Abstract
Biogeography patterns of marine tardigrades are poorly studied. Many species of marine tardigrades are considered endemic, but this high number may be an artifact resulting from skewed knowledge about marine tardigrade diversity in different regions of the world. On the other hand, some species of marine tardigrades are considered cosmopolitan. Most of these were described many years ago. Unfortunately, these early descriptions are very incomplete and omit many characteristics with diagnostically relevant value, thus, resulting in many types of these records of these species worldwide. The objective of this study is to report, for the first time, the presence of three species of marine tardigrades in the Atlantic shores of the Iberian Peninsula. These three species were previously described from other regions of the world and the biogeographic consequences of their presence on the study area are discussed. These records provide valuable insights about the biogeography of marine tardigrades in this region.
1. Introduction
Marine tardigrades are considered a frequent but minor taxa of the marine meiobenthos [1]. As for many other poorly studied taxa, our knowledge about the biogeographical patterns of marine tardigrades is very incomplete and sometimes contradictory. Kaczmarek et al. [2] reported that 68% of the marine tardigrade species were endemic, and, thus, showed a limited distribution to one or two contiguous FAO areas. However, this high number of endemic species may be an artifact resulting from skewed knowledge about marine tardigrade diversity in different regions of the world. For example, the Mediterranean fauna of marine tardigrades (mainly Italy) is probably the most extensively studied, and shows many endemic species [3]. However, the limited distribution of many of these endemic species may result from the lack of studies about this group in adjacent areas. For example, studies about marine tardigrades on the Atlantic shores of the Iberian Peninsula reported the presence of two species that previously had been only reported in the Mediterranean Sea [4,5]. Therefore, it can be expected that an increase in studies about marine tardigrades in poorly studied regions will increase the distribution area of many species currently considered endemic.
On the other hand, de Zio Grimaldi et al. [3] remarked on the high number of cosmopolitan species in the Italian fauna of marine tardigrades, while Kaczmarek et al. [2] reported that only 11% of the species of marine tardigrades are cosmopolitan. If one looks in detail at the marine tardigrade species with cosmopolitan distribution reported by de Zio Grimaldi et al. [3], one can see that all of them (Batillipes mirus Richters, 1909, Batillipes pennaki Marcus, 1946, Halechiniscus remanei Schultz, 1955, Orzeliscus belopus Du Bois Reimond Marcus, 1952 and Echiniscoides sigismundi (Schultze 1865) [6,7,8,9,10]) were described in the early days of the study of marine tardigrades. Unfortunately, these pioneer descriptions are very incomplete, and omit many characteristics with diagnostically relevant value. These general descriptions neglecting relevant diagnostic characteristics resulted in many types of these records of these species worldwide. However, a recent study that compared individuals of Bat. pennaki from different parts of the world detected morphological and morphometric differences that supports their separate identity [11]. Similarly, combining morphological and molecular methods, Faurby et al. [12,13] detected species-level differences between individuals of the genus Echiniscoides from different regions of the North Atlantic Ocean. Therefore, these recent studies suggest that a more detailed study of marine tardigrade species that are currently considered cosmopolitan will lead to the discovery of similar but distinct new species and to the denial of the previously presumed cosmopolitism.
The objective of this study is to report, for the first time, the presence of three species of marine tardigrades from the Atlantic shores of the Iberian Peninsula. These three species were previously described from other regions of the world and the biogeographic implications of their presence in the study area are discussed.
2. Materials and Methods
2.1. Studied Area and Sampling
Samples for this study were collected from two different localities (Ría de Ferrol and Ría de Vigo) both of them in the NW of the Iberian Peninsula (Figure 1). In Ría de Vigo, one sample was collected from an intertidal sandy beach at Toralla Island (To) using a core in April 2014. This (42°12′0.53″ N; 8°48′2.02″ W) was collected at low tidal level and was a medium-coarse sediment composed of siliceous sand and shell fragments. In Ría de Ferrol, several samples were collected from shallow subtidal sediments using a Van Veen grab in June 2014 (Fe1) and December 2014 (Fe2 and Fe3). The sample Fe1 (43°27′55.14″ N; 8°18′3.66″ W) was collected at 6 m depth and was a siliceous fine–medium sand. Sample Fe2 (43°26′55.00″ N; 8°18′47.00″ W) was collected at 16 m depth and was a clean biogenic gravel (mainly composed of pieces of mussel shells). Sample Fe3 (43°27′52.65″ N; 8°16′41.16″ W) was collected at 20 m depth and was a biogenic gravel mixed with silt and clay. All samples were preserved in formalin 4% neutralized with borax and stained with Rose of Bengal.
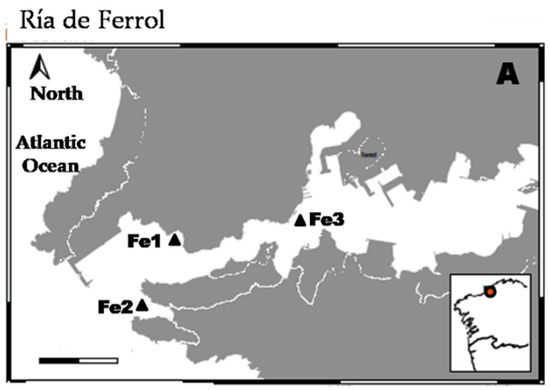
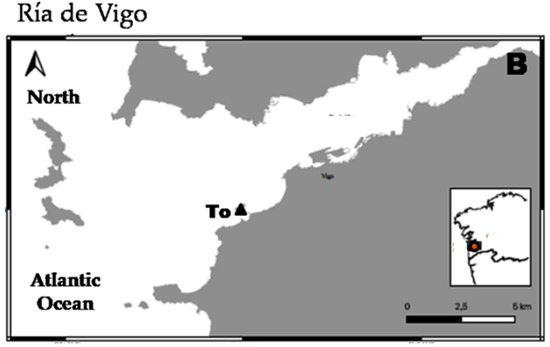
Figure 1.
Map showing sampling localities in Ría de Ferrol, scale bar = 2 km (A) and Ría de Vigo, scale bar = 5 km (B).
2.2. Laboratory Methodology and Systematics
In laboratory, tardigrades were extracted from the sediment by decantation through 0.5 mm and 40 µm mesh sieves. Tardigrades were sorted under a dissecting microscope (Leica S9D) with a magnification of ×30. All the specimens were transferred to microslides with coverslips. Specimens were infused in pure glycerine with some drops of formalin and sealed with nail varnish. Morphological terminology followed [14]. Measurements are given in micrometers (µm) and photomicrographs were made under ×100 oil immersion, using a Zeiss Axioimager-2 differential interference contrast microscope (DIC) equipped with digital cameras and using Zen Imaging Software. Species were identified based on original descriptions and later partial redescriptions. Following Perry et al. [15], three letter abbreviations for the names of genera were used to achieve the unambiguous recommendation of the International Code of Zoological Nomenclature.
3. Results
A total of 119 specimens from three species of tardigrades were found in sediment samples from the four surveyed sites (i.e., To, Fe1, Fe2, and Fe3).
Taxonomic Accounts
- Phylum Tardigrada Doyère, 1840 [16]
- Class Heterotardigrada Marcus, 1927 [17]
- Order Arthrotardigrada Marcus, 1927 [17]
- Family Batillipedidae Ramazzotti, 1962 [18] (emended by Gallo D’Addabbo et al., 2005 [19]
- Genus Batillipes Richters, 1909 [6]
3.1. Batillipes Marcelli Morone De Lucia, D’Addabbo Gallo and Grimaldi de Zio, 1988 [20]
Type locality: Ugento, Ionic Sea (Italy)
3.1.1. Material Examined
Ninety-five specimens were found in locality Fe1, including juveniles and adults. Specimens were deposited in the collection of tardigrades at the Department of Biology, Faculty of Sciences, University of Porto.
3.1.2. Morphological and Ecological Remarks
Specimens (Figure 2A) clearly correspond to the original description by Morone De Lucia et al. [20]. Primary clavae do not show any constriction and secondary clavae are conspicuous (Figure 2B). Toe arrangement pattern on feet of legs IV for the studied specimens (Figure 2C) corresponds to group A (the middle toes 3 and 4 are similar in length; toes 2 and 5 are the longest, and toes 1 and 6 of intermediate size) defined by Santos et al. [5].
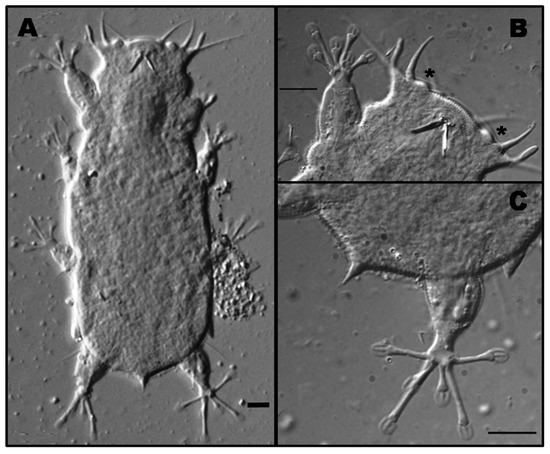
Figure 2.
Habitus of one of the studied animals (A). Detail of the head, asterisks indicate the presence of the secondary clava (B). Detail of the fourth leg of Bat. marcelli showing the toe pattern (C). Scale bars = 10 μm.
The cuticle show a dense fine punctation in the dorsal region of the body, while in the margin of the body punctuation is broader and less dense than in the dorsal region (Figure 3A). Lateral projection between legs III and IV is oriented backwards (Figure 3D) with a fine conical point also visible from dorsal view (Figure 3B). On legs IV, a small papilla is visible on the tibia (Figure 3C).
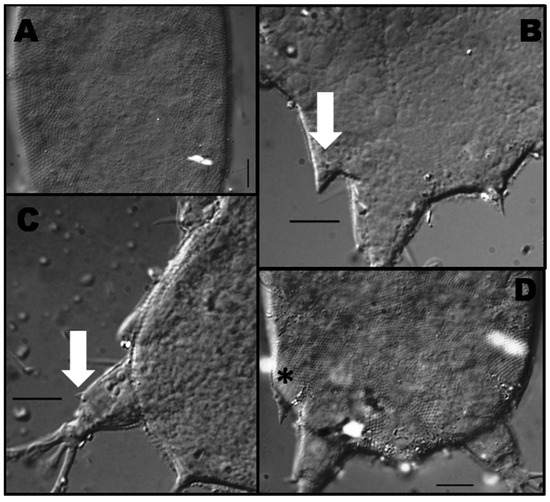
Figure 3.
Detail of the dorsal cuticle of Bat. marcelli between legs two and three (A). Dorsal view of the fourth lateral projection (white arrow) (B). Detail of the papilla on the fourth pair of legs (white arrow) (C). Ventral view of the fourth lateral projection (asterisk) (D). Scale bars = 10 μm.
Sensory spines are present on all legs; on legs IV, sensory spines have a basal portion and a cirrophore separated by a constriction from a van der Land’s organ. In the examined animals, caudal apparatus consists of a small spine (Figure 2A,C), in contrast with animals from the original description, where the caudal spine is longer [20]. In order to explore the variability of the length of the caudal spine, additional measurements were performed on 22 individuals collected in this study. Results of these additional measurements indicate a mean value of 6.9 ± 1.3 μm for the length of caudal spine, with a maximum value of 10 μm and a minimum of 3.8 μm. This is the only distinction between our specimens and the original description [20]. High variability in shape and/or size of the caudal apparatus is well-documented in the genus Batillipes [20]. However, the shape of the caudal apparatus in the studied individuals from Ferrol is identical to that reported in the original description [20]. This contrasts with other species such as Bat. africanus Morone De Lucia, D’Addabbo Gallo and Grimaldi de Zio, 1988 [20], where differences in shape and size in the caudal spine are detected among females, males, and larvae. Original description of Bat. marcelli was based on one adult female and a larva and, thus, minimal morphometric information was provided. In Appendix A, we included morphometric information about some of the individuals of Bat. marcelli collected in this study.
There is not detailed information about the habitat of Bat. marcelli. Individuals from the original description [20] were collected in shallow water (4–8 m depth) and individuals of subsequent records were collected at a maximum of 20 m depth [2]. Therefore, it seems that Bat. marcelli is a shallow-water species and our individuals were recorded within the defined depth range by other authors (i.e., 6 m depth). In previous studies, Bat. marcelli was recorded from sand, coarse sand, and fine sand mixed with mud [2]. In our study, individuals of Bat. marcelli were recorded from fine–medium sand.
3.1.3. Distribution
Until now, this species had only been reported from the Mediterranean Basin (Adriatic Sea, Alboran Sea, Ionian Sea, and Tyrrhenian Sea [2]). This is its first record for the Atlantic Ocean.
- Family: Halechiniscidae Thulin, 1928 [21] (amended by Fujimoto et al., 2017 [22])
- Subfamily: Halechiniscinae Thulin, 1928 [21] (amended by Grimaldi de Zio et al., 1990 [23])
- Genus: Halechiniscus Richters, 1908 [24] (amended by Grimaldi de Zio et al., 1990 [23])
3.2. Halechiniscus Remanei Remanei Schulz, 1955 [8]
Type locality: Baia, Gulf of Naples (Italy)
3.2.1. Material Examined
Four adult specimens were found at locality To. Specimens were deposited in the collection of tardigrades at the Department of Biology, Faculty of Sciences, University of Porto.
3.2.2. Morphological and Ecological Remarks
Specimens (Figure 4) clearly correspond to the original description [8].
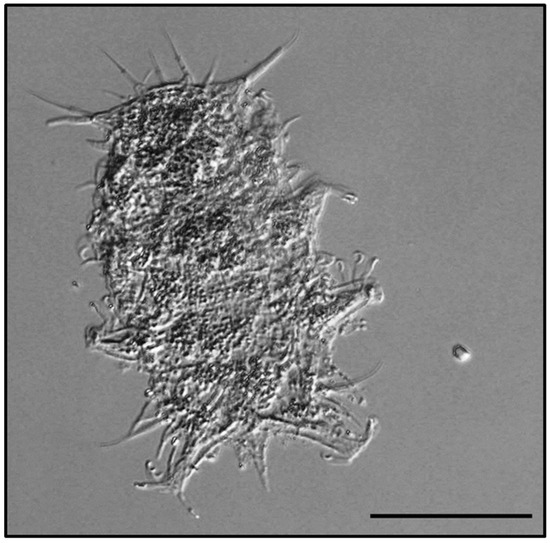
Figure 4.
Habitus of Hal. remanei remanei. Scale bar = 50 μm.
The studied individuals present all the head appendages, paired lateral cirri, primary clavae, external and internal cirri, and unpaired median cirrus (Figure 5A). Small conical auricular projections are present between the head and legs I (Figure 5A). Lateral projections between legs I and II and between legs II and III are also small and conical (Figure 5A). Lateral body projections between legs III and IV are also conical but bigger (Figure 5B). Sensory spines are present on all legs (Figure 5A) but, on legs IV, sensory spines are divided into two more or less equal parts (cirrophore and a papilla) with a van der Land’s body and ending in a thin tubular point (Figure 5B). Caudal apparatus consists of a strong conical spine (Figure 5B). These characteristics match the original description [8].
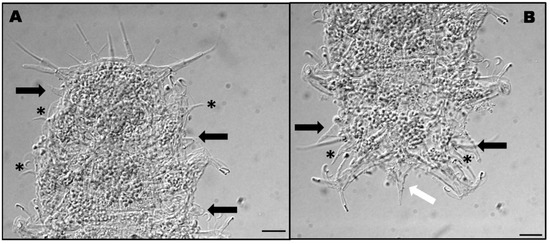
Figure 5.
Detail of the anterior part of the body of Hal. remanei remanei. Black arrows show body lateral projections. Asterisks indicate the position of sensory spines on legs I and II (A). Detail of the posterior part of the body of Hal. remanei remanei. Black arrows show the fourth body lateral projections. Asterisks indicate the position of sensory spines on legs IV. The white arrow shows the caudal apparatus (B). Scale bars = 10 μm.
The original description of Hal. remanei remanei provides little morphometric information, but measurements obtained from our individuals are within the range included in the original description (Appendix B).
Halechiniscus remanei remanei has been collected mainly from intertidal samples, as in this study, or from shallow water (less than 10 m depth), suggesting that it is basically an intertidal/shallow-water species [2]. However, there are a few records at more than 100 m depth [2]. Moreover, Hal. remanei remanei has been collected from many different sediment types but sand with high calcareous content, as in this study, is a frequent habitat for it.
3.2.3. Distribution
Halechiniscus remanei remanei has been reported from several latitudes and oceans such as the Mediterranean Basin (Adriatic Sea, Alboran Sea, Balearic Sea, Ionian Sea, Mediterranean Sea, Thyrrenian Sea); Atlantic Ocean; Caribbean Sea; Celtic Sea; North Sea; Pacific Ocean; Coral Sea; East China Sea; and the Indian Ocean, but mainly in the northern hemisphere, [2,25,26]. However, as suggested by Kaczmarek et al. [2], many of these records need thorough revision. Based on the literature, and summarized by Renaud-Mornant [27], individuals of Hal. remanei remanei from Australia have a small roundish anterior protuberance on the fourth lateral projection of the body and the distal part of the PIV is more lance-shaped instead of the spine form mentioned in the original description [8]. Individuals from the Indian Ocean seem to be bigger than those from the original description [28]. Finally, individuals from California reported as Hal. remanei remanei [29] are clearly different from the original description on PIV, which is terminated in a spade-shaped structure, and in having longer claws. However, specimens recorded in the Pacific coast of Costa Rica match the original description of Hal. remanei remanei [25].
- Family: Tanarctinae Renaud-Mornant, 1980 [30] (Family erected and amended by Fujimoto et al., 2017 [22])
- Genus: Actinarctus Schulz, 1935 [31]
3.3. Actinarctus Doryphorus Doryphorus Schulz, 1935 [31]
Type locality: Helgoland Island, North Sea (Germany)
3.3.1. Material Examined
Four specimens were found at locality Fe2 and sixteen in locality Fe3. All the individuals found were adults. Specimens were deposited in the collection of tardigrades at the Department of Biology, Faculty of Sciences, University of Porto.
3.3.2. Morphological and Ecological Remarks
Specimens (Figure 6A) clearly correspond to the original description by Schulz [31] and subsequent redescription by D’Addabbo Gallo et al. [32]. This species presents a very characteristic lateral membrane all around the body supported by very long pillars (Figure 6A). Actinarctus doryphorus doryphorus lacks secondary clavae but has a well-developed buccal clavae (Figure 6B). All the claws have a strong calcar and the two central claws have an accessory point (Figure 6C). All of these qualitative traits match previous descriptions. Morphometric information about some individuals of Act. doryphorus doryphorus collected in this study is included in Appendix C. Measurements of our individuals are similar to those by D’Addabbo Gallo et al. [32]. However, body size and some head appendages show slight differences.
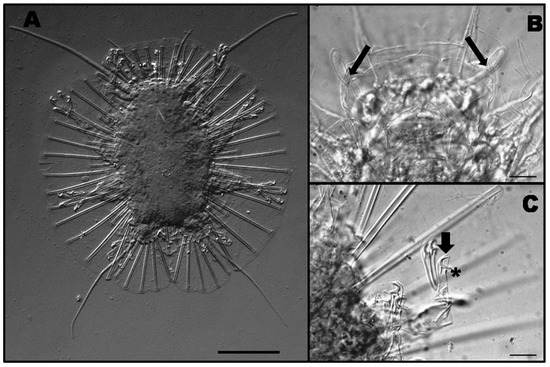
Figure 6.
Habitus of Act. doryphorus doryphorus. Scale bar = 50 μm (A). Detail of the buccal clavae (black arrows). Scale bar = 10 μm (B). Detail of the claw showing the calcar (asterisk) and the accessory point (black arrow). Scale bar = 10 μm (C).
Actinarctus doryphorus doryphorus was found from the intertidal to shallow water depth (about 25 m) [2]. Individuals collected in this study were within the previous depth range (i.e., 16 and 20 m depth). Our individuals were collected from biogenic coarse sand, in agreement with the majority of the previous records of this species [6].
3.3.3. Distribution
This species has been reported from several localities of the Mediterranean Basin (Adriatic Sea, Alboran Sea, Balearic Sea, Ionian Sea, and Ligurian Sea) and the Atlantic Ocean (North Sea and Celtic Sea) [2]. The record on the Atlantic shores of the Iberian Peninsula was expected, since the Iberian Peninsula is located between both areas where the species has been previously reported and proves the wide distribution of this species from the North Sea to the Mediterranean Sea.
4. Discussion
In this study, we report the presence of three species of marine tardigrades (Bat. marcelli, Hal. remanei remanei, and Act. doryphorus doryphorus) not previously known from the Iberian Peninsula. The presence of Bat. marcelli in Ria de Ferrol represents the first record of this species outside the Mediterranean Sea. Recent studies on the Atlantic shores of the Iberian Peninsula [4,5] also reported the presence of Bat. adriaticus Grimaldi de Zio, Morone De Lucia, D’Addabbo Gallo and Grimaldi, 1979 [33] and Styraconyx sardiniae D’Addabbo Gallo, Morone De Lucia and Grimaldi de Zio, 1989 [34] outside the Mediterranean Sea for the first time. These records suggest that the Atlantic shore of the Iberian Peninsula is the northern biogeographical boundary of these three Mediterranean species as they have not been found further north to date [35]. Additionally, two north boreal species, Styraconyx haploceros Thulin, 1942 [36] and Batillipes tubernatis Pollock (1971) [37], have also been recently reported on the Atlantic shores of the Iberian Peninsula [4,38], which suggests that it is, for now, their new southern biogeographical boundary. Therefore, these results suggest that the Atlantic shore of the Iberian Peninsula is an zone of overlap for boreal and Mediterranean marine tardigrades.
Halechiniscus remanei remanei is considered a cosmopolitan species, but this study reports its presence at the Iberian Peninsula for the first time. Morphological and morphometric study of the collected individuals fits perfectly with the available data from the original description. Despite the high number of records of Hal. remanei remanei worldwide, very few studies provide morphometric data or illustrate the collected individuals. The analysis of the few studies that provide some illustrations and/or morphometric information of records of Hal. remanei remanei from some distant regions (e.g., Australia, Indian Ocean, or California) suggest that the observed individuals are likely new species [27,29]. Similarly, Santos et al. [11] found that individuals previously identified as Bat. pennaki from Atlantic shores of America, Europe, and the Mediterranean Sea were in fact three different species with significant morphological and morphometric differences. Therefore, a detailed revision of the individuals previously identified as Hal. remanei remanei from different parts of the world is needed to clarify its real distribution.
Finally, the original description of Act. doryphorus doryphorus is based on material collected from the North Sea, but it also has been reported for many localities along the Mediterranean Sea and redescribed based on material from this region [20]. The record of Act. doryphorus doryphorus from the Atlantic shores of the Iberian Peninsula suggests the continuous distribution of this species from the North Sea and Celtic Sea to the Mediterranean.
5. Conclusions
Despite recent efforts, e.g., [4,5,11,38], knowledge about marine tardigrades in the Iberian Peninsula is still very scarce. These first records of three species along the Atlantic shores of the Iberian Peninsula suggest that this region may be of significant biogeographical importance for marine tardigrades. Future studies are needed to improve the knowledge about marine tardigrades in the Iberian Peninsula and, thus, obtain a better view of the biogeography of this group of invertebrates.
Author Contributions
Conceptualization, M.R., P.F. and P.V.; methodology, M.R., P.F. and P.V.; formal analysis, M.R., P.F. and P.V.; investigation, M.R., P.F. and P.V.; resources, M.R. and P.F.; writing—original draft preparation, M.R.; writing—review and editing, M.R., P.F. and P.V.; supervision, M.R. and P.F.; funding acquisition, M.R., P.F. and P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the FCT-Foundation for Science and Technology through national funds within the scope of UIDB/04423/2020, UIDP/04423/2020, and through the strategic project UID/MAR/04292/2019 granted to MARE. P.V. was hired through the Regulamento do Emprego Científico e Tecnológico—RJEC from the Portuguese Foundation for Science and Technology (FCT) program (CEECIND/03893/2018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to staff of the ‘Estación de Bioloxía Mariña de A Graña’ (University of Santiago de Compostela, Spain) for sampling assistance in Ría de Ferrol and to four anonymous referees for all the helpful comments and suggestions, which greatly improved this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A. Measurements in μm of Different Structures in the Studied Specimens of Batillipes marcelli
| Specimen | Bm1 Female | Bm2 Female | Bm3 Indet. | Bm4 Indet. |
| Body length | 123.7 | 144.0 | 92 | 177 |
| Body width | 52.3 | 56.7 | 34.5 | 78 |
| Buccal tube | 15.1 | 19 | ||
| Median cirrus | 11.3 | 11.5 | ||
| Internal cirri | 16.5 | 17.4 | 14.1 | 17.4 |
| External cirri | 13.5 | 15.7 | 10.6 | 17.5 |
| Lateral cirri | 21.8 | 21.7 | 18.8 | 23.6 |
| Primary clavae | 8.5 | 9.7 | 7.4 | 11.7 |
| Cirri E | 15.7 | 21.6 | 17.5 | 17.3 |
| Leg IV base | 5.9 | 6 | 4.7 | 5.8 |
| Leg IV spine | 7.8 | 5.3 | 4.4 | 7.1 |
| Leg III spine | 8.0 | 10.6 | ||
| Leg II spine | 8.9 | 10.7 | ||
| Leg I spine | 7.7 | 8.7 | 7.8 | |
| Projection 1 | 3.1 | 4.9 | 3.5 | |
| Projection 2 | 2.9 | 2.7 | ||
| Projection3 | 4.4 | 3.9 | 3.1 | 3.8 |
| Projection4 | 4.5 | 3.6 | 6.1 | 7.1 |
| Caudal appendage (L) | 6.6 | 6.5 | 3.8 | 5.6 |
| Leg I (toe 1) | 8.6 | 5.4 | 7.0 | |
| (toe 2) | 5.7 | 3.8 | 4.8 | |
| (toe 3) | 12.8 | 9.5 | 13.1 | |
| (toe 4) | 9.1 | 5 | 7.9 | |
| (toe 5) | 9.4 | 10 | 15.0 | |
| (toe 6) | 14.5 | 5.8 | 8.9 | |
| Leg IV (toe 1) | 12.2 | 11.7 | 9.4 | 12.6 |
| (toe 2) | 16.7 | 15.0 | 13.7 | 18.2 |
| (toe 3) | 8.2 | 7.1 | 7.6 | 11.3 |
| (toe 4) | 9.3 | 8.1 | 7.4 | 11.6 |
| (toe 5) | 16.7 | 17.4 | 13.7 | 21.0 |
| (toe 6) | 11.2 | 10 | 6.8 | 12.6 |
Appendix B. Measurements in μm of Different Structures in the Studied Specimens of Halechiniscus remanei remanei
| Specimen | Hr1 Male | Hr2 Indet. | Hr3 Indet. | Hr4 Indet. |
| Body length | 102.0 | 103.0 | 114.0 | 113.4 |
| Median cirrus | 21.9 | 13.7 | 15.7 | 16.4 |
| Internal cirri | 26.6 | 22.4 | 24.5 | 25.8 |
| External cirri | 22.5 | 21.7 | 15.2 | 16.3 |
| Lateral cirri | 29.2 | 27.2 | 30.3 | 32.0 |
| Primary clavae | 13.2 | 14.5 | 14.1 | 14.8 |
| Cirri E | 26.6 | 29.5 | 23.4 | |
| Leg IV base | 9.1 | 7.6 | 9.0 | |
| Leg IV spine | 4.6 | 5.3 | 6.1 | |
| Leg III spine | 10.0 | |||
| Leg II spine | 9.7 | 10.4 | ||
| Leg I spine | 6.7 | 9.4 | 9.8 | |
| Projection 1 | 5.4 | 5.1 | 5.9 | 9 |
| Projection 2 | 6.6 | 6.3 | ||
| Projection3 | 7.8 | 13.3 | 9.1 | |
| Projection4 | 9.9 | 14.4 | 13.1 | 15.9 |
| Caudal appendage (L) | 13.8 | 16.9 | 18.3 | |
| Claw | 3.6 | 3.9 | ||
| Digit and claw | 11.8 | 11.3 | 11.8 |
Appendix C. Measurements in μm of Different Structures in the Studied Specimens of Actinarctus doryphorus doryphorus
| Specimen | Ad1 Female | Ad2 Male | Ad3 Male | Ad4 Male |
| Body length | 119.1 | 76.1 | 98.8 | 114.7 |
| Body width | 58.7 | 38.1 | 52.1 | 71.9 |
| Median cirrus | 35.0 | |||
| Internal cirri | 82.8 | 58.9 | 67.2 | 78.4 |
| External cirri | 55.0 | 43.2 | 48.8 | 51.6 |
| Lateral cirri | 16.3 | 29.1 | 41.8 | 43.2 |
| Primary clavae | 12.1 | 92.2 | 150.1 | 170.5 |
| Buccal clavae | 24.8 | 24.9 | ||
| Cirri E | 48.4 | |||
| Leg IV | 96.3 | 95.7 | 1.5.5 | |
| Leg III spine | ||||
| Leg II spine | ||||
| Leg I spine | 11.2 | |||
| Frontal ala | 35.4 | 22.2 | 34.3 | 38.9 |
| Lateral ala | 71.3 | 49.7 | 62.2 | 64.8 |
| Caudal ala | 47.4 | 32.7 | 39.1 | 43.5 |
| Leg I | ||||
| External digit | 14.1 | 10.4 | 13.2 | 16.1 |
| Internal digit | 18.4 | 14.5 | 17.2 | 20.4 |
| Leg IV | ||||
| External digit | 18.5 | 12.4 | 17.3 | 16.0 |
| Internal digit | 22.7 | 16.4 | 22.1 | 22.3 |
References
- Nelson, D.R.; Bartels, P.J.; Guil, N. Tardigrade Ecology. In Water Bears: The Biology of Tardigrades; Schill, R.O., Ed.; Zoological Monographs 2; Springer Nature Switzerland AG: Cham, Switzerland, 2018. [Google Scholar]
- Kaczmarek, Ł.; Bartels, P.J.; Roszkowska, M.; Nelson, D.R. The Zoogeography of Marine Tardigrada. Zootaxa 2015, 4037, 1–189. [Google Scholar] [CrossRef]
- de Zio Grimaldi, S.; Gallo D’Addabbo, M.; Sandulli, R.; D’Addabbo, R. Checklist of the Italian marine Tardigrada. Meiofauna Mar. 2003, 12, 97–135. [Google Scholar]
- Rubal, M.; Veiga, P.; Fontoura, P.; Santos, E.; Sousa-Pinto, I. Biodiversity of marine tardigrades from the northern coast of Portugal (Iberian Peninsula). Zool. J. Linn. Soc. 2016, 178, 747–754. [Google Scholar] [CrossRef]
- Santos, E.; Rubal, M.; Veiga, P.; da Rocha, C.M.C.; Fontoura, P. Batillipes (Tardigrada, Arthrotardigrada) from the Portuguese coast with the description of two new species and a new dichotomous key for all species. Eur. J. Taxon. 2018, 425, 1–32. [Google Scholar] [CrossRef]
- Richters, F. Marine Tardigraden. Verh. Der Dtsch. Zool. Ges. 1909, 19, 84–95. [Google Scholar]
- Marcus, E. Batillipes pennaki, a new marine tardigrade from the North and South American Atlantic coast. Comun. Zoológicas Del Mus. Hist. Nat. Montev. 1946, 2, 1–3. [Google Scholar]
- Schulz, E. Studien an marinen tardigraden. Kiel. Meeresforsch. 1955, 11, 74–79. [Google Scholar]
- Du Bois-Reymond Marcus, E. On South American Malacopoda. Bol. Da Fac. De Filos. Ciências E Let. Da Univ. De São Paulo Ser. Zool. 1952, 17, 189–209. [Google Scholar]
- Schultze, M. 1865. Echiniscus sigismundi, ein Arctiscoide der Nordsee. Arch. Mikr. Anat. 1865, 1, 428–436. [Google Scholar] [CrossRef]
- Santos, E.; Veiga, P.; Rubal, M.; Bartels, P.J.; Da Rocha, C.M.C.; Fontoura, P. Batillipes pennaki Marcus, 1946 (Arthrotardigrada: Batillipedidae): Deciphering a species complex. Zootaxa 2019, 4648, 549–567. [Google Scholar] [CrossRef]
- Faurby, S.; Jørgensen, A.; Kristensen, R.M.; Funch, P. Phylogeography of North Atlantic intertidal tardigrades: Refugia, cryptic speciation and the history of the Mid-Atlantic Islands. J. Biogeogr. 2011, 38, 1613–1624. [Google Scholar] [CrossRef]
- Faurby, S.; Jørgensen, A.; Kristensen, R.M.; Funch, P. Distribution and speciation in marine intertidal tardigrades: Testing the roles of climatic and geographical isolation. J. Biogeogr. 2012, 39, 1596–1607. [Google Scholar] [CrossRef]
- Fontoura, P.; Bartels, P.J.; Jørgensen, A.; Kristensen, R.M.; Hansen, J.G. A dichotomous key to the genera of the Marine Heterotardigrades (Tardigrada). Zootaxa 2017, 4294, 1–45. [Google Scholar] [CrossRef]
- Perry, E.; Miller, W.R.; Kaczmarek, L. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 2019, 4608, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Doyère, L.M. Mémoire sur les Tardigrades. I. Ann. Sci. Nat. Série 2 1840, 14, 269–361. [Google Scholar]
- Marcus, E. Zur Anatomie und Ökologie mariner Tardigraden. Zool. Jahrbücher. Abt. Für Syst. Ökologie Und Geogr. Der Tiere 1927, 53, 487–588. [Google Scholar]
- Ramazzotti, G. Il Phylum Tardigrada. Mem. Dell’istituto Ital. Idrobiol. 1962, 14, 1–595. [Google Scholar]
- Gallo D’Addabbo, M.; Sandulli, R.; Grimaldi de Zio, S. New Batillipedidae (Tardigrada, Heterotardigrada) from the Orosei Gulf, Sardinia, Tyrrhenian Sea. Zool. Anz. 2005, 243, 219–225. [Google Scholar] [CrossRef]
- Morone De Lucia, R.M.; D’Addabo Gallo, M.; Grimaldi de Zio, S. Descrizione di due nuove specie di Batillipedidae (Tardigrada: Heterotardigrada). Cah. Biol. Mar. 1988, 29, 361–373. [Google Scholar]
- Thulin, G. Über die Phylogenie und das System der Tardigraden. Hered. Genet. Arkiv 1928, 11, 207–266. [Google Scholar] [CrossRef]
- Fujimoto, S.; Jørgensen, A.; Hansen, J.G. A molecular approach to Arthrotardigrade phylogeny (Heterotardigrada, Tardigrada). Zool. Scr. 2017, 46, 496–505. [Google Scholar] [CrossRef]
- Grimaldi de Zio, S.; D’Addabbo Gallo, M.; Morone De Lucia, R.M. Revision of the genus Halechiniscus (Halechiniscidae, Arthrotardigrada). Cah. Biol. Mar. 1990, 31, 271–279. [Google Scholar]
- Richters, F. Marine Tardigraden. Zool. Anz. 1908, 33, 77–85. [Google Scholar]
- Bartels, P.J.; Fontoura, P.; Nelson, D.R.; Orozco-Cubero, S.; Mioduchowska, M.; Gawlak, M.; Kaczmarek, Ł.; Cortés, J. A trans-isthmus survey of marine tardigrades from Costa Rica (Central America) with descriptions of seven new species. Mar. Biol. Res. 2021, 17, 120–166. [Google Scholar] [CrossRef]
- Miller, W.R.; Perry, E.S. The coastal marine Tardigrada of the Americas. Zootaxa 2016, 4126, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Mornant, J. Nouveaux Arthrotardigrades des Antilles. Bull. Muséum Natl. d’Histoire Nat. Série 4e 1984, 6, 975–988. [Google Scholar]
- Grimaldi de Zio, S.; D’Addabbo Gallo, M.; Morone De Lucia, M.R.; D’Addabbo, L. Marine Arthrotardigrada and Echiniscoidea (Tardigrada, Heterotardigrada) from the Indian Ocean. Boll. Zool. 1987, 4, 347–357. [Google Scholar] [CrossRef]
- McGinty, M. Batillipes gilmartini, a new marine tardigrade from a California beach. Pac. Sci. 1969, 23, 394–396. [Google Scholar]
- Renaud-Mornant, J. Description de trois espécies nouvelles du genre Tanarctus Renaud-Debyser, 1959, et création de la sous-famille des Tanarctinae subfam. nov. (Tardigrada, Heterotardigrada). Bull. Muséum Natl. d’Histoire Nat. Sér 4e 1980, 2, 129–141. [Google Scholar]
- Schulz, E. Actinarctus doryphorus, nov. gen. nov. spec. ein merkwürdiger Tardigrad aus der Nordsee. Zool. Anz. 1935, 111, 285–288. [Google Scholar]
- D’Addabbo Gallo, M.; Pietanza, R.; D’Addabbo, R.; Morone De Lucia, M.R.; Grimaldi de Zio, S. A redescription of Actinarctus doryphorus (Tardigrada, Heterotardigrada). Cah. Biol. Mar. 1999, 40, 21–27. [Google Scholar]
- Grimaldi de Zio, S.; Morone De Lucia, M.R.; D’Addabbo Gallo, M.; Grimaldi, P. Osservazioni su alcuni tardigradi di una spiaggia pugliese e descrizione di Batillipes adriaticus sp. nov. (Heterotardigrada). Thalass. Salentina 1979, 9, 39–50. [Google Scholar]
- D’Addabbo Gallo, M.; Morone De Lucia, M.R.; Grimaldi de Zio, S. Two new species of the genus Styraconyx (Tardigrada: Heterotardigrada). Cah. Biol. Mar. 1989, 30, 17–33. [Google Scholar]
- Bartels, P.J.; Kaczmarek, Ł.; Roszkowska, M.; Nelson, D.R. Interactive Map of Marine Tardigrades of the World. 2015. Available online: https://paul-bartels.shinyapps.io/marine-tardigrades/ (accessed on 19 January 2023).
- Thulin, G. Ein neuer mariner Tardigrad. Medd. Fran Göteborgs Musei Zool. Avd. 1942, 99, 1–10. [Google Scholar]
- Pollock, L.W. On some British marine Tardigrada, including two new species of Batillipes. J. Mar. Biol. Assoc. UK. 1971, 51, 93–103. [Google Scholar] [CrossRef]
- Santos, E.; Rubal, M.; Veiga, P.; Bartels, P.J.; da Rocha, C.M.C.; Fontoura, P. On the distribution of Batillipes tubernatis Pollock, 1971 (Arthrotardigrada: Batillipedidae) in the Atlantic Basin. Mar. Biodivers. 2019, 49, 621–631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).