Abstract
Wetlands are of great importance for biodiversity and nature conservation, especially geographically isolated wetlands (GIW). Yet literature about the ecological value of such GIW is missing, especially at the edge of the distribution of endangered species such as amphibians. In 2018 and 2022, we monitored amphibian communities in 15 isolated (GIW) and 12 non-isolated (nGIW) ponds by counting individuals using three methods: (1) capturing with hand nets, (2) visual counting, and (3) capturing with fyke traps. The three methods provided similar results, showing the great importance of GIW for amphibians, especially newts, whose abundance was 5–13 times greater in GIW compared to nGIW. The largest numbers of species and individuals (adults and larvae) were found in isolated wetlands (GIW). In non-isolated water bodies (nGIW) where more than 10 individuals of the Chinese sleeper Perccottus glenii, an alien invasive fish, were found, amphibians were not found at all. Importantly, between 2018 and 2022, the northern crested newt, T. cristatus, dramatically decreased in the nGIW. As a result of our work, it was revealed that the reconstruction of geographically isolated wetlands is very important for the conservation of amphibian biodiversity in a changing climate. One of the most effective measures aimed at protecting amphibians from negative factors—the spread of alien invasive fish species and diseases—is the reconstruction of wetlands in historically exploited landscapes with the creation of a wide range of water bodies yet broadly dominated by geographically isolated ponds.
1. Introduction
The significance of wetlands for biodiversity is very important. Wetlands are highly productive and biologically diverse systems that enhance water quality, control erosion, maintain stream flows, sequester carbon, and provide a home to at least one third of all threatened and endangered species [1]. In addition, wetland biodiversity is important for life to thrive for several reasons: swamp vegetation filters pollutants, improving water quality. Eventually, wetlands will provide livelihoods for one billion people. Their shallow waters, abundance of nutrients, and significant primary productivity make them ideal for organisms that form the base of the food web, upon which many species depend. Animals, such as amphibians, heavily depend on wetlands for all or part of their life cycles, meaning that their survival is directly dependent on the presence and condition of wetlands. Especially for the conservation of populations of native amphibians, wetlands are of the utmost importance. In order to maintain healthy amphibian populations, wetland habitat must be protected.
A watershed contains multiple habitats, all of which are affected by changes in climate, hydrology, land use, and water quality. Since no habitat is isolated from its surroundings, the protection of amphibian species must take place at both the large-scale watershed level and at the smaller scale of individual wetlands. Nowadays, more amphibian species are in need of protection due to their sensitivity to environmental quality; population decline and range reduction are therefore observed globally [2,3]. There are many reasons for this: anthropogenic influence (fragmentation, destruction, and pollution of wetlands), climate change (which, for example, leads to the reduction of amphibian spawning grounds), biological invasions (especially impacts caused by predatory fish and reptiles), diseases (e.g., the devastating fungi species Batrachochytrium dendrobatidis), and other agents that can cause changes in the structure of populations and the appearance of anatomical and physiological anomalies amongst individuals [4,5,6,7,8,9,10].
Finding solutions to counter amphibian declines and extinctions is one of the greatest conservation challenges of the century, which comes with alarming and serious implications for the health of ecosystems globally. Today, there is a large body of literature devoted to the protection of amphibians in wetlands in various countries: the USA, England, Scotland, Wales, Switzerland, Estonia, Ukraine, etc. [11,12,13,14]. The issue of classifying wetlands according to the complexity and structure of hydrosystems as well as geographical isolation has been analyzed in particular detail ([12,15]; https://www.ramsar.org/, accessed on 1 January 2023). The most important wetlands for amphibian protection are inland wetlands, which are indicators of the state of the environment because these species are very sensitive to any changes, and especially to detrimental processes taking place in geographically isolated wetlands [14,15]. The term geographically isolated wetlands (GIW, or isolated ponds) were first used in an historical context as a result of both hydrologic and biotic expressions within an isolation-connectivity continuum [16,17]. Therefore, it is quite interesting to monitor recently reconstructed wetlands, where one can observe succession processes and the appearance and/or disappearance of amphibian populations and analyze the underlying mechanisms of these processes. The origin of isolated wetlands can be very diverse, being associated with both natural activity (e.g., drying up of hydrosystems; European beaver (Castor fiber L., 1758) and anthropogenic activity (e.g., habitat fragmentation, intensive land use, and the creation of artificial reservoirs). Of all the listed transformations of wetlands, the most benign and important for the conservation of amphibians and preserving spawning areas are those due to beaver (C. fiber) activities.
The reconstruction of water bodies in historically exploited areas is a promising way to preserve native fauna and flora. Indeed, historical water bodies such as ponds or lakes have met the water demands of populations for centuries, and a community management system has sustained them for a long period of time together with the biodiversity they have been supporting. At the same time, in the present context of climate change and globalization-driven biological invasions, the question arises: how can we prevent the spread of alien invasive predators and exotic infectious agents in recreated wetlands while at the same time desiring to increase the number of amphibian spawning sites? The answer to this question may be to create and/or restore isolated reservoirs and at the same time prevent or control the entry of predators, which could harm amphibians that have settled and eventually breed there [9]. Surprisingly, the literature lacks information and data on practical results considering the creation or restoration of wetlands and on the impact of the degree of their isolation on the number of amphibians and fish found there [3,12,18,19,20]. Therefore, the purpose of our work is to study the influence of the degree of isolation of wetlands on the native amphibian communities and to assess the degree of risk associated with the appearance of predatory fish, especially invasive ones such as the Chinese sleeper Perccottus glenii Dybowski, 1877.
2. Materials and Methods
2.1. Description of Wetlands
The study site was located in the south of Latvia in the Silene Nature Park (Natura 2000 territory LV0300400; 55.691217 N; 26.771125 E) forest between three large lakes: Richu, Sila, and Sitas. Silene Nature Park is located in the South Eastern part of Latvia, in the Demenes and Skrudalienas parishes of Augsdaugavas county, in the border zone with the Republic of Belarus. The area of the Silene Nature Park is 3825 ha. The territory of the park includes the Nature Reserve “Ilgas” with an area of 157 ha, which was established in 1999 (code LV0526300). Forests occupy the largest part of the park’s territory, or 58.8% of the total area. The territory of the nature park is characterized by a large proportion of wetlands, water bodies, and watercourses, which occupy 26.6% of the territory, the largest of which are Riču and Sila lakes. Swamps occupy 1.7%.
The investigated ponds have been restored or created between 2004 and 2018 in groups in historically exploited wetlands, where they previously existed and were suitable for amphibians (Table 1). Its ecosystem is primarily the result of Eurasian beaver (C. fiber) activity, which has produced an intricate system of shallow (up to 1 m) natural and artificial waterbodies with silt and sand sediments. The following characteristics were used to describe the pond: coordinates, dimensions (L—length, B—width, S—surface, and Dmax—maximum depth), vegetation (E—emergent, SE—submerged, and F—floating), % of area (depending on water level in a particular year or season), and wetland history and restoration (natural wetland destroying history (WD) and the method used for wetland restoration (WR)). The wetlands are overgrown with aquatic and hydrophilic macrophytes, such as Myriophyllum spicatum L., Phragmites australis (Cav.) Trin. ex Steud., Hydrocharis morsusranae L., Potamogeton sp., Carex sp., and Juncus sp.

Table 1.
The study wetlands of Silene Nature Park with dimensions (L—length, B—width, S—surface, and Dmax—maximum depth), vegetation (E—emergent, SE—submerged, and F—floating), % of area (depending on water level in a particular year or season), and wetland history and restoration (natural wetland destroying history (WD), and the method used for wetland restoration (WR)).
In this study, 40 sites with 27 reconstructed or created ponds in historical lowlands and stream valleys were analyzed.
During the reconstruction of the ponds in the floodplain, the main components of the hydraulic systems were identified, which made it possible to classify the ponds according to the degree of isolation from water contacts (“open waters”). The ponds were arranged in series: LV.1–LV.2—geographically isolated wetlands (GIW) and LV.3–LV.4—non-isolated wetlands (nGIW) (Figure 1). Since the reconstruction of isolated wetlands (GIW) and during the yearly monitoring, no contact of wetlands LV.1–LV.2 with other water systems has been identified, and water bodies have been surrounded by higher uplands (“wetlands that are completely surrounded by upland”—as defined by S.G. Leibowitz, T.L. Nadeau and R.W. Tiner, 2003 [16,17]) that are higher than the water level. Conversely, non-isolated wetlands (nGIW) have been noted to be in contact with streams or rivers, especially during spring floods.
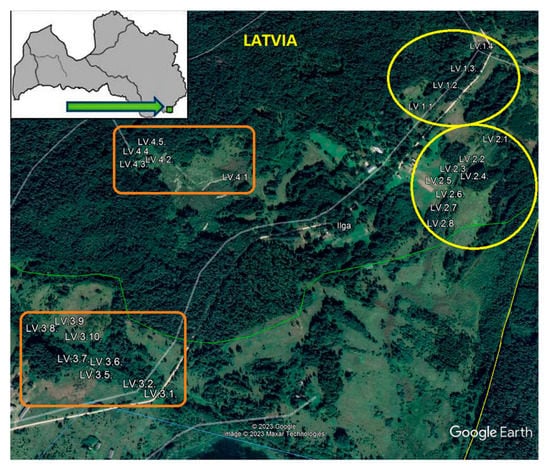
Figure 1.
A map of the location of ponds in the Silene Nature Park, South East of Latvia: yellow ovals—geographically isolated wetlands (GIW) (isolated ponds); orange rectangles—non-isolated ponds (nGIW) (the green arrow indicates the location of the study area).
2.2. Amphibian Recording Methods
A standard method for establishing animal population dynamics models was implemented following the protocol described below [21,22,23,24].
2.2.1. Capturing Newt Larvae Using a Hand Net
The catching of newt larvae was carried out in accordance with the state-approved newt monitoring methodology [22] using a hand net with an opening diameter of 0.5 m (square −0.2 m2). In all ponds, regardless of their size, we blindly netted an area of a standard size (volume 0.2 m2 × 10 m), at the same distance from the shore and depth (0.5 m), and approximately equally overgrown with small-leaved vegetation, which made it possible to compare the density of newt larvae in ponds of different area and depth. The projection square where the newts were caught was 10 m long and 1 m wide (10 m2). To prevent the individual impact of catchers, all nettings were made by the same researcher. Captured newt species and captured fish were noted—the number of individuals per 10 net sweeps per pond (mean/pond).
2.2.2. Visual Counting of Green Water Frogs While Netting Newt Larvae
While netting newt larvae on the same shore site of the netting, the number of green frogs (Pelophylax Fitzinger, 1843) was visually counted [24]. The area of the visual observation was 10 m long and 1 m wide (10 m2).
Capturing newt larvae using a hand net and visual counting green frogs was performed in 10 ponds (5 GIW and 5 nGIW) in 2018, and in 14 ponds (8 GIW and 6 nGIW) in 2022.
2.2.3. Bycatch Capturing Amphibians with Fyke Traps
In part of another monitoring program targeting the European pond turtle Emys orbicularis, fyke traps were deployed in 32 places at 19 reconstructed ponds, where amphibians were captured as bycatch (Figure 2). This trapping protocol consisted of monthly sessions of 5 consecutive days from May to September 2022 on the entire study sites, where traps were set approximately every 100 m apart in every large water body or one per every smaller water body with a length less than 100 m. The traps were provided with an identity plate, a float, and a bait, all moored to a geolocated fixed point. The bait (fresh herring, Clupea harengus) was placed in a small box with holes to prevent the bait from being eaten by captured animals during one trapping session. All traps were checked every day between 11h00 a.m. and 16h00 p.m. and set again. Any amphibian captures were noted before immediate release on site with no further manipulation. The mesh of the trap (1 cm in diameter) prevented the capture of small individuals, so only adult frogs and large tadpoles were caught. The traps were placed horizontally at the water’s surface, so that they were operating only at the near-surface layer (30–40 cm). In total, 24 traps were placed in geographically isolated ponds and 12 traps in non-isolated ponds.

Figure 2.
Fyke traps in the study: (a) prepared and used for catching; (b) deployed in a pond.
Due to differences in the sizes of the surveyed ponds, the numbers of amphibians caught or visually counted in areas of the same area or the number of amphibians per 36 traps per day from each pond were averaged and compared.
The data were processed using the Statistica v. 10 and PAST v. 4 software for Windows. To study the influence of various factors, a standard set of techniques was used. NPMANOVA (Non-Parametric MANOVA, also known as PERMANOVA) was used as a non-parametric test of significant difference between two or more groups, based on Euclidean distances. PERMANOVA assumes no distribution and is rank-based; therefore, this is an appropriate statistical method for analyzing our data. The significance was computed by permutation of group membership with 9999 replicates [25]. Only verified and statistically significant values are presented in the article. Wetlands and associated data series were analyzed separately as isolated and non-isolated wetlands. Separately, the influence of various factors on the differences in the samples, as well as their dependencies on the area of the reservoir, were considered. The test statistic used was a pseudo F-ratio, similar to the F-ratio in ANOVA. It compares the total sum of squared dissimilarities (or ranked dissimilarities) among objects belonging to different groups to that of objects belonging to the same group. It is generally accepted that any separation between groups is not significant if more than ~5% of the permuted F-statistics have values greater than that of the observed statistic (i.e., p-value > 0.05, Supplementary Table S1).
3. Results
Based on the monitoring accomplished in 2018 and 2022, nine species of amphibians were identified: Triturus cristatus (Laurenti, 1768)—TC; Lissotriton vulgaris (Linnaeus, 1758)—LV; Bombina bombina (Linnaeus, 1761)—Bom; Bufo bufo (Linnaeus, 1758)—Buf; Pelobates fuscus (Laurenti, 1768)—PF; Rana temporaria Linnaeus, 1758—RT; and representative species of Pelophylax esculentus complex — Pel: hybrid Pelophylax esculentus (Linnaeus, 1758)—PE; between parent species—Pelophylax lessonae (Camerano, 1882)—PL; and Pelophylax ridibundus (Pallas, 1771)—PR.
The methods outlined in “2.2.1. Capturing newt larvae using a hand net” and “2.2.2. Visual counting green frogs” revealed that there were significantly more amphibians in isolated than in non-isolated water ponds (PERMANOVA, p ˂ 0.05; Table 2, Figure 3). The most numerous species was Pelophylax. In 2018 and 2022, the total number of amphibian individuals captured was 97 and 148, respectively, for 10 and 14 ponds. Between 2018 and 2022, the number of amphibians increased, but only in isolated ponds, whereas it decreased in non-isolated ponds. The northern crested newt, T. cristatus, was the most susceptible, with its numbers decreasing significantly by 1.6-fold in isolated ponds over 4 years (p ˂ 0.05), while it totally disappeared in some non-isolated ponds. Inversely, the number of northern smooth newts, L. vulgaris, increased on average by 5.7-fold over 4 years in isolated ponds, whereas it only slightly decreased in non-isolated ponds (although the newt disappeared from some ponds where fish were found in 2022). The most numerous amphibian species in isolated ponds was the Pelophylax esculentus complex, yet its numbers dropped between 2018 and 2022.

Table 2.
The numbers of individuals and the average number of individuals of newts and frogs Pelophylax identified in Silene Nature Park, South East of Latvia, during the monitoring periods 2018 and 2022 (method 2.2.1, using a net—the number of individuals per 10 net sweeps per pond, and method 2.2.2—the number of individuals of Pelophylax per 10 m2, mean/pond) in isolated wetlands GIW and non-isolated ponds nGIW (N—number).
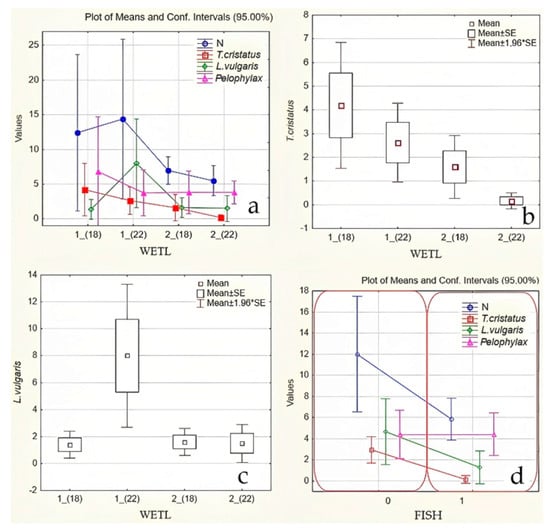
Figure 3.
Dynamics of the abundance of three taxa of amphibians (newts (method 2.2.1) and frogs Pelophylax (method 2.2.2), mean/pond, Table 2) identified in Silene Nature Park, South East of Latvia, during the monitoring periods 2018 and 2022: (a) the average number of individuals of newts and frogs Pelophylax (N); (b) the average number of individuals of T. cristatus; (c) the average number of individuals of L. vulgaris, depending on the isolation of the pond (“wetl”): (1) geographically isolated wetlands (GIW) and (2) non-isolated ponds (nGIW); and (d) the dependence of the average number of individuals of newts and frogs Pelophylax, on the presence of fish (b, “Fish”): 0 = no fish and 1 = presence of fish. Abscises refer to 2018—“(18)” and 2022—“(22)” (Table 2).
Importantly, the number of invasive fish also increased between 2018 and 2022: in 2018, only the Chinese sleeper was captured in one non-isolated pond, whereas in 2022, three additional fish species (Tinca tinca (L., 1758); Esox lucius L., 1758; and Misgurnus fossilis (L., 1758)) were found in six non-isolated ponds (Figure S1).
The method outlined in “2.2.3. Bycatch capturing amphibians with fyke traps” permitted the identification of additional amphibian species, except the small-sized newt, L. vulgaris (Figure 4 and Figure 5). This method confirms the results obtained using the previous hand net method with two additional species, but most importantly, a higher abundance of adults (by 4.5 times, p ˂ 0.05) and larvae (by 19.4 times, p ˂ 0.05) in isolated ponds compared to non-isolated ponds.
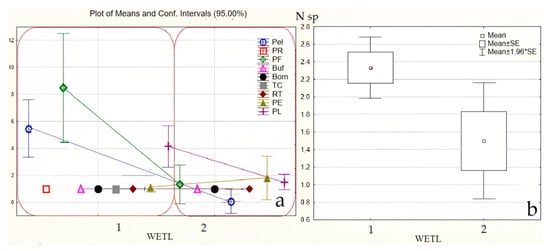
Figure 4.
Number of individuals of different amphibian species counted in Silene Nature Park, South East of Latvia, in 2022 using method 2.2.3 (a, mean/pond, designations in the text) and the average number of amphibian species (“N sp”, mean/pond, b), “wetl”: 1—geographically isolated wetlands (GIW); 2—non-isolated ponds (nGIW) (see mat. and methods).
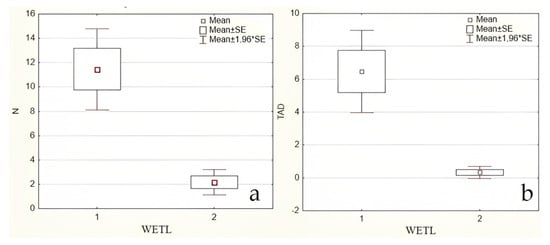
Figure 5.
The number of amphibian individuals counted in Silene Nature Park, South East of Latvia, in 2022 using method 2.2.3 (the number of amphibian (all) - “N”, mean/pond, a), and the number of amphibian larvae (“TAD”, mean/pond, b) caught using traps, depending on wetland isolation (“wetl”): 1—geographically isolated wetlands (GIW); 2—non-isolated ponds (nGIW).
Importantly, in isolated reservoirs where predatory fish were not found, the number of larvae and young individuals of amphibians was observed only in sporadic numbers (Figure S2).
4. Discussion
Analyzing the difference between the ponds, our results show that the size of the water body did not greatly affect either the species diversity or the number of individuals (Figure S3). This is why applying an appropriate and unified methodology for assessing biodiversity and the state of amphibian populations is very important [21,22,23,24]. So, to study and compare the composition of amphibians, sites with similar conditions were selected in all wetlands, regardless of their total surface area or average/maximum depth. It was the surveyed area of the wetland itself that was the same in area or depth everywhere, both in a large lake and in a small pond. Such methodologically very important points make it possible to assess the state of a given wetland, since amphibians as bioindicators can be associated with the state of the environment and, in terms of their population characteristics, reflect the ecological significance of the wetland during monitoring [8,26]. This is especially important at the present time of climate change, where there are fewer and fewer places suitable for amphibians to breed.
This study shows that a combination of different methods provides complementary results for monitoring amphibians. For instance, traps permit the identification of more amphibian and fish species than direct sweeps with a net. Naturally, in addition to amphibians, traps permit the capture of more species, including invertebrates, fish, and reptiles (Natrix natrix (L., 1758) and Emys orbicularis (L., 1758)). For both types of capture (net and trap), we found differences in amphibian biodiversity between geographically isolated and non-isolated wetlands. Namely, in isolated ponds, the number of amphibian species and their specific abundance (adults and larvae) were higher than in non-isolated ponds. In the context of massive, ongoing losses of aquatic habitats and increased impacts caused by aggressive invasive species, such isolated water bodies can provide alternative breeding sites for amphibians. These elements are usually associated with traditional agriculture and livestock in rural areas and thus represent a valuable cultural heritage that is enriched by their added value as ecological refugia for a wide diversity of species. Some amphibian species can successfully exploit such habitats, thus representing important targets for conservation actions.
In our study, the most numerous individuals were adults of P. lessonae and larvae of L. vulgaris and P. fuscus in isolated ponds, where their numbers were 2.7–5.3-fold higher in 2022 than in 2018 (p ˂ 0.05). Moreover, the presence of larvae and young individuals can serve as an indicator of the environmental state, or, in other words, “the health” of wetlands. Representatives of these species also suffer from the activity of predatory fish. As such, the average number of amphibian larvae in isolated ponds was 19.4-fold higher (p ˂ 0.05, Figure 5b) than in non-isolated ponds. Since such a vulnerable and sensitive species as T. cristatus survived only in isolated water bodies at the time of our 4 year study, the protection of newts is obviously of primary conservation importance. This is even more urgent considering that the recent appearance of alien invasive predatory fish such as the Chinese sleeper P. glenii appears to be an important driver of the abundance of amphibians in Latvian wetlands. Consistently, field observations revealed newt larvae to strongly suffer from Chinese sleeper when occurring together in artificial reservoirs.
All these features of wetlands should be taken into account when creating protected areas and ecological networks (Natura 2000, https://www.daba.gov.lv/en [18]), and they must also be included in programs and management plans for the protection of amphibians and their habitats [12,14]. Very often, protected areas include only homogeneous landscapes, while at the same time more species may occur in areas adjacent to the reserve than in the reserve itself. Moreover, as far as amphibians are concerned, it appears very important to ensure that wetlands contain more natural elements, such as greater diversity and stratification of plant elements (from herbaceous shrubs to trees) from wetlands to adjacent terrestrial areas [13]. This is particularly important to consider when reconstructing reservoirs since each species has its own habitat requirements [13]. Additionally, a wide variety of vegetation cover both in the water and in adjacent terrestrial parts of wetlands, especially the presence of ruderal vegetation, is very important for amphibians [13]. The diversity and combination of aquatic and terrestrial environments allow for protection not only from the emergence of new invasive species (animals and plants) by creating isolated ponds but also to ensure the conservation of amphibians in the context of climate change and global drought.
5. Conclusions
This study involving nine species of amphibians revealed that the reconstruction of wetlands is highly relevant for the conservation of European wetlands native biodiversity. In the course of our research, all three representatives of the hybrid Pelophylax esculentus complex were found. Moreover, we show that geographically isolated wetlands reveal the largest specific diversity and abundance compared to non-isolated ponds. In water bodies where more than 10 individuals of alien invasive predatory fish occur, amphibians were not found at all. Newts (T. cristatus) are especially sensitive to water quality and to the presence of predators. Therefore, there is an urgent need to develop plans for the protection of amphibians, especially newts. One of the most effective measures aimed at protecting amphibians from negative factors—the spread of unwanted invasive predatory fish and diseases—is the reconstruction of wetlands in historically exploited landscapes with the creation of a wide range of water bodies yet broadly dominated by geographically isolated ponds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15030461/s1, Figure S1: Dynamics of the abundance of newts in Silene Nature Park, South East of Latvia, during the monitoring periods 2018 and 2022 using method 2.2.1 (average number of individuals per 10 net sweeps per pond, mean/pond): a—T. cristatus, b—L. vulgaris, depending on the presence of alien predatory fish (“Fish”): 1—no fish; 2—presence of fish; Figure S2: Dynamics of the abundance of amphibians in Silene Nature Park, South East of Latvia, in 2022 using method 2.2.3 (mean/pond): a—average number of individuals of nine amphibian species (N—average number of individuals), b—the average number of individuals of each species separately (designations in the text), depending on the presence of alien predatory fish (“Fish”): 0 = no fish and 1 fish (N = 1), 10 fish (N = 10), and 12 fish (N = 12); Figure S3: The dependence of the area (S, m2) of the reservoir on the number of amphibians (N) in Silene Nature Park, South East of Latvia, in 2022 using method 2.2.3: a—Extrapolation coefficient dependence of the number of amphibians on the area of the reservoir (N/S) in wetlands (“wetl”: 1—geographically isolated wetlands (GIW); 2—non-isolated ponds (nGIW)); b—the number of amphibians (N) found depending on the surface area (S) of the water bodies; Table S1. Result of the one-way NPMANOVA statistical method for testing the difference between the data (the average number of individuals of amphibians) of two types of wetlands (geographically isolated wetlands (GIW) and non-isolated wetlands (nGIW)) in Silene Nature Park, South East of Latvia, using methods 2.2.1–2.2.3.
Author Contributions
Conceptualization, O.N., M.P. and J.-Y.G.; data curation, O.N., M.P. and A.S.; formal analysis, O.N. and M.P.; investigation, O.N., M.P., A.G., I.P., A.M., J.-Y.G., K.T., A.S. and A.Č.; methodology, O.N., A.Č. and M.P.; project administration, A.S., O.N., A.Č., J.-Y.G., K.T. and M.P.; resources, O.N., M.P. and A.Č.; software, O.N. and M.P.; supervision, O.N. and M.P.; validation, O.N., V.T., A.Č. and M.P.; visualization, O.N. and M.P.; writing—original draft and writing—review and editing, author: O.N., V.T., M.P., A.Č., J.-Y.G., K.T., A.G., I.P., A.M. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was partly funded by the project Emys-R https://emysr.cnrs.fr (accessed on 1 March 2023) through the 2020–2021 Biodiversa and Water JPI joint call for research proposals, under the BiodivRestore ERA-Net COFUND programme, and with the funding organizations: the Agence Nationale de la Recherche (ANR, France), the Bundesministerium für Bildung und Forschung (BMBF, Germany), the State Education Development Agency (VIAA, Latvia), the National Science Center (NSC, Poland), and the project “Ecological and socioeconomic thresholds as a basis for defining adaptive management triggers in Latvian pond aquaculture” (lzp-2021/1-0247).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Table 1, https://emysr.cnrs.fr (accessed on 1 March 2023).
Acknowledgments
We thank the FLPP project (# 16-00-F02201-000002) for cooperation, projects LIFE-Bombina, LIFE-HerpetoLatvia, and LVAFA for the creation and restoration of the ponds. We thank the project “The mobile complex of Daugavpils University pond aquaculture scientific laboratories” (16-00-F02201-000002) for the possibility of using the mobile complex of scientific laboratories for research purposes.
Conflicts of Interest
The authors declared no conflict of interest.
References
- García-Muñoz, E.; Gilbert, J.D.; Parra, G.; Guerrero, F. Wetlands classification for amphibian conservation in Mediterranean landscapes. Biodivers. Conserv. 2010, 19, 901–911. [Google Scholar] [CrossRef]
- Beebee, T.J.; Griffiths, R.A. The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 2005, 125, 271–285. [Google Scholar] [CrossRef]
- Grant, E.H.C.; Muths, E.; Schmidt, B.R.; Petrovan, S.O. Amphibian conservation in the Anthropocene. Biol. Conserv. 2019, 236, 543–547. [Google Scholar] [CrossRef]
- Pupina, A.; Pupins, M.; Nekrasova, O.; Tytar, V.; Kozynenko, I.; Marushchak, O. Species distribution modelling: Bombina bombina (Linnaeus, 1761) and its important invasive threat Perccottus glenii (Dybowski, 1877) in Latvia under global climate change. J. Environ. Res. Eng. Manag. 2018, 74, 79–86. [Google Scholar] [CrossRef]
- Bosch, J.; Fernández-Beaskoetxea, S.; Garner, T.W.; Carrascal, L.M. Long-term monitoring of an amphibian community after a climate change-and infectious disease-driven species extirpation. Glob. Chang. Biol. 2018, 24, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Tytar, V.; Nekrasova, O.; Pupins, M.; Skute, A.; Marushchak, O.; Čeirāns, A.; Kozynenko, I. Identifying Environmental Refuges (“Coldspots”) from Infection by Batrachochytrium dendrobatidis of Amphibians in Eastern Europe. Biol. Life Sci. Forum 2021, 2, 36. [Google Scholar] [CrossRef]
- Tytar, V.; Nekrasova, O.; Pupins, M.; Čeirāns, A.; Skute, A. Modelling the range expansion of pumpkinseed Lepomis gibbosus across Europe, with a special focus on Ukraine and Latvia. North-West. J. Zool. 2022, 18, 143–150. [Google Scholar]
- Marushchak, O.Y.; Nekrasova, O.D.; Tytar, V.M.; Smirnov, N.A.; Korshunov, O.V.; Pupins, M.; Mykytynets, G.; Skute, A.; Henle, K.; Kaiser, H.A. GIS approach to the study of colour anomalies in amphibians of Ukraine reveals the deleterious effect of human impacts. Herpetol. Notes 2021, 14, 1239–1251. Available online: https://www.biotaxa.org/hn/article/view/62048 (accessed on 1 January 2023).
- Pupins, M.; Nekrasova, O.; Marushchak, O.; Tytar, V.; Theissinger, K.; Čeirāns, A.; Skute, A.; Georges, J.-Y. Potential Threat of an Invasive Fish Species for Two Native Newts Inhabiting Wetlands of Europe Vulnerable to Climate Change. Diversity 2023, 15, 201. [Google Scholar] [CrossRef]
- Nekrasova, O.; Tytar, V.; Pupins, M.; Čeirāns, A. Range expansion of the alien red-eared slider Trachemys scripta (Reptilia, Testudines) in Eastern Europe, with special reference to Latvia and Ukraine. BioInvasions Rec. 2022, 11, 287–295. [Google Scholar] [CrossRef]
- Dubrovsky, Y.V.; Nekrasova, O.D. Vertebrate species richness analysis in pond complexes. In Proceedings of the ZOOCENOSIS–2007: Biodiversity and Role of Animals in Ecosystems. The IV International Conference, Dnipropetrovsk, Ukraine, 9–12 October 2007; Dnipropetrovsk University Press: Dnipropetrovsk, Ukraine; pp. 17–19. (In Russian). [Google Scholar]
- Burrow, A.K.; Lance, S. Restoration of Geographically Isolated Wetlands: An Amphibian-Centric Review of Methods and Effectiveness. Diversity 2022, 14, 879. [Google Scholar] [CrossRef]
- Siffert, O.; Pellet, J.; Ramseier, P.; Tobler, U.; Bergamini, A.; Schmidt, B.R. Where Land and Water Meet: Making Amphibian Breeding Sites Attractive for Amphibians. Diversity 2022, 14, 834. [Google Scholar] [CrossRef]
- Nekrasova, O.D.; Marushchak, O.Y.; Vasyliuk, O.V.; Oskyrko, O.S. Herpetofauna of habitats from Bern Convention Resolution № 4 in Ukraine. In Proceedings of the Conference Proceedings: Classification of Vegetation and Biotopes of Ukraine: The Third Ukrainian Scientific-Theoretical, Kyiv, Ukraine, 19–21 April 2018; Didukh, Y.P., Dubyna, D.V., Eds.; Kholodny Institute of Botany NAS: Kyiv, Ukraine, 2018; pp. 55–63. (In Ukrainian). [Google Scholar]
- Marushchak, O.; Nekrasova, O.; Pupins, M.; Tytar, V.; Ceirans, A. The role and importance of the protected areas’ (Emerald Network) development for amphibians and reptiles on the example of Ukraine in the context of various factors’ influence. J. Environ. Res. Eng. Manag. 2019, 1, 154–158. [Google Scholar] [CrossRef]
- Leibowitz, S.G.; Nadeau, T.L. Isolated wetlands: State-of-the-science and future directions. Wetlands 2003, 23, 663–684. [Google Scholar] [CrossRef]
- Tiner, R.W. Geographically isolated wetlands of the United States. Wetlands 2003, 23, 494–516. [Google Scholar] [CrossRef]
- Nekrasova, O.; Marushchak, O.; Pupins, M.; Tytar, V.; Georges, J.-Y.; Theissinger, K.; Ceirans, A.; Skute, A. Modeling the influence of invasive fish species Perccottus glenii (Dybowski, 1877) on the distribution of newts in Eastern Europe, exemplified by Lissotriton vulgaris (Linnaeus, 1758) and Triturus cristatus (Laurenti, 1768), using a GIS approach. In Proceedings of the 2nd International Electronic Conference on Diversity (IECD 2022)—New Insights into the Biodiversity of Plants, Animals and Microbes, 15–31 March 2022; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Rannap, R.; Lõhmus, A.; Briggs, L. Restoring Ponds for Amphibians: A Success Story. Hydrobiologia 2009, 634, 87–95. [Google Scholar] [CrossRef]
- Catenazzi, A. State of the world's amphibians. Annu. Rev. Environ. Resour. 2015, 40, 91–119. [Google Scholar] [CrossRef]
- Heyer, R.; Donnelly, M.A.; Foster, M.; Mcdiarmid, R. (Eds.) Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Smithsonian Institution: Washington, DC, USA, 2014; p. 320. [Google Scholar]
- Čeirāns, A.; Pupins, M. Amphibian and Reptile Background Monitoring Manual, 2nd ed.; Latgale Ecological Society; Daugavpils University: Daugavpils, Latvia, 2020; 34p. (In Latvian) [Google Scholar]
- Čeirāns, A.; Pupins, M. Amphibian and Reptile Natura 2000 Monitoring Manual, 2nd ed.; Latgale Ecological Society; Daugavpils University: Daugavpils, Latvia, 2020; 29p. (In Latvian) [Google Scholar]
- Pupins, M.; Čeirāns, A.; Nekrasova, O.; Theissinger, K.; Georges, J.-Y. Method of collecting green frogs for scientific and environmental studies by hand net catching. Daugavpils Latg. Ecol. Soc. 2022, 1–11. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Calderon, M.R.; González, S.P.; Pérez-Iglesias, J.M.; Jofré, M.B. Anthropogenic impacts on rivers: Use of multiple indicators to assess environmental quality status. Hydrobiologia 2022, 850, 469–487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).