phycol. J. 1987, 22, 11–22. [Google Scholar] [CrossRef]
- Heathcote, A.J. Anthropogenic Eutrophication and Ecosystem Functioning in Freshwater Lakes. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2013; pp. 1–158. [Google Scholar]
- Hutchinson, G.E. The Ecological Theater and the Evolutionary Play; Yale University Press: New Haven, CT, USA, 1965. [Google Scholar]
- Tilman, D. Resource competition between planktonic algae: An experimental and theoretical approach. Ecology 1977, 58, 338–348. [Google Scholar] [CrossRef]
- Pichrtová, M.; Holzinger, A.; Kulichová, J.; Ryšánek, D.; Šoljaková, T.; Trumhová, K.; Nemcova, Y. Molecular and morphological diversity of Zygnema and Zygnemopsis (Zygnematophyceae, Streptophyta) from Svalbard (High Arctic). Eur. J. Phycol. 2018, 53, 492–508. [Google Scholar] [CrossRef]
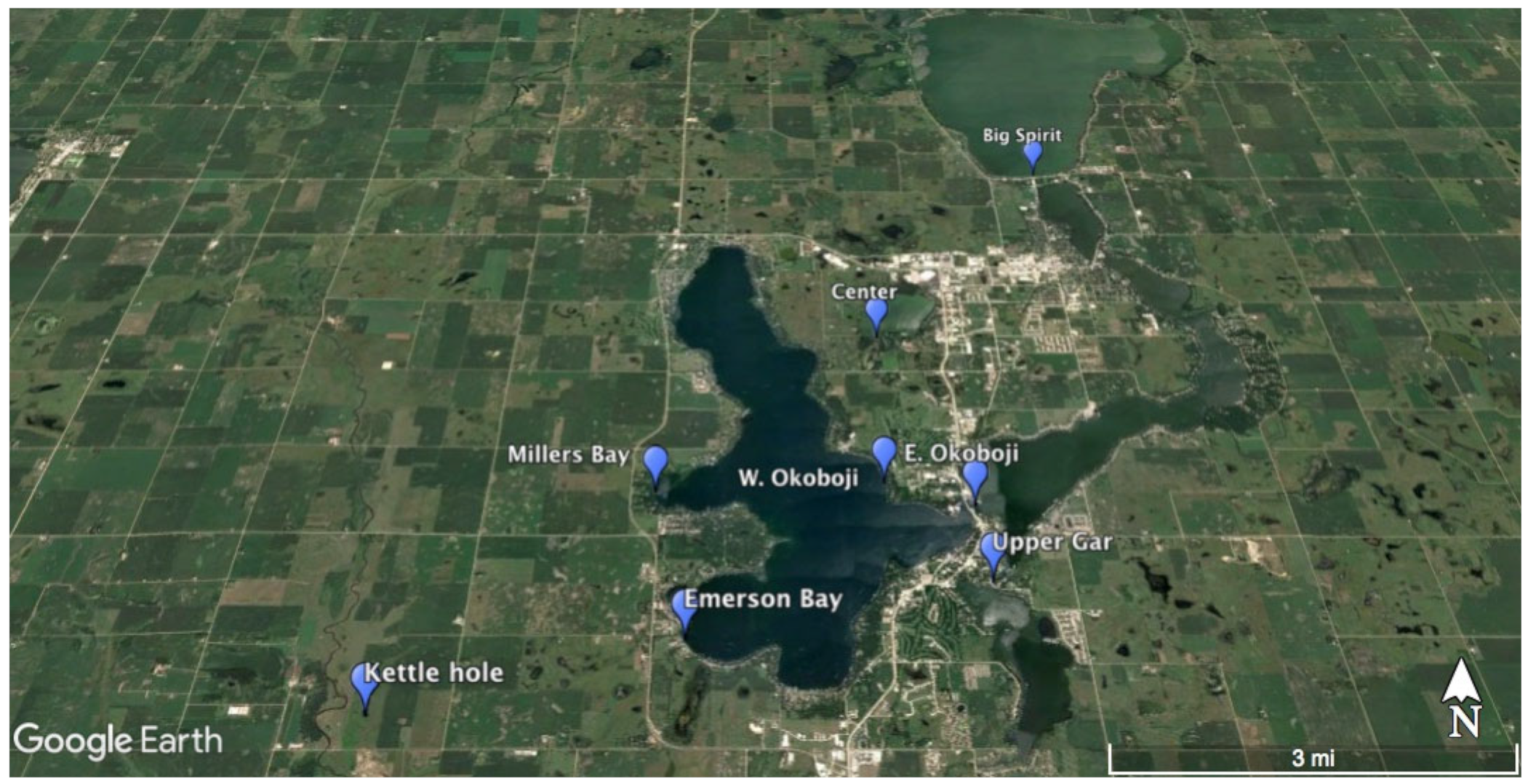



| Site | Latitude | Longitude |
|---|---|---|
| Center Lake | 43.406908° | −95.140512° |
| Emerson Bay | 43.356369° | −95.174774° |
| Freda Haffner Kettlehole State Preserve | 43.346369° | −95.221853° |
| Millers Bay | 43.378333° | −95.181111° |
| East Okoboji Lake | 43.376111° | −95.126111° |
| West Okoboji Lake | 43.379920° | −95.141378° |
| Big Spirit Lake | 43.445810° | −95.102304° |
| Upper Gar Lake | 43.364444° | −95.125000° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, K.M.; Manoylov, K.M. Living Algal Community Voucher and Taxonomy in Dickinson County Iowa: A Case for Collection-Based Research. Diversity 2024, 16, 21. https://doi.org/10.3390/d16010021
Johnson KM, Manoylov KM. Living Algal Community Voucher and Taxonomy in Dickinson County Iowa: A Case for Collection-Based Research. Diversity. 2024; 16(1):21. https://doi.org/10.3390/d16010021
Chicago/Turabian StyleJohnson, Katherine M., and Kalina M. Manoylov. 2024. "Living Algal Community Voucher and Taxonomy in Dickinson County Iowa: A Case for Collection-Based Research" Diversity 16, no. 1: 21. https://doi.org/10.3390/d16010021
APA StyleJohnson, K. M., & Manoylov, K. M. (2024). Living Algal Community Voucher and Taxonomy in Dickinson County Iowa: A Case for Collection-Based Research. Diversity, 16(1), 21. https://doi.org/10.3390/d16010021






