Assessing the Diversity of Ant-Associated Silverfish (Insecta: Zygentoma) in Mediterranean Countries: The Most Important Hotspot for Lepismatidae in Western Palaearctic
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Studied Species
2.3. Bibliographic Revision and Sources of Information
2.4. New Material Studied
2.5. Keys to Myrmecophilous Zygentoma of the Mediterranean Area
2.6. Nomenclature and Authorship of Species
2.7. Distribution Maps and Analysis
3. Results and Taxonomic Discussion
3.1. Commented List of Ant-Associated Silverfish from Mediterranean Countries
- Family Lepismatidae.
- Subfamily Acrotelsatinae.
- Genus Lepismina Gervais, 1844 (Figure 1).
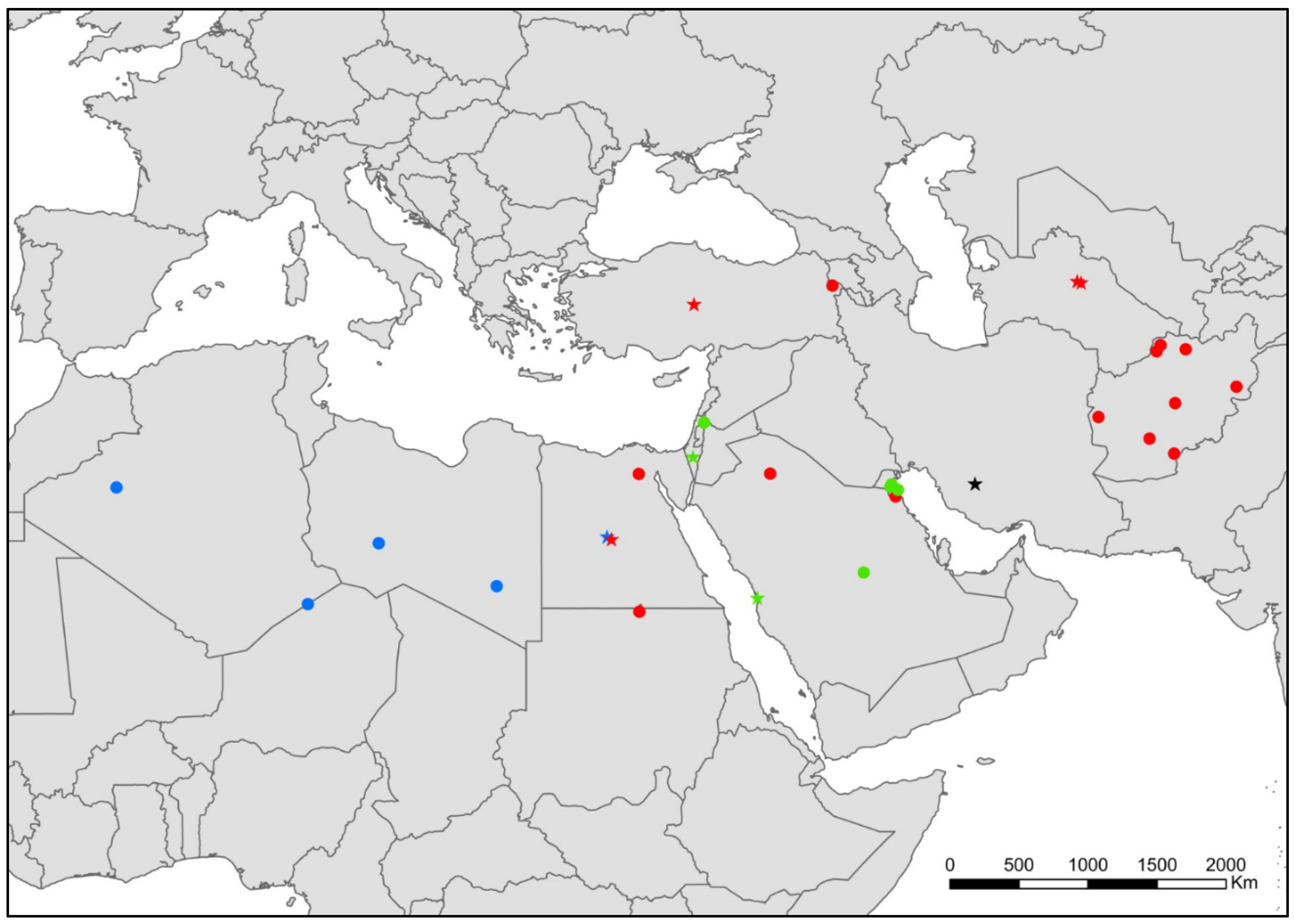
- Lepismina audouinii (Lucas, 1840) (Figure 1).
- Lepismina aurisetosa Wahlgren, 1906 (Figure 1).
- Lepismina persica Escherich, 1905 (Figure 1).
- Subfamily Lepismatinae.
- Genus Lepisma Linnaeus, 1758 (Figure 2).
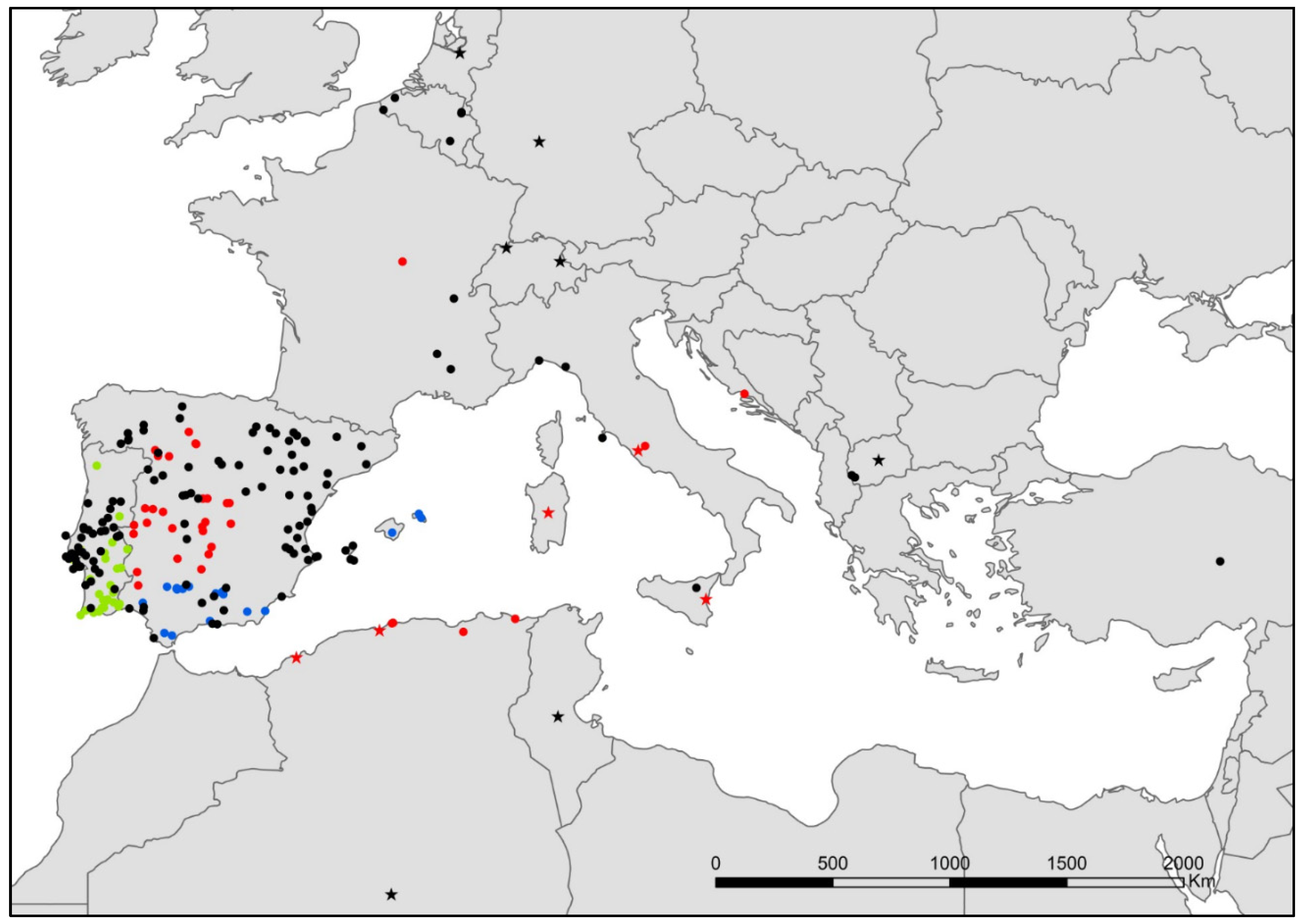
- Lepisma chlorosoma Lucas, 1846 (Figure 2).
- Lepisma baetica Molero-Baltanás, Gaju-Ricart, Bach de Roca & Mendes, 1994 (Figure 2).
- Lepisma saccharinum Linnaeus, 1758 (Figure 2).
- Genera Neoasterolepisma Mendes, 1988 and Tricholepisma Paclt, 1967.
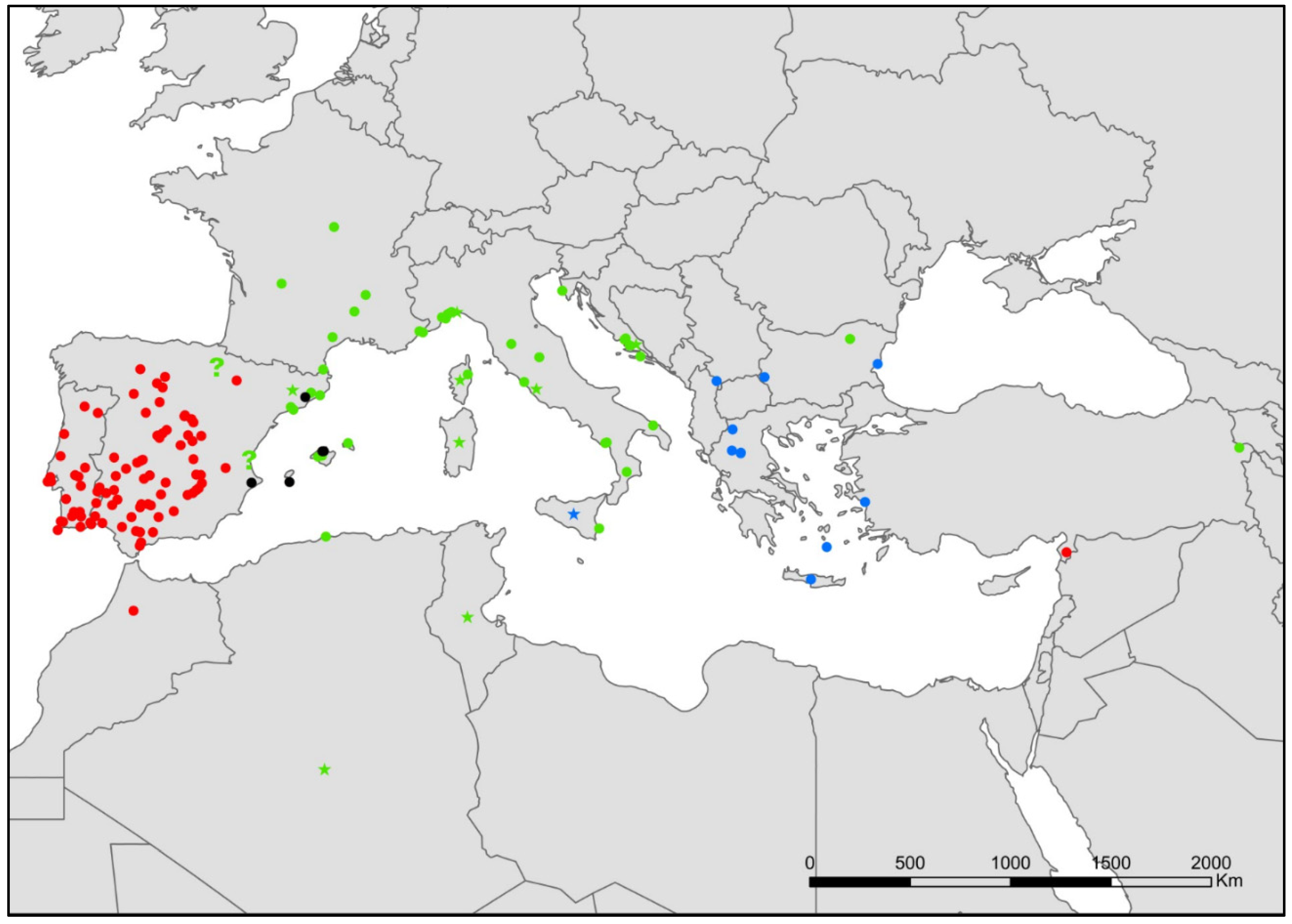
- Neoasterolepisma balcanicum (Stach, 1922) (Figure 3).
- Neoasterolepisma balearicum Molero-Baltanás, Bach de Roca & Gaju-Ricart, 1997.
- Neoasterolepisma lusitanum (Wygodzinsky, 1945) (Figure 3).
- Tricholepisma aureum (Dufour, 1831) (Figure 3).
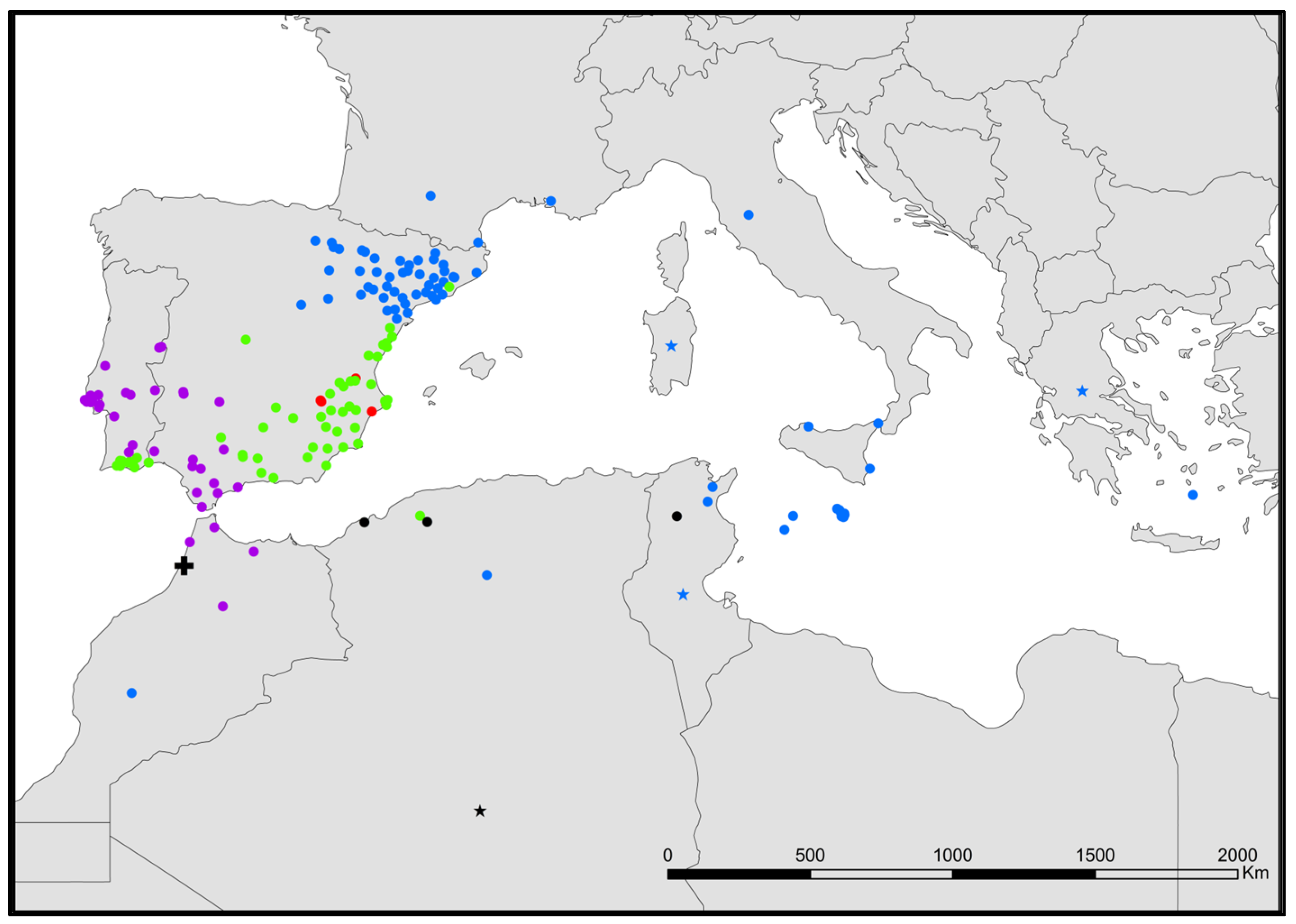
- Neoasterolepisma gauthieri (Wygodzinsky, 1941) (Figure 5).
- Neoasterolepisma calvum Molero-Baltanás, Mendes, Gaju-Ricart & Bach de Roca, 1994 stat. nov. (Figure 5).
- Neoasterolepisma crassipes (Escherich, 1905) (Figure 5).
- Neoasterolepisma soerenseni (Silvestri, 1908) (Figure 5).
- Neoasterolepisma foreli (Moniez, 1894) (Figure 5).
- Neoasterolepisma imitans (Mendes, 1980) (Figure 5).

- Neoasterolepisma delator Molero-Baltanás, Bach de Roca & Gaju-Ricart, 1996 (Figure 6).
- Neoasterolepisma hespericum Molero-Baltanás, Bach de Roca & Gaju-Ricart 1996 (Figure 6).
- Neoasterolepisma spectabile (Wygodzinsky, 1945) (Figure 6).
- Neoasterolepisma spectabiloides Mendes, 1988 (Figure 6).
- Neoasterolepisma wasmanni (Moniez, 1894) (Figure 6).
- Tricholepisma gyriniforme (Lucas, 1846) (Figure 6).
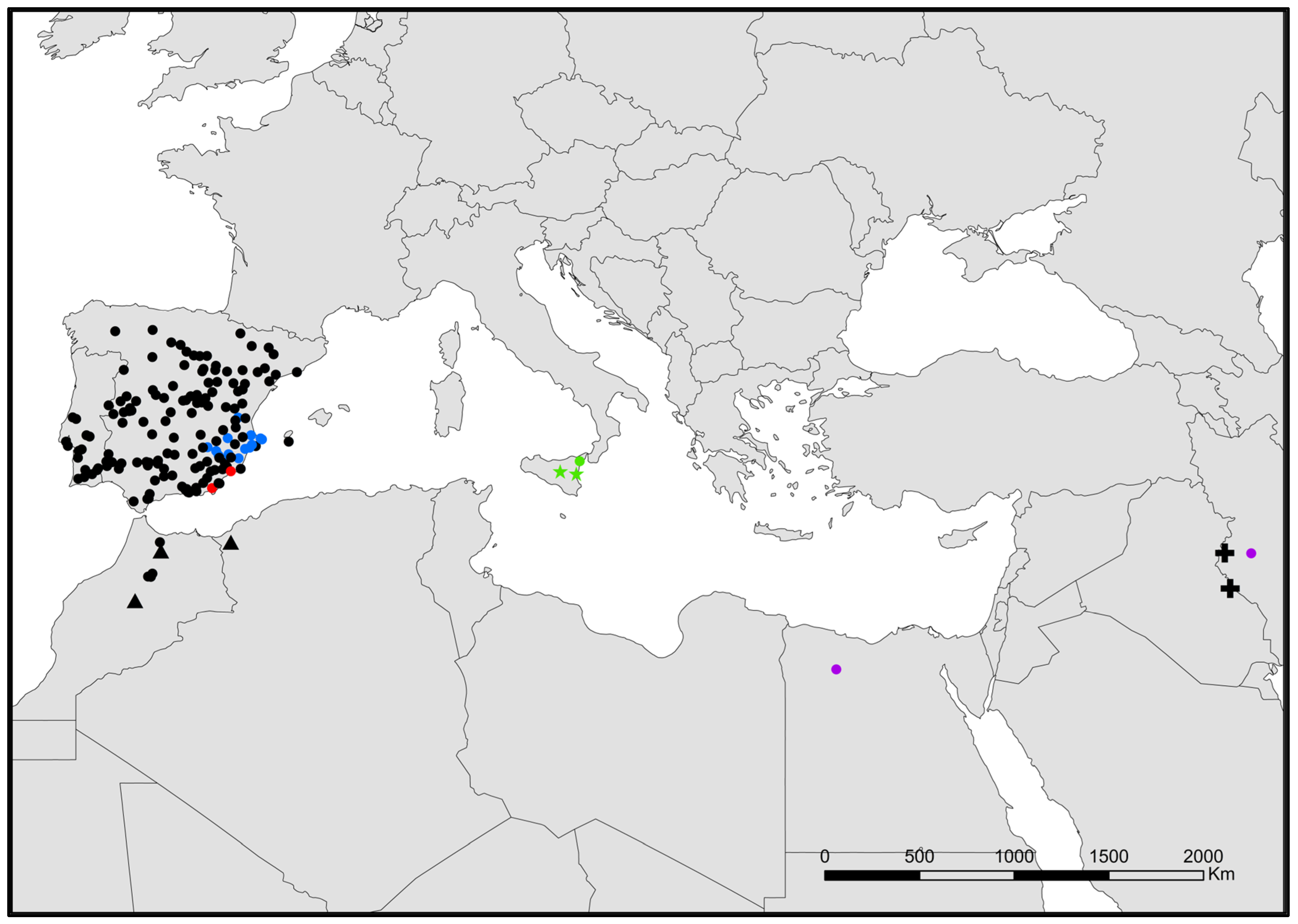
- Neoasterolepisma angustothoracicum (Grassi and Rovelli, 1889) (Figure 7).
- Neoasterolepisma curtiseta Mendes, 1988 (Figure 7).
- Neoasterolepisma pallidum Molero-Baltanás, Gaju-Ricart & Bach de Roca, 1995 (Figure 7).
- Neoasterolepisma evansi (Silvestri, 1923) (Figure 7).
- Neoasterolepisma priesneri (Stach, 1946) (Figure 7).
- Neoasterolepisma stachi (Wygodzinsky, 1941) (Figure 7).
- Tricholepisma indalicum Molero-Baltanás, Bach de Roca & Gaju-Ricart 1995 (Figure 7).
- Neoasterolepisma cf. myrmecobium (Silvestri, 1908).
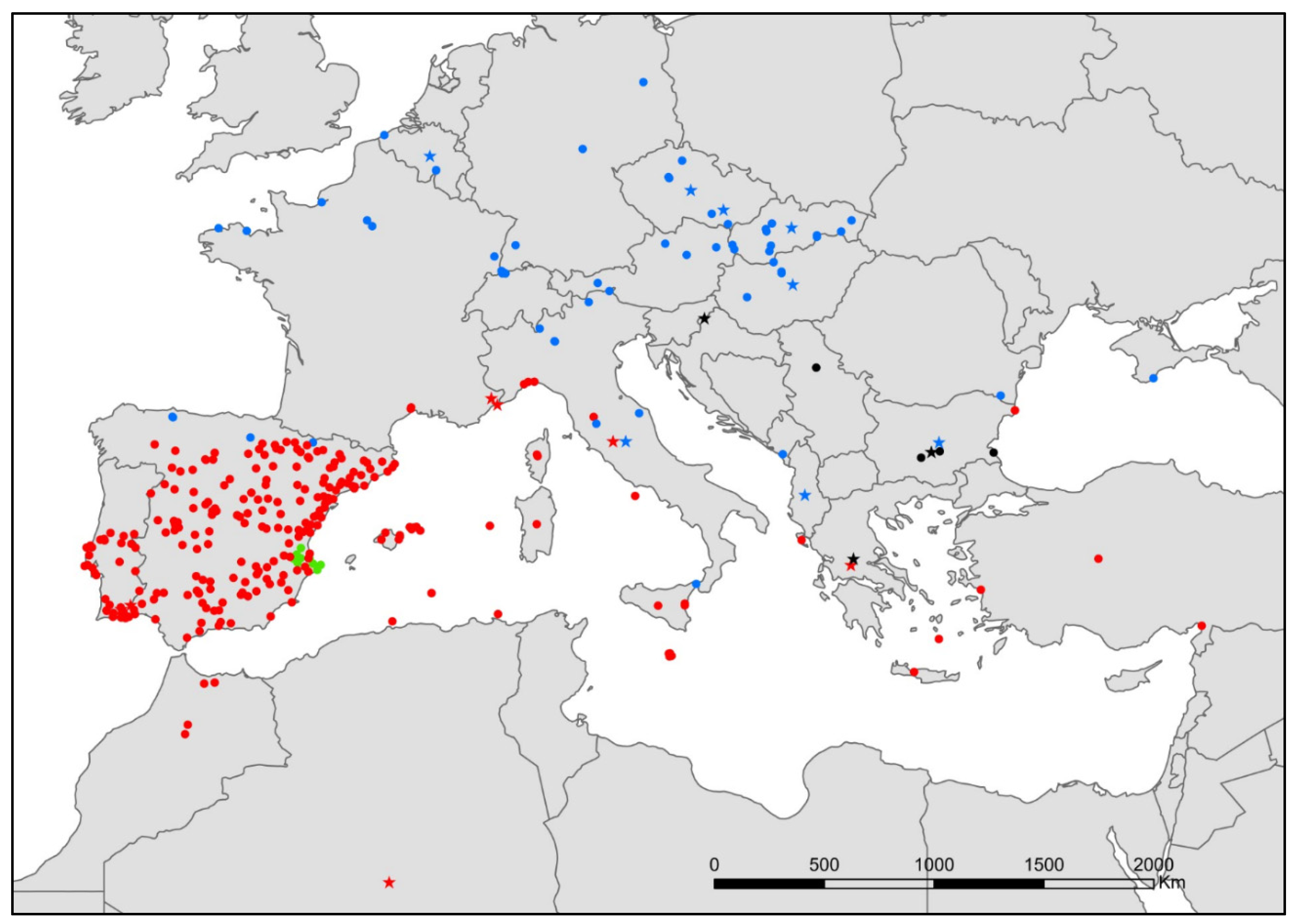
- Atelura formicaria Heyden, 1855 (Figure 8).
- Atelura montana (Stach, 1946) (Figure 8).
- Atelura valenciana Molero-Baltanás, Gaju-Ricart, Bach de Roca & Mendes, 1998 (Figure 8).
- Proatelurina pseudolepisma (Grassi, 1887) (Figure 8).
- Subfamily Coletiniinae (Figure 9).
- Genus Coletinia Wygodzinsky, 1980.
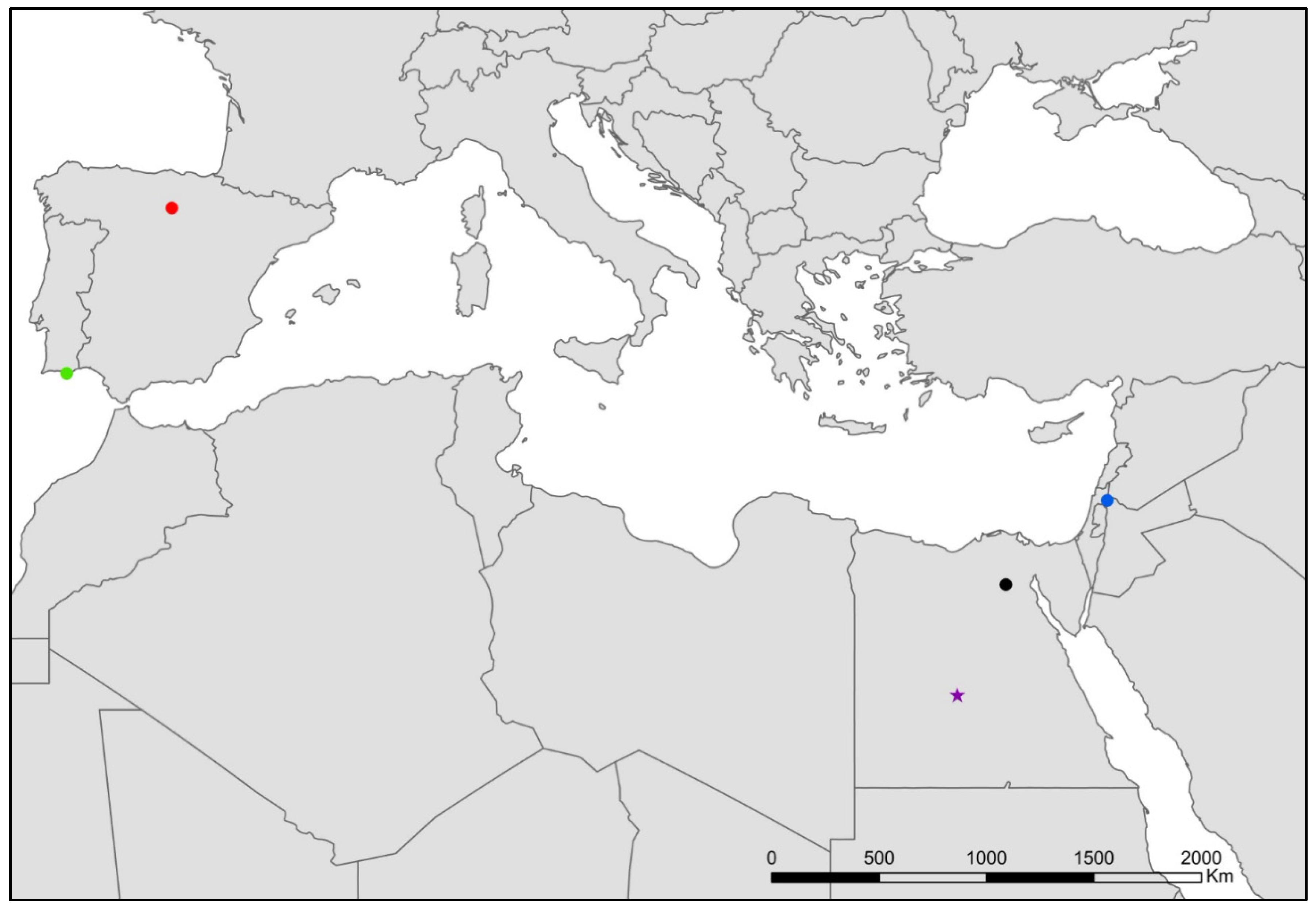
- Coletinia maggi (Grassi, 1887) (Figure 9).
- Coletinia mendesi Wygodzinsky, 1980 (Figure 9).
3.2. Possibly Ant-Associated Silverfish without Enough Information
- Lepismina pluriseta Wygodzinsky, 1942. (Figure 1).
- Neoasterolepisma necrophilum Mendes, 1992 (Figure 10).
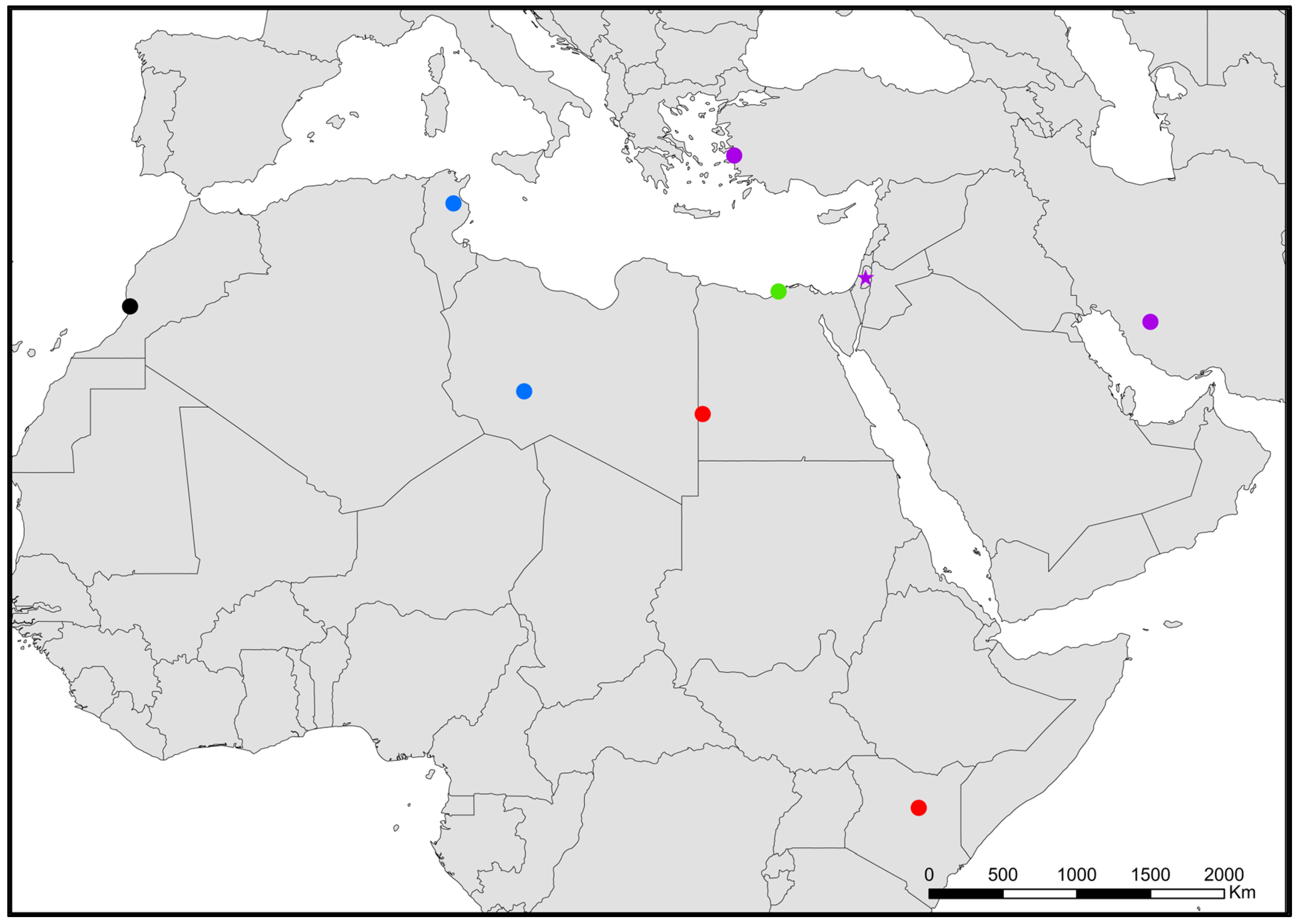
- Neoasterolepisma palmonii (Wygodzinsky, 1942) (Figure 10).
- Neoasterolepisma paucisetosum (Stach, 1935) (Figure 10).
- Neoasterolepisma santschii (Silvestri, 1908) (Figure 10).
- Neoasterolepisma scorpius Mendes, 1993 (Figure 10).
- Grassiella leuca Stach, 1935 (Figure 9).
3.3. Identification Key
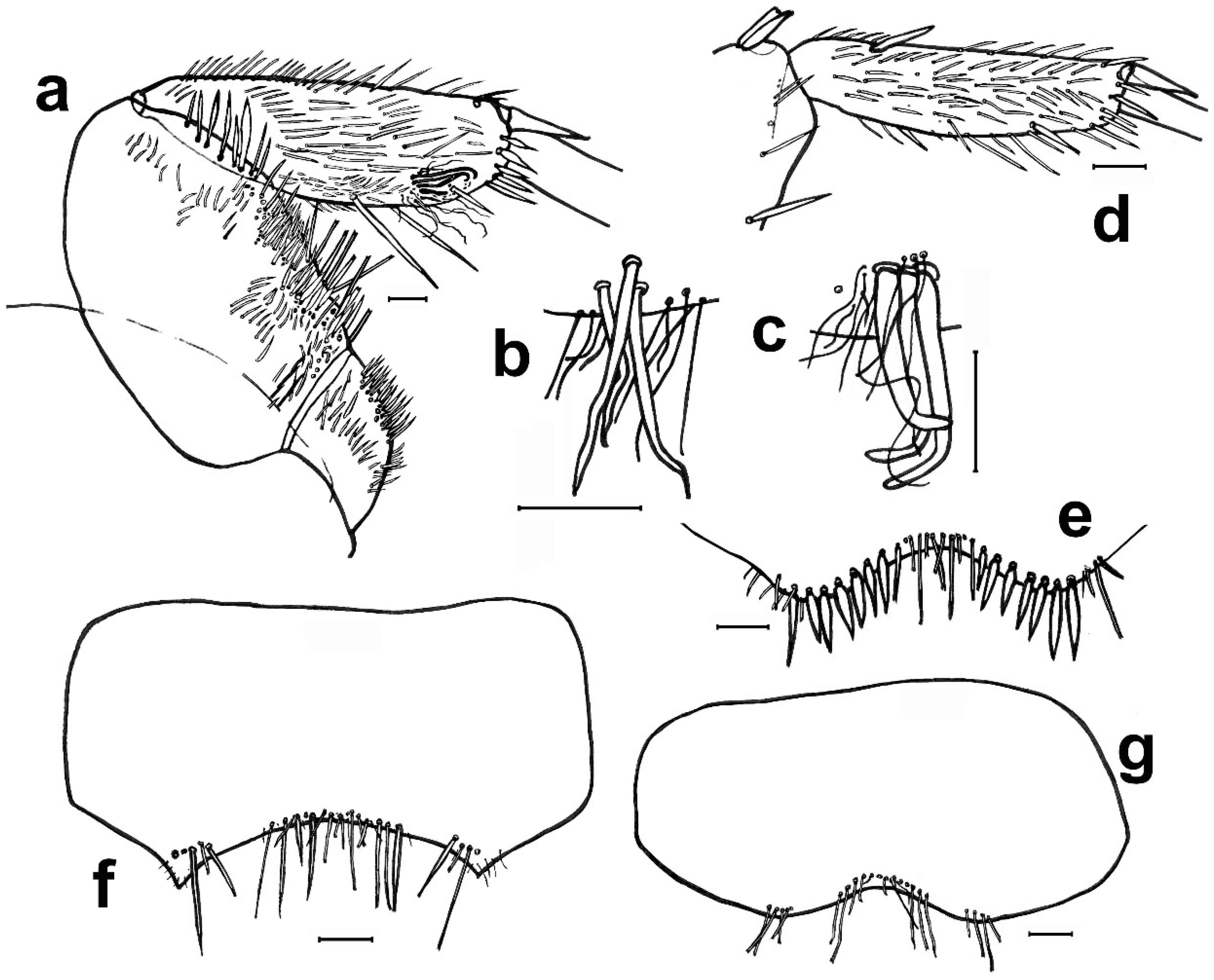
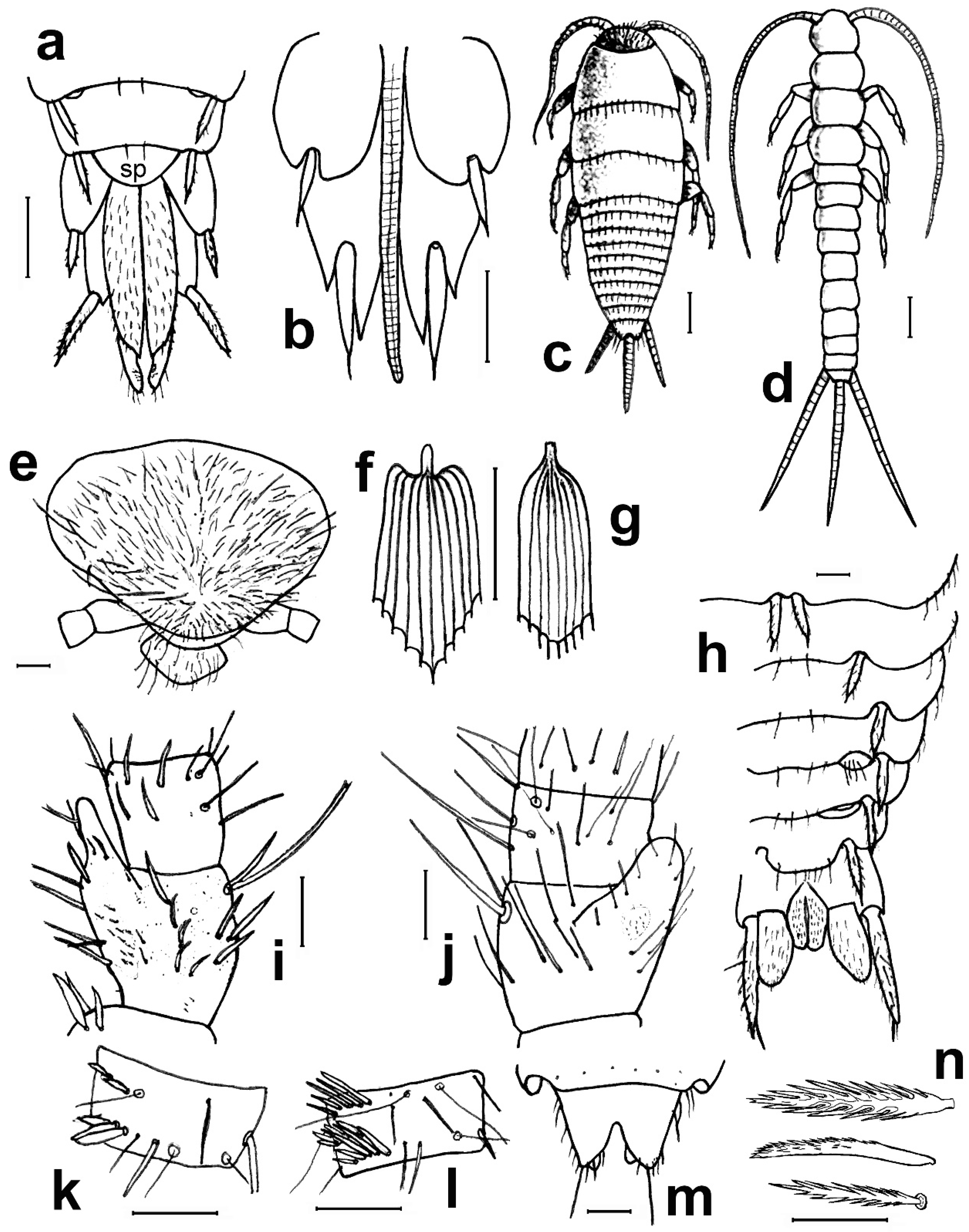
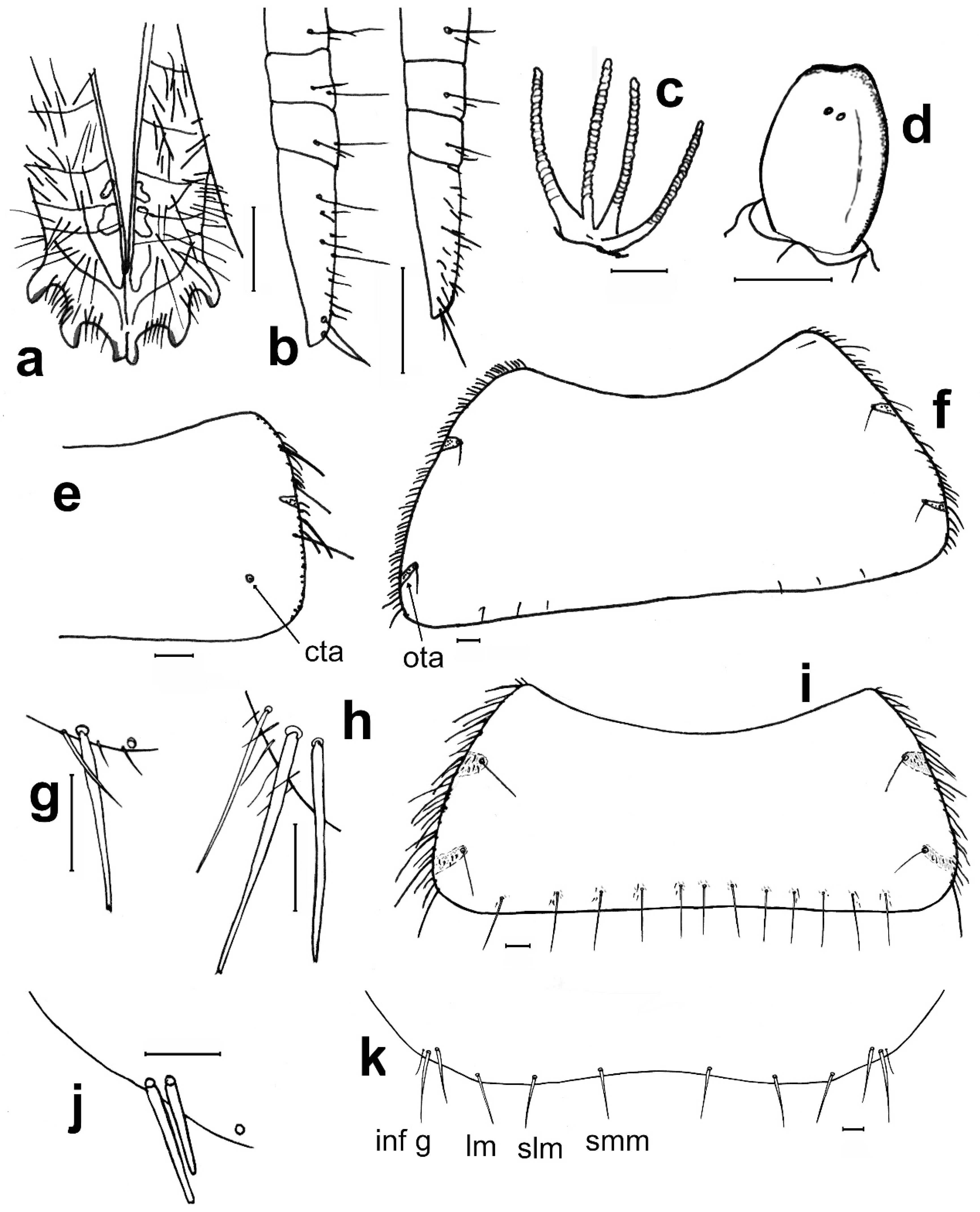
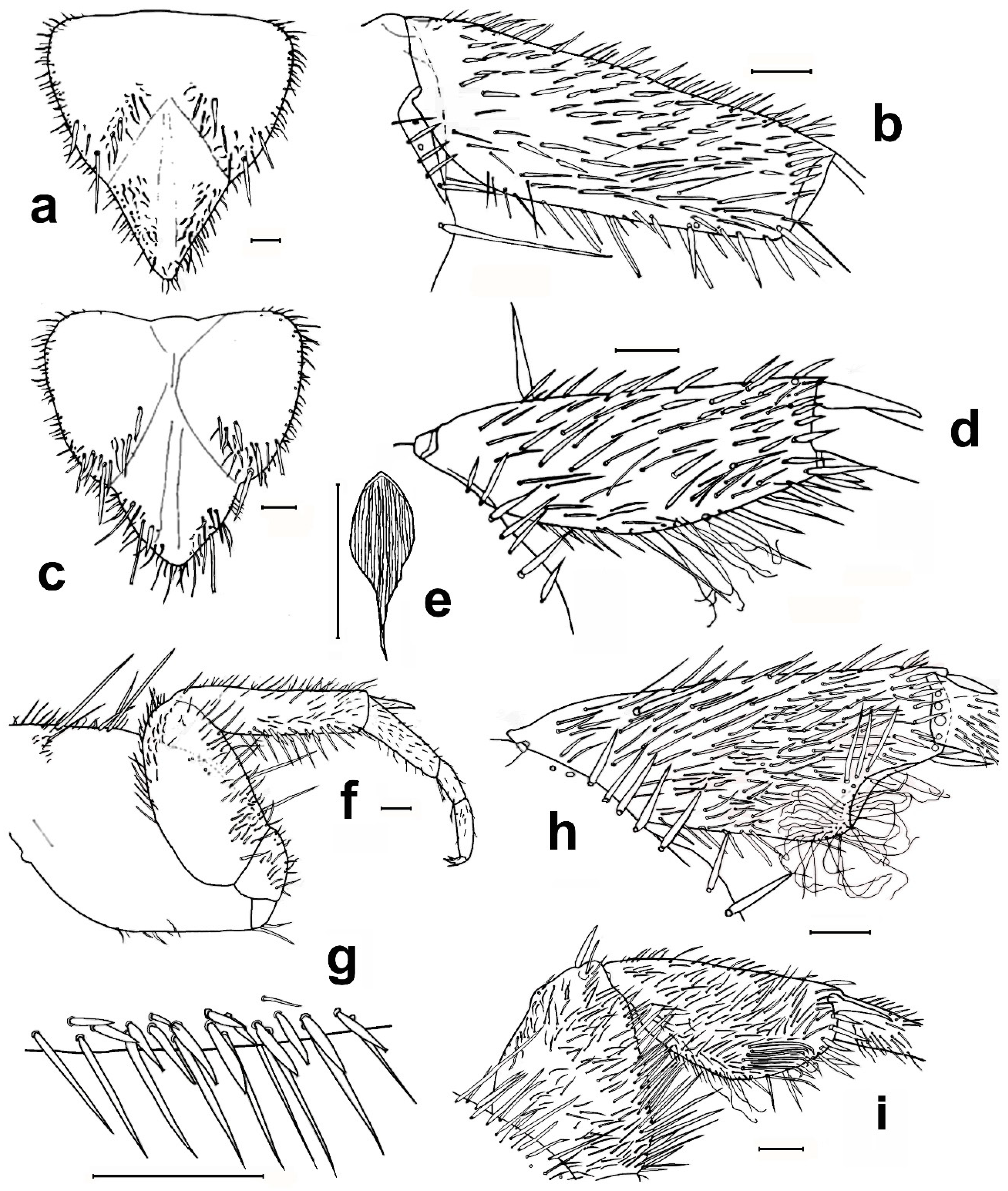
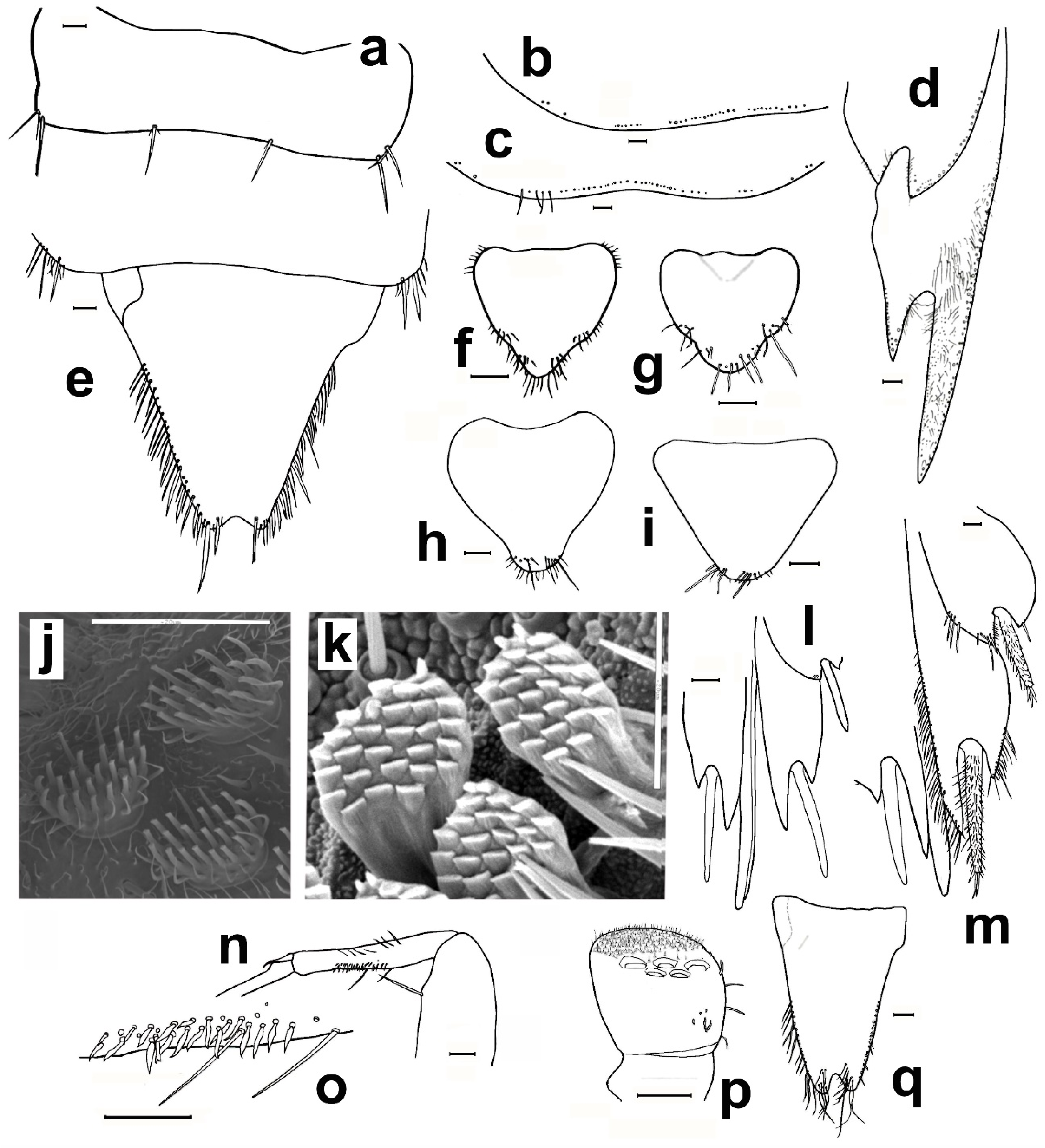
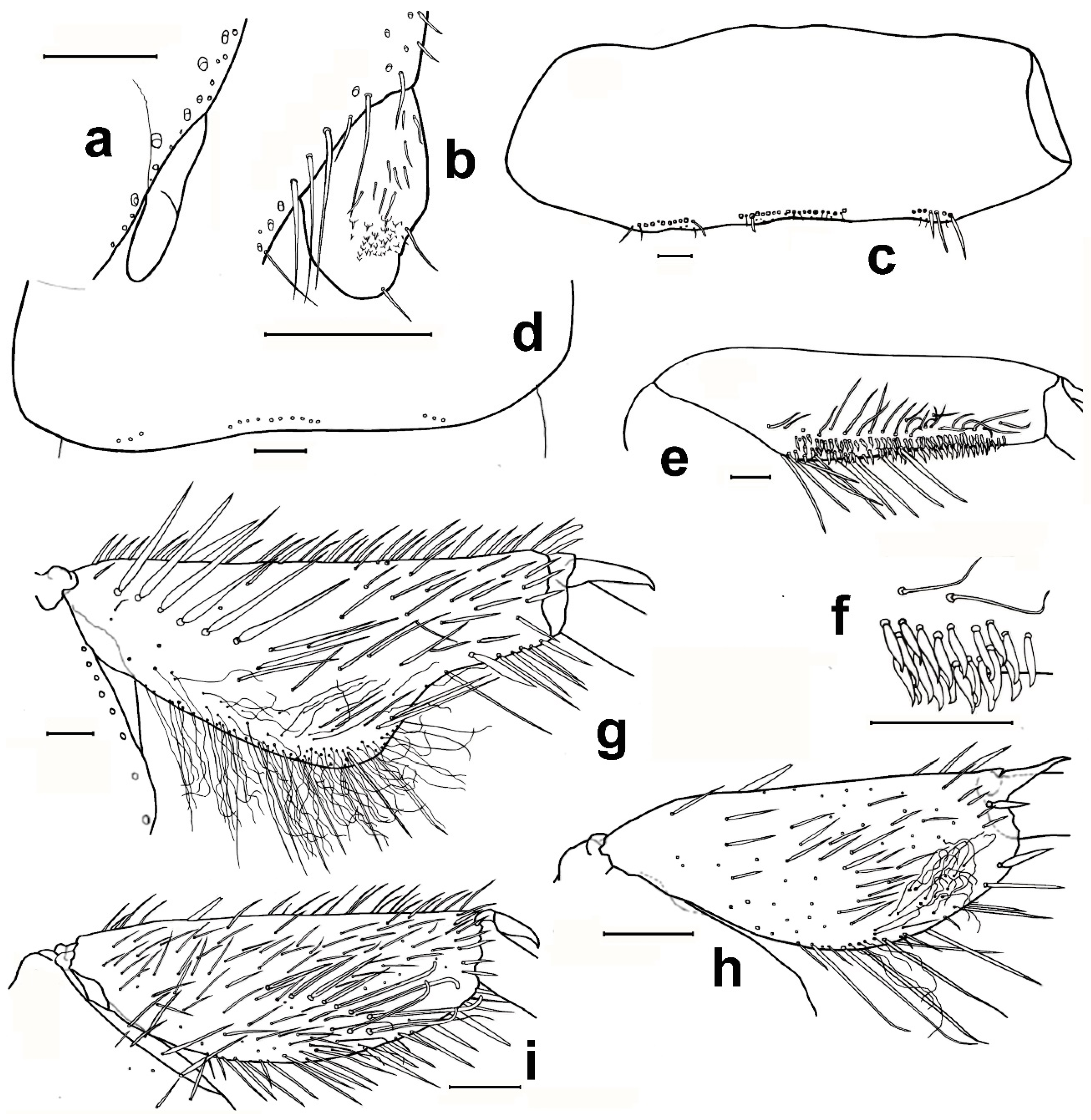
- 1. Head without eyes. Some abdominal sternites with 1 + 1 vesicles in their hind margin (Figure 11h). Eighth abdominal sternite of females divided in two lateral coxites and a median subgenital plate (Figure 11a) ……………………………… Family Nicoletiidae (2).—Head with black compound eyes. Abdominal sternites without vesicles. Eighth abdominal sternite of females divided in two lateral coxites without a median subgenital plate (Figure 11b) ……………………………………………… Family Lepismatidae (9).2. Body short, ovoid or limuloid, with thorax wider than abdomen (Figure 11c), covered with scales (Figure 11f,g) …………………………………………… Subfamily Atelurinae (3).—Body cylindric, elongated, with thorax not clearly wider than abdomen (Figure 11d), without scales …… Subfamily Coletiniinae–Genus Coletinia Wygodzinsky, 1980. * …………………………………………………………… For distinguishing all Coletinia species see [10].3. Head with setae restricted to the frontal area. Cephalic capsule covered with scales only. Male antennal pedicellus with an inner distal apophysis ……………………………… (4).—Head densely setose and without scales (Figure 11e). Antenna of male without pedicellar apophysis. Abdominal vesicles only on urosternite VI, with a medial tuft of long, often deeply cleft robust setae; pseudovesicles on urosternite VII. Seven pairs of abdominal styli, on segments III-IX (Figure 11h) ………………………………………………… Tribe ……………………………………………………………… Atopatelurini–Genus Arabiatelura Mendes, 1995 (8).4. Urosternite II without vesicles; these are present on abdominal segments IV–VII or V–VII. Styli on abdominal segments IV–IX (6 pairs) …………………………………………………………………………………………… Tribe Grassiellini–Genus Grassiella–Grassiella leuca Stach, 1935.—Urosternite II with vesicles, that are absent on the remaining abdominal segments (although pseudovesicles occur on urosternite VII) ………………………… Tribe Atelurini (5).5. Eight pairs of abdominal styli (present on segments II-IX) ……………………………………………………………………………………… genus Atelura Heyden, 1855 (6).—Three pairs of abdominal styli (present on segments VII-IX) ………………………………………………………………………………… Proatelurina pseudolepisma (Grassi, 1887).6. Pedicellar apophysis of male thumb-shaped and bent, its distal part thinner and forming a more or less right angle with the basal part (Figure 11i). Endemic of eastern Spain … Atelura valenciana Molero-Baltanás, Gaju-Ricart, Bach de Roca & Mendes, 1998.—Pedicellar apophysis of male more or less cylindrical or ovoid, slightly narrowed but not bent in its apical part (Figure 11j) ……………………………………………………… (7).7. In adult males, the pedicellar apophysis is bigger (surpassing the middle of the first flagellomere, sometimes attaining its apex or even the base of the second), the urotergite X has about 30 pegs (or a higher number), and a shallower concavity of its posterior margin. Basal divisions of the cerci with two–three rows of pegs, each division with a higher number of pegs (about 10; Figure 11l) …………………… Atelura montana (Stach, 1946).—In adult males, the pedicellar apophysis is smaller (at most reaching the middle of the first flagellomere), the urotergite X has about 20 pegs (or a lower number), and a deeper concavity of its posterior margin. Basal divisions of the cerci with one–two rows of pegs, each division with a lower number of pegs (about six; Figure 11k) …………………………………………………………………………………… Atelura formicaria Heyden, 1855.8. Pedicel longer than wide. Urotergite X with a deep notch in its hind margin, its ventral surface with a rounded little membranous projection in each side of the posterolateral angles (Figure 11m). Paramera about three times longer than wide ………………………………………………………… Arabiatelura palaestinensis Mendes, 1955.—Pedicel wider than long. Urotergite X with a shallower notch in its hind margin, its ventral surface lacking membranous projections. Paramera about 2.5 times longer than wide …………………………………………… Arabiatelura spinifera (Stach, 1935).9. Sternal plates of thorax not very developed, not overlapping the base of coxae. Macrochaetae plumose (Figure 11n). Abdominal sternites without combs of macrochaetae. Ovipositor with apical fossorial spines (Figure 12a) ………………………………………………………………………………………………… Subfamily Acrotelsatinae–Genus Lepismina Gervais, 1844 (10).—Sternal plates of thorax well developed, overlapping the bases of coxae. Macrochaetae smooth, bifid apically. Abdominal sternites with combs of macrochaetae. Apex of the ovipositor more or less acute, but without apical fossorial spines (Figure 12b) ……………………………………………………………………………………………… Subfamily Lepismatinae (12).10. Scales black on dorsal and ventral surfaces … Lepismina audouinii (Lucas, 1840).—Scales black on dorsal side, those of the ventral side bright, lighter ……………………… (11).11. Urotergite I with submedian macrochaetae ………………………………………………………………………… Lepismina persica Escherich, 1905/L. pluriseta Wygodzinsky, 1942.—Urotergite I without submedian macrochaetae ……………………………………………………………………………………………………………………… Lepismina aurisetosa. Wahlgren, 1906.12. Antennae with asteriform sensilla (Figure 12c). Posterior trichobothrial areas of the pronotum open, in contact with the lateral margin of the notum (Figure 12f) …………………… Genera Neoasterolepisma Mendes, 1988 and Tricholepisma Paclt, 1967(15).—Antenna without asteriform sensilla, with Silvestri’s sensilla (globular basiconic; Figure 12d). Posterior trichobothrial areas of the pronotum closed (surrounded by scales and not in contact with the margin of the notum; Figure 12e) …………………………………………………………………………………………… Genus Lepisma L. 1758 (13).13. Dorsal scales uniformly greyish. Epidermic pigment absent or light yellowish …………………………………………………………………… Lepisma saccharinum L. 1758.—Dorsal scales blackish or greyish, except one row of white scales at the posterior margin of each thoracic notum ………………………………………………………… (14).14. Infralateral group of the urotergites consisting of only one macrochaeta and an additional thin acute smaller seta (Figure 12g) …………………… Lepisma chlorosoma Lucas, 1846.—Infralateral group of the urotergites consisting of two macrochaetae and an additional outer thin acute smaller seta (Figure 12h) ………………………………………………………………………… Lepisma baetica Molero-Baltanás, Gaju-Ricart, Bach de Roca & Mendes, 1994.15. Hind margin of thoracic nota with a row of more or less short bifid macrochaetae (Figure 12i) ……………………………………………… Genus Tricholepisma Paclt, 1967 (16).—Hind margin of thoracic nota without macrochaetae, only with some thin and acute setulae (Figure 12f) or bare ………………………… Genus Neoasterolepisma Mendes, 1988 (18).16. Infralateral groups of urotergites consisting of two macrochaetae, lacking a thinner outer seta (Figure 12j). Urotergite IX only with infralateral groups of macrochaetae, its hind margin lacking additional setae. Males without modified metatibiae. Endemic of south-eastern Spain …………………………………………………………………………………… Tricholepisma indalicum Molero-Baltanás, Bach de Roca & Gaju-Ricart, 1995.—Infralateral groups of urotergites at least with two macrochaetae and a thinner outer seta (Figure 12h). Urotergite IX with at least 1 + 1 macrochaetae in its hind margin additional to the infralateral groups. Males with modified metatibiae …………… (17).17. Transverse row of macrochaetae of the posterior margin of the tergites interrupted in the middle line. Urosternites with combs of numerous setae, some of the more internal of the lateral combs and the more external of the medial combs of segments V–VIII short and spiniform (Figure 4e). Hind leg of the male with modified chaetotaxy on the ventral side of trochanter, femur, and tibiae, with a dense field of straight spines; metatibiae in its apical half with a group of strong and more or less hooked spines (Figure 4a–c) ………………………………………………… Tricholepisma aureum (Dufour, 1831).—Transverse row of macrochaetae of the posterior margin of the tergites continuous. All urosternal macrochaetae thin. Metatibiae ventrally dilated and with several ciliary setae but lacking a group of strong hooked spines; femora and trochanters not modified ……………………………………………………………………… Tricholepisma gyriniforme (Lucas, 1846).18. Urosternite VII with pseudostyli (Figure 4f) ………………………………………………………… (19).—Urosternite VII without pseudostyli (Figure 4g) …………………………………………………………… (24).19. Dorsal side of the body with abundant scales with its apical part ending in an acute point, as prolongation of central rays (Figure 13e) ……………………………………… (20).—Dorsal side without pointed scales ……………………………………………………………………… (21).20. Prosternum about as wide as long, or slightly longer than its width at the base; its lateral margins are slightly or hardly constricted, and its apex is sharp (Figure 13a). Metatibiae of males not ventrally dilated and devoid of ciliary setae, quite similar to that of the female and with a diameter slightly larger in its basal part than in the middle or distal zone (Figure 13b). Parameres very small, straight and slender, almost devoid of glandular area …………………………………………………… Neoasterolepisma soerenseni (Silvestri, 1908).—Prosternum wider at its base than long, usually with a very sharp constriction on its lateral margins; less acutely angled apex (Figure 13c). Metatibiae of adult males convex or slightly ventrally dilated, wider in its middle or distal zone than in its basal part, and usually with some ciliary setae inserted on the dilation (Figure 13d). Parameres more developed of usual size, with curved apex and provided with a distinct glandular area …………………………………………………………………… Neoasterolepisma foreli (Moniez, 1894).21. Metatibiae of adult males with parallel sides, ventrally with a row of fine and robust setae and a row parallel to this of short spiniform setae ending in a small button and lacking ciliary setae (Figure 13f,g). Urotergite X as long as wide at its base or slightly shorter, with a deep and semi-circular posterior notch …………………………………………………………… Neoasterolepisma imitans (Mendes, 1980).—Metatibiae of adult males with convex ventral side, their specialized chaetotaxy different, provided with ciliary setae. Urotergite X longer than wide at its base, its posterior notch wide and shallow ……………………………………………………………………………… (22).22. Metatibiae of adult males with a subtriangular expansion on the distal part of its ventral side (Figure 13h). Robust setae of the tibiae acute, not with curved apex …………… (23).—Metatibiae of adult males ventrally dilated, the expansion is uniformly curved, and the width of the tibia is higher in the middle area (Figure 13i) Robust setae of the male metatibiae with curved apex. ………………………………………… Neoasterolepisma crassipes (Escherich, 1905).23. Urotergite I with 1 + 1 infralateral groups of macrochaetae, 1 + 1 lateral isolated lateral macrochaetae and 1 + 1 submedian macrochaetae. Urotergite IX with 1 + 1 infralateral groups of macrochaetae and 1 + 1 isolated submedian macrochaetae (Figure 14a) …………………………………………… Neoasterolepisma gauthieri (Wygodzinsky, 1941).—Urotergite I with 1 + 1 infralateral groups of macrochaetae and 1 + 1 submedian macrochaetae, lacking lateral macrochaetae. Urotergite IX only with 1 + 1 infralateral groups of macrochaetae, lacking submedian macrochaetae (Figure 14e) ………………………………… Neoasterolepisma calvum Molero-Baltanás, Mendes, Gaju-Ricart & Bach de Roca, 1994.24. Posterior urosternites (usually V–VII or V–VIII) with some of the more external setae of medial comb and some of the more internal of lateral combs, short and more robust and sclerotized, spiniform (Figure 4e) ………………………………………………………… (25).—All urosternites with combs of macrochaetae, if some of them spiniform, not sclerotized and thin (Figure 4g) .………………………………………………………………………… (27).25. Metatibiae of adult males with their ventral side slightly convex, and with a specialized chaetotaxy consisting of abundant fine setae externally and internally and a group of three–four spiniform setae, at the beginning of the ultimate ventral third, accompanied by long, slightly dense cilia. Metafemur of males with a dense field of strong spines (similar to that of Tricholepisma aureum,Figure 4a–c) ……………………………………………………………………………………………… (26).—Metatibiae of adult males not clearly convex, its ventral side more or less parallel to the dorsal side, but with a specialized chaetotaxy completely different, consisting of one–two ventral rows of fine, erect spines and a row of long and strong setae (similar to Figure 13f,g). Metafemur of males not modified, without a dense field of strong spines …………………………………………………………………………… Neoasterolepisma lusitanum (Wygodzinsky, 1945).26. Urotergite I with 1 + 1 infralateral groups of macrochaetae and 1 + 1 submedian macrochaetae, lacking lateral macrochaetae. Apart from infralateral groups, the urotergites II-VIII have only 3 + 3 isolated macrochaetae on their posterior margins (1 + 1 sublateral, 1 + 1 lateral and 1 + 1 submedian, as in Figure 12k ……………………………………………………… Neoasterolepisma balcanicum (Stach, 1922).—Urotergite I with 1 + 1 infralateral groups of macrochaetae, 1 + 1 lateral isolated lateral macrochaetae and 1 + 1 submedian macrochaetae. Apart from infralateral groups, the urotergites II–VIII have a higher number of macrochaetae on their posterior margins, usually 5–6 + 5–6 ……………………………………………………………………………… Neoasterolepisma balearicum Molero-Baltanás, Bach de Roca & Gaju-Ricart, 1997.27. Submedian macrochaetae on urotergites I–VIII substituted by groups of setae; urotergite I–IV with 1 + 1 submedian groups of 12–18 setae, clearly isolated on the more anterior tergites, very close on V. On VI and VII, the median groups are merged, constituting a single band (Figure 14b,c). On VIII, the setae of median groups are very dense forming a sagittal brush ……………………………………………………………………………… Neoasterolepisma evansi (Silvestri, 1923).—Without these characteristics, i.e., chaetotaxy of urotergites typical, usually with 1 + 1 infralateral groups (of one or two setae), 1 + 1 lateral, 1 + 1 sublateral and 1 + 1 submedian macrochaetae (Figure 12k) …………………………………………………………………………………………… (28).28. Labial papillae of type “aufgelöst” (disperse sensilla, such as those of Figure 5j). Median combs of urosternites with only three macrochaetae ………………………………………………………………………………………………………………………… Neoasterolepisma paucisetosa (Stach, 1935).—Labial papillae compact (Figure 14k). Median combs of urosternites with a higher number of macrochaetae ………………………………………… (29).29. Median combs of urosternites with 25–34 setae, the lateral combs with 12–14; the distance between them about 0.5 the width of the latter. Inner process of coxite IX of females about five times longer than wide at its base (Figure 14d) …………………………………………………………… Neoasterolepisma priesneri (Stach, 1946).—Median combs of urosternites in general with much fewer setae, a maximum of 27 and the distance separating them from the lateral combs much greater and these with fewer setae. Inner process of coxite IX of females generally less elongate …………………………………… (30).30. Infralateral group of urotergites with only one isolated macrochaeta or one bifid macrochaeta and an outer tiny, small seta (as in Figure 12g) ………………………… (31).—Infralateral group of urotergites with at least two bifid macrochaetae ……………………… (33).31. Infralateral group of urotergites with only one isolated macrochaeta, without an outer tiny, small seta. Median combs of urosternites III–VII with 12–15 macrochaetae. Open trichobothrial areas long and narrow .………………………………………………………………………………… Neoasterolepisma santschi (Silvestri, 1908).—Infralateral group of urotergites consisting of one macrochaeta and an outer thin acute seta. Median combs of urosternites with four–seven macrochaetae. Open trichobothrial areas shorter and wider ………………………………………………………………………………………… (32).32. Prosternum as long as wide, with a more acute apical region (Figure 14f). Metasternum constrained towards the apical region (Figure 14h). Distance between the subapical combs of the metasternum similar or higher than the width of a comb …………………………………………………… Neoasterolepisma myrmecobium (Silvestri, 1908).—Prosternum wider than long, with a rounded apical region (Figure 14g). Apical part of the metasternum rounded, without a constriction in lateral margins (Figure 14i). Distance between the subapical combs of the metasternum shorter than the width of a comb. Up to now, endemic to Sicily ………………………………………………… Neoasterolepisma angustothoracicum (Grassi & Rovelli, 1889).33. Infralateral group of urotergites II–VIII with two bifid macrochaetae only …… (34).—Infralateral group of urotergites II–VIII with two macrochaetae and an outer tiny acute seta, usually shorter than the bifid macrochaetae ………………………………… (35).34. Median combs of urosternites with four–six macrochaetae. Prosternum slightly wider at its base than long (ratio L/W about 0.9). Hind margin of metanotum not clearly concave. Coxite VIII of females with only one comb consisting of two macrochaetae (Figure 14l). Inner process of the coxite IX of adult females about 2.5 times longer than wide at its base ………………………………………………………… Neoasterolepisma scorpius Mendes, 1993.—Median combs of urosternites with a higher number of macrochaetae (9–27). Prosternum about as wide as long. Hind margin of metanotum clearly concave. Coxite VIII of females with two combs of macrochaetae, the lower one with a higher number of macrochaetae (Figure 14m). Inner process of the coxite IX of adult females about 3.5–4 times longer than wide at its base …………………………………………………… Neoasterolepisma curtiseta Mendes, 1988.35. Distance between median and lateral urosternal combs more than four times the width of a lateral comb; these consisting of a low number of macrochaetae (two–four). Metatibiae of males not modified in respect to those of females ………………………………………………Neoasterolepisma stachi (Wygodzinsky, 1941).—Distance between median and lateral urosternal combs lower, at most four times the width of a lateral comb; these frequently consisting of a higher number of macrochaetae (3–15). Metatibiae of males frequently modified in shape and/or chaetotaxy respect to those of females ……………………………………………………………………………………… (36).36. Metatibiae long in both sexes, more than four times longer than wide (Figure 4d and Figure 14n). Coxites VIII of females with only one comb of setae …………………………………… (37).—Metatibiae shorter, at most four times longer than wide; frequently, their ratio length/width is lower. Coxites VIII of females with one or (frequently) two combs of setae ……………………………………………………………… (38).37. Metatibiae of males modified in chaetotaxy respect to those of females (Figure 14n,o). Apical article of labial palps similar in both sexes. Urotergite I with 1 + 1 submedian macrochaetae …………………………………… Neoasterolepisma spectabiloides Mendes, 1988.—Metatibiae of males not modified in respect to those of females (Figure 4d). Apical article of labial palps of males with a dense glandular field (Figure 14p). Urotergite I without submedian macrochaetae ……………………………………………………… Neoasterolepisma pallidum Molero-Baltanás, Gaju-Ricart & Bach de Roca, 1995.38. Metatibiae of males not modified in respect to those of females ……………………… (39).—Metatibiae of males modified in chaetotaxy and shape respect to those of females ……………………………………………………………………………………………………… (40).39. Lateral urosternal combs with 3–5 macrochaetae; distance between urosternal combs similar to the width of a lateral comb (Figure 15c). Prosternum as long as wide or even a little longer than wide. Males with long and thin cilia in the notch of the hind margin of the tenth urotergite (Figure 14q). Paramera very reduced and glabrous (Figure 15a) ……………………………………………………………… Neoasterolepisma palmonii (Wygodzinsky, 1942).—Lateral urosternal combs with 6–11 macrochaetae; distance between urosternal combs more than twice the width of a lateral comb (Figure 15d). Prosternum wider than long (ratio length/width about 0.8–0.85). Hind margin of the tenth urotergite without setae, only with one macrochaeta and one thin seta in each posterolateral corner. Paramera developed and with several thin setae (Figure 15b) ………………………………………………………………………… Neoasterolepisma necrophilum Mendes, 1992.40. Metatibiae of males with parallel sides and modified chaetotaxy (Figure 15e) consisting of numerous rows of short and robust spiniform setae and with a row of long strong setae, lacking ciliary or hooked setae on its ventral side (Figure 15f). Metasternum with rounded apex, its combs in the antedistal position. Inner process of coxite IX of the female about five times longer than wide at its base, stylus IX not reaching the apex of coxite IX ……………………………………… Neoasterolepisma spectabile (Wygodzinsky, 1945).—Metatibiae of males with its ventral side dilated, convex, with specialized chaetotaxy including ciliary and/or hooked setae on its ventral side. Metasternum usually with the distal region truncate, the combs in a terminal or almost apical position. Inner process of coxites IX of the females proportionally shorter, the respective styli always longer ……………………………………………………………………………………………………………… (41).41. Urotergite X as long or longer than its width at the base in adults. Tibia III of males with a subtriangular dilation and its maximum width usually on the apical half of its length; the contour of the apical half of the ventral margin of this article is slightly concave. A large number of ciliary setae are inserted on the dilation, and an oblique row of strong, straight setae is inserted laterally (Figure 15g). Male urotergites often with supernumerary setae. Ovipositor with more than 25 divisions. Dorsal scales usually yellowish …………………………………………………………… Neoasterolepisma wasmanni (Moniez, 1894).—Urotergite X wider at the base than long. Tibia III of males slightly domed ventrally, its maximum width about the middle of its length. With or without ciliary setae, and with an oblique row of strong setae whose apex may be straight, ciliary, or curved. Urotergites of males with the usual chaetotaxy (i.e., 1 + 1 infralateral groups of two macrochaetae and one thin outer seta, and 3 + 3 isolated macrochaetae). Ovipositor with less than 25 divisions. Dorsal scales frequently greyish, although sometimes they can be yellowish ………………………………………………………………………………………………………………… (42).42. Metatibiae of adult males presenting several ciliary setae with curly apex. The most distal strong setae of the oblique series have a straight or ciliary apex (Figure 15h). Coxites VIII— of females with a single comb of macrochaetae. Prosternum less than 0.8 times as long as its maximum width. Head capsule with more or less intense brownish purple epidermic pigment …………………………………………………………………………………… Neoasterolepisma hespericum Molero-Baltanás, Bach de Roca & Gaju-Ricart, 1996.—Metatibiae of males devoid of ciliary setae, at most with some setae with a very fine and almost straight apex. The macrochaetae of the oblique series have a strong, hooked apex (Figure 15i). Coxite VIII of females presenting two combs, sometimes the upper one with only one or two setae. Prosternum 0.9–1.1 times as long as its maximum width. Epidermic pigment absent or very diffuse …………………………………………………………………… Neoasterolepisma delator Molero-Baltanás, Bach de Roca & Gaju-Ricart, 1996.
3.4. Diversity and Endemicity of Mediterranean Countries
3.5. Diversity Comparation with Other Non-Mediterranean Areas
3.6. State of Art of Publications
4. General Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | ALB | ALG | BOS | BUL | CRO | CYP | EGY | FRA | UK * | GRE | IRAQ | ISR | ITA | JOR | LEB | LIB | MAL | MON | MONT | MOR | MAC | PAL | POR | SAN | SER | SLO | SPA | SYR | TUN | TUR |
| Family Lepismatidae | ||||||||||||||||||||||||||||||
| Lepismina audouinii | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||||
| Lepismina aurisetosa | ⬤ | ⬤ | ||||||||||||||||||||||||||||
| Lepismina persica | ⬤ | |||||||||||||||||||||||||||||
| Lepismina pluriseta * | ⬤ | |||||||||||||||||||||||||||||
| Lepisma chlorosoma | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||
| Lepisma baetica | ⬤ | |||||||||||||||||||||||||||||
| Lepisma saccharinum | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ||||||||||||||||||||||
| Neoasterolepisma angustothoracicum | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma balcanicum | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||
| Neoasterolepisma balearicum | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma calvum | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma crassipes | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ||||||||||||||||||||||
| Neoasterolepisma curtiseta | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||||
| Neoasterolepisma delator | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma evansi | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma foreli | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||||
| Neoasterolepisma gauthieri | ⬤ | ⬤ | ||||||||||||||||||||||||||||
| Neoasterolepisma hespericum | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||||
| Neoasterolepisma imitans | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma lusitanum | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||||
| Neoasterolepisma necrophilum * | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma pallidum | ⬤ | |||||||||||||||||||||||||||||
| Species | ALB | ALG | BOS | BUL | CRO | CYP | EGY | FRA | UK * | GRE | IRAQ | ISR | ITA | JOR | LEB | LIB | MAL | MON | MONT | MOR | MAC | PAL | POR | SAN | SER | SLO | SPA | SYR | TUN | TUR |
| Family Lepismatidae | ||||||||||||||||||||||||||||||
| Neoasterolepisma palmonii * | ⬤ | ⬤ | ||||||||||||||||||||||||||||
| Neoasterolepisma paucisetosum * | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma priesneri | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma santschii * | ⬤ | ⬤ | ||||||||||||||||||||||||||||
| Neoasterolepisma scorpius * | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma soerenseni | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||||
| Neoasterolepisma spectabile | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||||
| Neoasterolepisma spectabiloides | ⬤ | |||||||||||||||||||||||||||||
| Neoasterolepisma stachi | ⬤ | ⬤ | ||||||||||||||||||||||||||||
| Neoasterolepisma wasmanni | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ||||||||||||||||
| Tricholepisma aureum | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||
| Tricholepisma gyriniformis | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||
| Tricholepisma indalicum | ⬤ | |||||||||||||||||||||||||||||
| Family Nicoletiidae | ||||||||||||||||||||||||||||||
| Arabiatelura palaestinensis * | ⬤ | |||||||||||||||||||||||||||||
| Arabiatelura spinifera * | ⬤ | |||||||||||||||||||||||||||||
| Atelura formicaria | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||||||
| Atelura montana | ⬤ | ⬤ | ||||||||||||||||||||||||||||
| Atelura valenciana | ⬤ | |||||||||||||||||||||||||||||
| Coletinia maggi | ⬤ | |||||||||||||||||||||||||||||
| Coletinia mendesi | ⬤ | |||||||||||||||||||||||||||||
| Grasiella leuca * | ⬤ | |||||||||||||||||||||||||||||
| Proatelurina pseudolepisma | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |||||||||||||||||||||
| Total myrmecophilous species | 2 | 11 | 0 | 2 | 2 | 1 | 5 | 6 | 0 | 6 | 1 | 1 | 10 | 1 | 0 | 3 | 2 | 1 | 1 | 11 | 1 | 1 | 11 | 0 | 0 | 0 | 21 | 0 | 4 | 6 |
| Total species | 2 | 11 | 0 | 2 | 2 | 1 | 9 | 6 | 0 | 6 | 1 | 3 | 10 | 1 | 0 | 4 | 2 | 1 | 1 | 12 | 1 | 2 | 11 | 0 | 0 | 0 | 21 | 0 | 5 | 7 |
| Diversity ratio | 5.7 | 31.4 | 0.0 | 5.7 | 5.7 | 2.9 | 14.3 | 17.1 | 0.0 | 17.1 | 2.9 | 2.9 | 28.6 | 2.9 | 0.0 | 8.6 | 5.7 | 2.9 | 2.9 | 31.4 | 2.9 | 2.9 | 31.4 | 0.0 | 0.0 | 0.0 | 60.0 | 0.0 | 11.4 | 17.1 |
References
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G.; et al. Habitat loss and extinction in the hotspots of biodiversity. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Blondel, J.; Aronson, J.; Bodiou, J.-Y.; Boeuf, G. The Mediterranean Region: Biological Diversity in Space and Time; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Cuttelod, A.; García, N.; Malak, D.A.; Temple, H.; Katariy, V. The Mediterranean: A biodiversity hotspot under threat. In The 2008 Review of The IUCN Red List of Threatened Species; Vié, J.-C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Mittermeier, R.A.; Gil, P.R.; Hoffmann, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Ecoregions, 2nd ed.; Cemex: Mexico City, Mexico, 2004. [Google Scholar]
- Tierno de Figueroa, J.M.; López-Rodríguez, M.J.; Fenoglio, S.; Sánchez-Castillo, P.; Fochetti, R. Freshwater biodiversity in the rivers of the Mediterranean Basin. Hydrobiologia 2013, 719, 137–186. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.C.; Wall, D.H. Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. In Soil Microbiology, Ecology and Biochemistry; Paul, E.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 111–149. [Google Scholar]
- Kulma, M.; Molero-Baltanás, R.; Petrtýl, M.; Patoka, J. Invasion of synanthropic silverfish continues: First established populations of Ctenolepisma calvum (Ritter, 1910) revealed in the Czech Republic. BioInvasions Rec. 2022, 11, 110–123. [Google Scholar] [CrossRef]
- Molero-Baltanás, R.; Gaju-Ricart, M.; Fišer, Ž.; de Roca, C.B.; Mendes, L.F. Three new species of European Coletinia Wygodzinsky (Zygentoma, Nicoletiidae), with additional records and an updated identification key. Eur. J. Taxon. 2022, 798, 127–161. [Google Scholar] [CrossRef]
- Mendes, L.F. Les rapports inter-specifiques chez les Thysanoures. I. La myrmécophilie. In Proceedings of the IX International Colloquium on Soil Zoology, Moscow, Russia, 1 August 1987; pp. 686–691. [Google Scholar]
- Mendes, L.F. Biodiversity of the Thysanurans (Microcoryphia and Zygentoma). In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2018; Volume II, pp. 155–198. [Google Scholar]
- Molero-Baltanás, R.; Bach de Roca, C.; Tinaut, A.; Pérez, J.D.; Gaju-Ricart, M. Symbiotic relationships between silverfish (Zygentoma: Lepismatidae, Nicoletiidae) and ants (Hymenoptera: Formicidae) in the Western Palaearctic. A quantitative analysis of data from Spain. Myrmecol. News 2017, 24, 107–122. [Google Scholar]
- Rivas-Martínez, S.; Sáenz, S.; Penas, A. Worldwide Bioclimatic Classification System. Glob. Geobot. 2011, 1, 1—634+4 Maps. [Google Scholar] [CrossRef]
- Claus, R.; Vantieghem, P.; Molero-Baltanás, R.; Parmentier, T. Established populations of the indoor silverfish Lepisma saccharinum (Insecta: Zygentoma) in red wood ant nests. Belg. J. Zool. 2022, 152, 45–53. [Google Scholar] [CrossRef]
- Mendes, L.F. Revisão do género Lepisma Lin., 1758 s.lat. (Zygentoma, Lepismatidae), Boletim da Sociedade Portuguesa de Entomologia; Fundo de Apoio a Comunidade Científica da Junta Nacional de Investigaçao Científica e Tecnológica; Fundaçao Calouste Gulbenkian: Lisboa, Portugal, 1988; Volume 2, pp. 1–236. [Google Scholar]
- Molero-Baltanás, R.; Gaju-Ricart, M.; Bach de Roca, C. On the taxonomic use of the distribution pattern of the antennal asteriform sensilla in Neoasterolepisma and Tricholepisma (Insecta, Zygentoma, Lepismatidae). Pedobiologia 2000, 44, 248–256. [Google Scholar] [CrossRef]
- Escherich, K. Das System der Lepismatiden. Zoologica 1905, 43, 1–164. [Google Scholar]
- Stach, J. Die Lepismatiden-Fauna Ägyptens. Ann. Musei Zool. Pol. Warszawa 1935, 11, 27–111. [Google Scholar]
- Molero-Baltanás, R. Case 3704. Lepisma Linnaeus, 1758 (Insecta, Zygentoma, Lepismatidae): Proposed reversal of Direction 71 (1957) regarding the gender of the name. Bull. Zool. Nomencl. 2016, 73, 7–16. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature. Opinion 2427 (Case 3704)–Lepisma Linnaeus, 1758 (Insecta, Zygentoma, Lepismatidae): Direction 71 (1957) reversed. Bull. Zool. Nomencl. 2018, 75, 290–294. [Google Scholar] [CrossRef]
- Wygodzinsky, P. Second contribution towards the knowledge of Diplura and Thysanura from Palestine. Rev. Bras. De Biol. 1942, 2, 29–46. [Google Scholar]
- Irish, J. Lepismatidae (Thysanura: Insecta) of the Arabian Peninsular. Fauna Saudi Arab. 1991, 12, 225–241. [Google Scholar]
- Paclt, J. Thysanura. Fam. Lepidotrichidae, Maindroniidae, Lepismatidae, Genera Insectorum 218; Anver. Imprimerie et Editions Mercuris: Toronto, ON, USA, 1967; pp. 1–86. [Google Scholar]
- Mendes, L.F. Tisanuros (Microcoryphia E Zygentoma: Insecta) de Portugal. Novos dados e Consideraçóes. Comunicações do Instituto de Investigacao Cientifica Tropical. 2002, 3, 1–47. [Google Scholar]
- Paclt, J. Neue Beiträge zur Kenntnis der Apterygoten-Sammlung des Zoologischen Staatsinstituts und Zoologischen Museums Hamburg. II. Lepismatidae und Maindroniidae. Entomol. Mitt. Aus Dem Zool. Mus. Hambg. 1966, 57, 147–162. [Google Scholar]
- Dufour, L. Description et figures de deux especies nouvelles du genre Lepisma. Ann. Des Sci. Nat. Zool. 1831, 22, 419–421. [Google Scholar]
- Molero-Baltanás, R.; Mendes, L.F.; Gaju-Ricart, M.; Bach de Roca, C. Nova nota sobre os Neoasterolepisma (Zygentoma: Lepismatidae) ibero-norteafricanos. García De Orta Série De Zool. 1994, 20, 149–158. [Google Scholar]
- Molero-Baltanás, R.; Bach de Roca, C.; Gaju-Ricart, M. El género Tricholepisma en España: Descripción de T. indalica n. sp. (Zygentoma: Lepismatidae). In Avances en Entomología Ibérica; Zarazaga, A., Ed.; Asociación Española de Entomología: Madrid, Spain, 1995; pp. 353–364. [Google Scholar]
- Parmentier, T.; Gaju-Ricart, M.; Wenseleers, T.; Molero-Baltanás, R. Chemical and behavioural strategies along the spectrum of host specificity in ant-associated silverfish. BMC Zool. 2022, 7, 23. [Google Scholar] [CrossRef]
- Molero-Baltanás, R.; Bach de Roca, C.; Gaju-Ricart, M. Sobre Neoasterolepisma wasmanni (Moniez, 1894) y la identidad de Lepisma iberica Stach, 1930, con descripción de dos nuevas especies ibéricas de Neoasterolepisma (Apterygota: Zygentoma: Lepismatidae). Graellsia 1996, 52, 37–55. [Google Scholar] [CrossRef]
- Mendes, L.F.; Bach de Roca, C.; Gaju-Ricart, M.; Molero-Baltanás, R. Trichotriuroides boneti gen. et sp. n (Zygentoma, Nicoletiidae) and new data on Zygentoma in the collection of the Museo Nacional de Ciencias Naturales in Madrid (Spain). EOS. Rev. Española Entomol. 1994, 69, 21–29. [Google Scholar]
- Kaplin, V.G. New species of Lepismatidae (Thysanura) from Turkmenia. Entomol. Obozr. 1980, 59, 276–286. (In Russian) [Google Scholar]
- Kaplin, V.G. About bristletails fauna of Russia and problems of its study. In Invertebrate Animals of the Southern Transurals and Adjacent Territories; Kurgan University Press: Kurgan, Russia, 1998; pp. 175–177. (In Russian) [Google Scholar]
- Wygodzinsky, P. Apuntes sobre “Thysanura” americanas (Apterygota, Insecta). Acta Zool. Lilloana 1952, 11, 435–458. [Google Scholar]
- Escherich, K. Beiträge zur Kenntnis der Thysanuren. I Reihe. Zoologischer Anzeiger (XXVI) 1903, 697, 345–366. [Google Scholar]
- Molero-Baltanás, R.; Fanciulli, P.P.; Frati, F.; Carapelli, A.; Gaju-Ricart, M. New data on the Zygentoma (Insecta: Apterygota) from Italy. Pedobiologia 2000, 44, 320–332. [Google Scholar] [CrossRef]
- Kahrarian, M.; Molero-Baltanás, R.; Monshizadeh, M.; Shenavaee, M. A faunistic study on Lepismatidae (Zygentoma) in Kermanshah (Iran). Entomol. Gen. 2014, 35, 53–60. [Google Scholar] [CrossRef]
- Kahrarian, M.; Molero-Baltanás, R. The first report of the family Protrinemuridae and Neoasterolepisma priesneri (Stach, 1946) (Insecta: Zygentoma) for Iran. Turk. J. Zool. 2015, 39, 956–957. [Google Scholar] [CrossRef]
- Silvestri, F. Primo contributo alla conoscenza dei Tisanuri del Peru e descrizione di un genere e due specie dell’Argentina settentrionale. Boll. Del Lab. Di Entomol. E Agrar. Della Fac. Agrar. Portici 1940, 4, 444–458. [Google Scholar]
- Wygodzinsky, P. Beitrag zur Kenntnis der Machilida und Thysanura der Türkei. Opuscula Entomol. 1959, 14, 36–54. [Google Scholar]
- Wygodzinsky, P. Notes et descriptions de Machilida et Thysanura paléarctiques. Rev. Française D’entomologie 1958, 25, 298–315. [Google Scholar]
- Mendes, L.F. Note sur les Zygentoma (Insecta: Apterygota) de L’Europe et du Bassin Mediterraneen. Arq. Mus. Bocage Lisbon 1980, 7, 215–260. [Google Scholar]
- Heyden, C. Nachricht űber eine in Gesellschaft der Ameisen lebende Lepismene. Stettin. Entomol. Ztg. 1855, 16, 368–370. [Google Scholar]
- Meineke, T. Ein weiterer Fund von Atelura formicaria Von Heyden, 1855 in Thüringen (Zygentoma, Nicoletiidae, Atelurinae) und Synopsis der Lebensweise und Verbreitung. Entomol. Nachr. Ber. 2016, 60, 45–51. (In German) [Google Scholar]
- Molero-Baltanás, R.; Gaju-Ricart, M.; Bach de Roca, C.; Mendes, L.F. Description of Atelura valenciana n.sp. (Insecta, Zygentoma) and distribution and myrmecophilic relationships of Proatelurina pseudolepisma in the Iberian peninsula. Miscel-Lània Zoològica 1998, 21, 101–117. [Google Scholar]
- Mendes, L.F. Nova nota sobre os Tisanuros (Apterygota, Microcoryphia e Zygentoma) da Europe e da Bacia Mediterrânica. Bol. Soc. Port. Entomol. 1981, 18, 1–9. [Google Scholar]
- Silvestri, F. Contributo alla conoscenza dei Lepismidae e Machilidae (Thysanura) della Bulgaria. Mitt. Aus Den K. Nat. Inst. Sofia Bulg. 1942, 15, 27–32. [Google Scholar]
- Gilgado, J.D.; Ortuño, V.M. Intra- and inter-population polymorphism in Coletinia maggi (Grassi, 1887) (Zygentoma: Nicoletiidae), an inhabitant of soil, mesovoid shallow substratum (MSS) and caves. A challenge for the strict classification of subterranean fauna? Zootaxa 2015, 3920, 85–100. [Google Scholar] [CrossRef]
- Mendes, L.F. Nota preliminar sobre os Tisanuros (Microcoryphia e Zygentoma) do Algarve (Portugal). Bol. Soc. Port. Entomol. 1985, 1, 239–262. [Google Scholar]
- Mendes, L.F. A review of the Thysanura of Israel with new data on the Microcoryphia and Zygentoma. In Soil Fauna of Israel; Decu, V., Nitzu, E., Por, F.D., Dimentman, C.H., Eds.; Academiei Romane; Israel Academy of Sciences and Humanities: Bucarest, Romania, 1995; pp. 87–110. [Google Scholar]
- Mendes, L.F. Description of the male of Lasiotheus Paclt, 1963, its implication in Atelurinae supra-generic taxonomy and keys for the genera (Insecta: Zygentoma). Zootaxa 2012, 3573, 18–32. [Google Scholar] [CrossRef]
- Glasier, J.R.N.; Poore, A.G.B.; Eldridge, D.J. Do mutualistic associations have broader host ranges than neutral or antagonistic associations? A test using myrmecophiles as model organisms. Insectes Sociaux 2018, 65, 639–648. [Google Scholar] [CrossRef]
- Hölldobler, B.; Kwapich, C.L. The Guests of Ants: How Myrmecophiles Interact with Their Hosts; Harvard University Press: Cambridge, MA, USA, 2022; p. 400. [Google Scholar]
- Witte, V.; Schliessmann, D.; Hashim, R. Fine tuning of social integration by two myrmecophiles of the ponerine army ant, Leptogenys distinguenda. J. Chem. Ecol. 2010, 35, 355–367. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robla, J.; Gaju-Ricart, M.; Molero-Baltanás, R. Assessing the Diversity of Ant-Associated Silverfish (Insecta: Zygentoma) in Mediterranean Countries: The Most Important Hotspot for Lepismatidae in Western Palaearctic. Diversity 2023, 15, 635. https://doi.org/10.3390/d15050635
Robla J, Gaju-Ricart M, Molero-Baltanás R. Assessing the Diversity of Ant-Associated Silverfish (Insecta: Zygentoma) in Mediterranean Countries: The Most Important Hotspot for Lepismatidae in Western Palaearctic. Diversity. 2023; 15(5):635. https://doi.org/10.3390/d15050635
Chicago/Turabian StyleRobla, Jairo, Miquel Gaju-Ricart, and Rafael Molero-Baltanás. 2023. "Assessing the Diversity of Ant-Associated Silverfish (Insecta: Zygentoma) in Mediterranean Countries: The Most Important Hotspot for Lepismatidae in Western Palaearctic" Diversity 15, no. 5: 635. https://doi.org/10.3390/d15050635
APA StyleRobla, J., Gaju-Ricart, M., & Molero-Baltanás, R. (2023). Assessing the Diversity of Ant-Associated Silverfish (Insecta: Zygentoma) in Mediterranean Countries: The Most Important Hotspot for Lepismatidae in Western Palaearctic. Diversity, 15(5), 635. https://doi.org/10.3390/d15050635








