Abstract
The lime leaf miner Phyllonorycter issikii (Kumata) (Lepidoptera: Gracillariidae) is an east Asian pest, which has recently distributed across the Palaearctic. Here, we provide the novel data on the diversity of its parasitoids in the Russian Far East (native region) and western Siberia (invaded region). Overall, 19 parasitoids from the Eulophidae (17 species) and Braconidae (2 species) reared from the Ph. issikii larvae and pupae were identified based on morphology and/or DNA barcoding. Among them, 12 species were detected in the Primorskiy Territory (Russian Far East) and 10 species in the Novosibirsk Province (Western Siberia), with only 3 shared species, namely Chrysocharis laomedon (Walker), Elachertus inunctus Nees and Sympiesis gordius (Walker). Pleurotroppopsis japonica (Kamijo) is a novel record for Russia, whereas the other eight eulophids are novel for the Novosibisk Province and two for the Primorskiy Territory. The eulophid Mischotetrastichus nadezhdae (Kostjukov) was recorded as a parasitoid of Ph. issikii for the first time. Four new species were described from the Primorskiy Territory: Achrysocharoides nagasawi sp. nov., A. carinatus sp. nov., Cirrospilus ussuriensis sp. nov., Pholetesor nataliae sp. nov. For all parasitoids, the distribution and hosts are listed; the majority of the species are illustrated. In addition, a checklist of the Ph. issikii parasitoids counting 79 species is compiled for the Palaearctic region.
1. Introduction
The lime leaf miner Phyllonorycter issikii (Kumata, 1963) is a micromoth from the family Gracillariidae (Lepidoptera) originating from east Asia (Russian Far East, China, Korea, Japan) and presently known as an invasive pest of limes (Tilia spp., Malvaceae) in the western Palaearctic [1,2,3,4]. Over the last few decades, the species has spread across the European part of Russia and penetrated into many European countries. Furthermore, it invaded western Siberia, even though the lime has a significantly disjunct natural range there [4,5,6]. In addition to the fact that Ph. issikii is known mainly as a leaf mining pest, causing notable damage to limes in urban areas in the invaded regions, it is also able to attack trees in the wild, affecting the health of the local lime groves [7]. A recent study of herbarium specimens collected in the south of the Russian Far East over the last 2.5 centuries revealed numerous mines on leaves of aboriginal limes (Tilia amurensis Rupr., T. mandshurica Rupr. and Maxim.), suggesting population density increases in Ph. issikii in this region at the beginning of the 20th century [4]. Prior to our study, no data were known on the pest outbreaks in its native range.
Hymenopteran parasitoids are important natural enemies of Gracillariidae moths. The moths of this family share many parasitoid species, including those developing on the leaf miners from other families and orders (Coleoptera, Hymenoptera, Diptera) [8]. As a result, parasitoid complexes can respond to the abundance of their hosts and develop new trophic associations, shifting to newly invaded leaf mining species [9].
In the invaded range, the parasitoids of the lime leaf miner have been studied intensively in the European part of Russia and partially in some European countries (Bulgaria, Croatia, Hungary, Slovakia, Ukraine), where, overall, 73 species have been recorded up to recently [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Some data on parasitoids attacking larvae and pupae of the moth in its native range are known only from Japan, where 16 species were documented [9,31,32,33,34,35]. Hardly any information on parasitoids of Ph. issikii is available from Asian Russia, except for our early record of a few parasitic wasps reared from larvae of the invasive pest in Novosibirsk [5,6] and opportunistic records from Tyumen [23]. At the same time, Asian Russia represents a highly important territory for studying Ph. issikii parasitoids, bearing in mind that the moth is native to the Russian Far East but invasive in western Siberia.
Here, we explored the diversity of parasitoids developing in the larvae and pupae of Ph. issikii in Siberia and the Russian Far East. As a result, a list of 19 parasitoids from Asian Russia is provided, and four new parasitoid species from the Russian Far East are described. For all species, distributional and trophic data are given; some species are illustrated. In addition, a checklist of parasitoids attacking Ph. issikii is compiled.
2. Materials and Methods
2.1. Sampling Leaf Mines
The study was carried out in June–July 2020–2021 in two regions of Asian Russia: Primorskiy Territory (the Russian Far East), where Phyllonorycter issikii is a native species [36], and Novosibirsk Region (western Siberia), where it is known as an alien pest since 2008 [5,6]. In both regions, the mined leaves were sampled in botanical gardens and the adjacent territory; in the Russian Far East, sampling was conducted in the Mountain-Taiga (Gornotayozhnaya) Station (MTS) of the Far Eastern Branch of the Russian Academy of Sciences (Gornotaezhnoe, Ussuriysk District) and in western Siberia—in the Central Siberian Botanical Garden (CSBS) of the Siberian Branch of the Russian Academy of Sciences (Novosibirsk) (Figure 1 and Figure 2). The following lime species were surveyed in the study: Tilia amurensis, T. mandshurica, T. taquetii C. K. Schneider (MTS), T. platyphyllos Scop. and T. cordata Mill. (CSBG). The survey was timed to the development of the first generation of Ph. issikii with either late instar larvae or pupae present in the leaf mines.
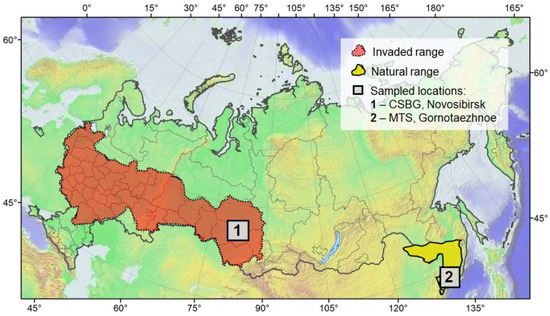
Figure 1.
The studied localities in the Russian Far East and in western Siberia. MTS—the botanical garden of the Mountain-Taiga Station, Gornotaezhnoe, Ussuriysk District, Primorskiy Territory; CSBG—the Central Siberian Botanical Garden, Novosibirsk.

Figure 2.
The studied biotopes in Siberia (A,B) and the Russian Far East (C,D). The grove of Tilia cordata (A) with the foci of Phyllonorycter issikii and numerous mines on the leaves (a,B) in the forest park zone adjacent to the Central Siberian Botanical Garden (Novosibirsk). Tilia amurensis in the undergrowth of a mixed forest predominated by Mongolian oak Quercus mongolica (C), with rare Ph. issikii mines (c,D) around the Mountain-Taiga Station (Gornotaezhnoe, Ussuriysk District, Primorskiy Territory).
In CSBG and the neighboring forest park area where the moth had an outbreaking density (>2 mines per leaf) in the studied years (Figure 2), the leaves with mines were collected randomly from 30 trees from low branches on the perimeter of a tree crown. In MTS and the surrounding forest where the moth experienced low density (<10 mines per 100 leaves) (Figure 2), all leaves carrying mines were sampled to obtain representative material for the rearing parasitoids. Mostly young limes (from 0.5 to 4 m height) were checked for the presence of leaf mines, as in older trees, the low branches were inaccessible. In total, around 400 young trees were surveyed in MTS and the surrounding area.
2.2. Rearing Parasitoids
In our study, all parasitoids were reared from the larvae and pupae of Ph. issikii. To achieve that, the mines were cut from the leaves and placed in Petri dishes (90 mm in diameter) lined with filter paper, 25–30 mines per dish. To maintain humidity, a segment of a cotton pad was fixed on the lid and moisturized every third day. In total, about 900 mined leaves and 1800 individual mines were utilized in the research. The mines were maintained in 60 dishes, which were kept under constant conditions (temperature 22–25 °C, humidity 65%, photoperiod 18:6 h) and checked every second day to sample the emerged parasitoids. The date of parasitoid emergence (par. em.) was documented. Parasitoid adults were preserved in 95% ethanol and stored in the freezer at −20 °C prior to morphological and molecular genetic studies.
2.3. DNA Barcoding
The parasitoid specimens were DNA barcoded in order to genetically characterize the species, define the nearest neighbors, assess the intra- and interspecific divergence and highlight problematic taxonomic cases. The representative specimens of parasitoids collected in a series from the studied regions were transferred into a genetic plate with 96 wells (Eppendorf, Sample submission kit, BOLD System, CCDB, Guelph, ON, Canada) filled with 0.1 mm of 95% ethanol. To avoid cross-contamination during the transfer of insects to the wells, the forceps were cleaned with 95% ethanol, and their tips were treated over a flame after each manipulation.
Overall, 64 specimens of parasitoids of 14 morphologically identified species were DNA barcoded (Supplementary Material Table S1). The remaining 5 species grown from the Ph. issikii larvae and pupae were not subjected to DNA barcoding in our study. The non-destructive protocol of DNA extraction was applied to save the specimens for morphological study. The mitochondrial cytochrome oxidase I gene (mtDNA COI, 658 bp) was sequenced in the specimens using the primer set C_LepFolF/C_LepFolR, following the standard high-throughput protocol [37]. DNA barcoding was carried out at the Canadian Center for DNA barcoding (CCDB) at the University of Guelph (Canada). The specimen data are given in Table S1. The sequences, trace files, biogeographic data and photographs of the vouchers were deposited in the Barcode of Life Data Systems (BOLD) [38] and the National Centre for Biotechnology Information (NCBI). All data are publicly accessible in BOLD using the link dx.doi.org/10.5883/DS-ISSIKPAR.
For phylogenetic analysis, ten sequences of nine parasitoid species showing close genetic and/or morphological relatedness to Ph. issikii parasitoids from Asian Russia were used in the analysis: seven from Europe (from England to Belarus), i.e., Achrysocharoides cilla (Walker, 1839) (1 sequence), Chrysocharis laomedon (Walker, 1839) (1), Colastes braconius Haliday, 1833 (1), Euplectrus sp. (1), Pholetesor circumscriptus (Nees, 1834) (2), Pnigalio pectinicornis (Linnaeus, 1758) (1), Pnigalio soemius (Walker, 1839) (1), and two from Asia (Japan and China, respectively), i.e., Sympiesis gordius (Walker, 1839) (1) and Pediobius cassidae Erdös, 1958 (1) (Table S1).
Barcode index numbers (BINs) were retrieved for each species in BOLD [39]. The sequences were aligned in BioEdit 7.2.5 [40]. A maximum likelihood (ML) tree was built in MEGA X [41] using the maximum likelihood method, the Kimura two-parameter model and a bootstrap method (1000 iterations). Intra- and interspecific genetic distances were estimated using the same approaches.
The DNA barcodes of leaf mining flies Aulagromyza populi (Kaltenbach, 1864) from Siberia (process ID ISSIK113-14) and Phytomyza sp. from the Russian Far East (ISSIK125-14) grown from the leaf mines on Populus balsamifera Linnaeus and Malus sp., obtained earlier by the last author (Table S1), were used to root the phylogenetic tree.
2.4. Morphology
The adults of parasitoids were identified based on their morphological characteristics using the keys for Eulophidae [12,31,32,34,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] and Braconidae [79,80,81].
Different resources were followed for the terminology of morphological features of Eulophidae [43,49,65,71,82] and Braconidae [83,84]. The abbreviations F1–F3 are used for funicular segments, C1–C3 for claval segments, OOL for the distance between the posterior ocellus and the eye, POL for the distance between the posterior ocelli, MV for the length of the marginal vein, ST for the length of the stigmal vein and PM for the length of the postmarginal vein.
All specimens (including types) are deposited in the Zoological Institute of the Russian Academy of Sciences (Saint Petersburg, Russia; ZISP).
2.5. Imaging
The photographs of biotopes and leaf mines were taken with a smart phone digital camera Xiaomi 11 Lite (Beijing, China). The parasitoid specimens were examined using an Olympus SZ51 stereomicroscope. Photographs of parasitoid adults were taken with a Canon EOS 70D digital camera mounted on an Olympus SZX10 microscope (Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia). Image stacking was performed using Helicon Focus 5.0 (Kharkiv, Ukraine; https://helicon-focus.software.informer.com/5.0/, accessed on 1 August 2022). The figures were corrected using the Adobe Photoshop CS6 program.
3. Results
3.1. Molecular Data
Overall, 64 specimens were identified to 14 species using DNA barcoding (Figure 3). Among them, there were 12 species of Eulophidae, Chrysocharis laomedon, Pleurotroppopsis japonica, Pediobius cassidae, Achrysocharoides cilla, Mischotetrastichus nadezhdae, Cirrospilus ussuriensis sp. n., Pnigalio soemius, Pnigalio pectinicornis, Sympiesis gordius, S. laevifrons, Elachertus fenestratus and Elachertus inunctus, and 2 species of Braconidae, Colastes braconius and Pholetesor nataliae sp. n. They formed distinguishable clusters where the sequences of the same species publicly available in BOLD were added; furthermore, all identified species were assigned to unique BINs in BOLD (Figure 3). As an exception, the specimens identified in the study as Sympiesis gordius and S. laevifrons entered one cluster on the COI tree under one BIN name (BOLD:AAE2642) with minimal pairwise distances of 0.17%, whereas the maximal intraspecific distances reached 2.03% in S. laevifrons and 2.46 in S. gordius (Table 1).
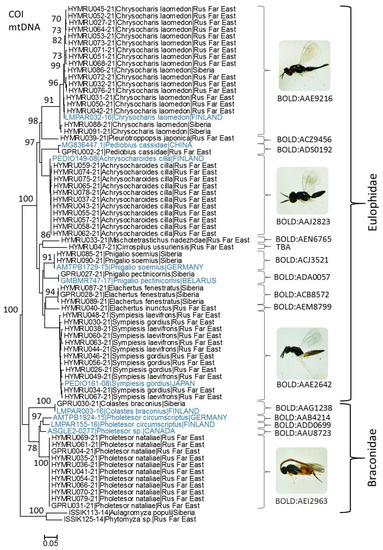
Figure 3.
Maximum likelihood tree showing the relatedness of hymenopteran parasitoids grown from larvae and pupae of Phyllonorycter issikii in Asian Russia in 2020–2021, with closest neighbors in BOLD (borrowed sequences are indicated in blue). Each specimen is indicated by the BOLD process ID (begins with HYMRU or GPRU), followed by species name, country or macroregion (Siberia, Rus Far East = Russian Far East). Bootstrap values > 70 are indicated next to the corresponding branches. BIN numbers are given next to each cluster. The images of adults are provided for most frequently recorded species.

Table 1.
Intra- and interspecific divergences in COI mtDNA gene among Eulophinae (Eulophidae) parasitoid species of Phyllonorycter issikii. Minimal pairwise distances are given for each species pair; values in square brackets represent maximal intraspecific distances; [―] no data because a single specimen was sequenced.
In other related species of Eulophinae used in the study, in particular Elachertus spp., Pnigalio spp., interspecific divergences varied from 9.99% (between Pnigalio soemius and P. pectinicornis) to 10.50% between Elachertus inunctus and E. fenestratus (Table 1). Within this subfamily, the Asian species Mischotetrastichus nadezhdae (process ID: HYMRU033-21) and Cirrospilus ussuriensis sp. n. (process ID: HYMRU047-21) were DNA barcoded for the first time.
In the sequenced species from the subfamily Entedoninae, a notable intraspecific divergence (93.97%) was detected in Chrysocharis laomedon (Table 2). All 18 specimens of Ch. laomedon sequenced from the Russian Far East (15 sequences) and Siberia (3) and the sequence of this species from Finland (process ID: LMPAR032-16) were assigned in BOLD to one BIN (BOLD:AAE9216).

Table 2.
Intra- and interspecific divergences in COI mtDNA gene among Entedoninae (Eulophidae) parasitoid species of Phyllonorycter issikii. Minimal pairwise distances are given for each species pair; values in square brackets represent maximal intraspecific distances; [―] no data because a single specimen was sequenced.
In Braconidae, only two species were sequenced in the study: Pholetesor nataliae sp. n. and Colastes braconius from the Russian Far East and western Siberia, respectively (Figure 3, Table 3). In GenBank, the nearest neighbor of Ph. nataliae appeared to be Pholetesor sp. from Canada (process ID: ASGLE2-0277); the minimal pairwise distance between these two species reached 6.98% (Table 3).

Table 3.
Intra- and interspecific divergences in COI mtDNA gene among Braconidae parasitoid species of Phyllonorycter issikii, including three related species of Pholetesor from BOLD (indicated by *) for comparison. Minimal pairwise distances are given for each species pair; values in square brackets represent maximal intraspecific distances; [―] no data because a single specimen was sequenced.
In fact, Ph. nataliae is morphologically highly similar to Ph. circumscriptus; however, genetically, these species are significantly divergent. The minimal genetic distance between Ph. nataliae and Ph. circumscriptus from Germany (process ID: AMTPB1924-15) was 7.95%, and between Ph. nataliae and Ph. circumscriptus from Finland (process ID: LMPAR155-16), it reached 8.08% (Table 3). Notably, all Pholetesor spp. involved in the analysis were assigned to different BINs in BOLD (Figure 3). The maximal intraspecific divergence within Ph. nataliae did not exceed 0.33%, as based on the analysis of 11 sequences (Table 3).
3.2. List of Parasitoids of Phyllonorycter issikii from the Asian Part of Russia
Order Hymenoptera Linnaeus, 1758
Superfamily Chalcidoidea Latreille, 1817
Family Eulophidae Westwood, 1829
Subfamily Eulophinae Westwood, 1829
Cirrospilus Westwood, 1832
The specimen of the genus Cirrospilus was identified using the keys from different sources [12,43,47,54,55,64,76].
Cirrospilus ussuriensis Kosheleva, sp. nov.
Figure 4A–D,F
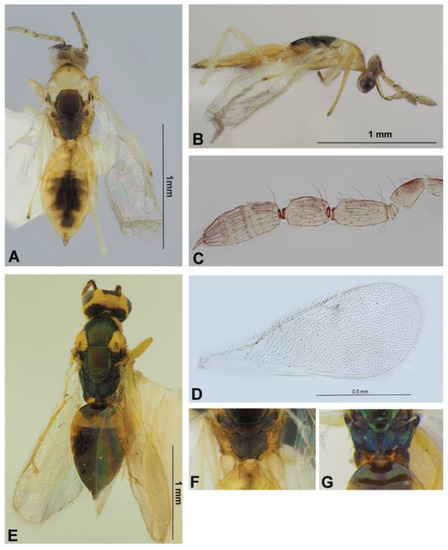
Figure 4.
Eulophinae (females): Cirrospilus ussuriensis sp. nov. (A–D,F) and C. diallus Walker, 1838 (E,G). Habitus, dorsal view (A,E) and lateral view (B), flagellum (C), fore wing (D), dorsellum and propodeum (F,G).
Type material. Holotype: female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from the Phyllonorycter issikii mine on Tilia amurensis, 07.VII.2021 (leaf mine coll.), N. Kirichenko coll., “MTS-21-6-12, DNA barcoded: sample ID NK866, process ID HYMRU047-21”.
Description. Female. Body length 1.6 mm. Body mostly pale yellow with the following parts brownish with or without greenish metallic reflection: mid lobe of mesoscutum pale yellowish, lateral lobes of mesoscutum and axilla pale yellow to whitish, scutellum, dorsellum and ocellar triangle brownish with greenish metallic reflection, pronotum medially with brown-green spot, lateral panel of metanotum, median part of propodeum, gaster medially and ovipositor sheaths brown. Antenna light brown to pale yellowish with upper scape and pedicel brown. Legs pale yellow to white, with apices of tarsi darker. Wings subhyaline with venation whitish (Figure 4A).
Head collapsed, vertex distorted; in this state in dorsal view about 2.4 times as broad as long, POL about 2 times OOL. Malar space about 0.6 times height of eye. Antenna inserted near the lower level of eyes; scape reaching top of vertex, 4.0 times as long as broad; pedicellus plus flagellum about 1.5 times as breadth of head; F1 1.4 times as long as pedicellus and about twice as long as broad; F2 thicker than F1 and 1.4 times as long as broad; clava about as long as F1 plus F2, and 2.7 times as long as broad. Funicle with sensilla disposed in two irregular rows, claval segments with sensilla disposed in one row (Figure 4C).
Mesosoma 1.75 times as long as broad; mid lobe of mesoscutum with two pairs of whitish bristles, with engraved reticulations. Scutellum quadrate, reticulate as in mid lobe of mesoscutum, with sublateral grooved lines, with two pairs of bristles, distant between anterior and posterior bristles about 0.6 times distances between sublateral grooved lines. Dorsellum reticulation as in scutellum, rounded posteriorly, 0.7 times as long as median length of propodeum (Figure 4A). Propodeum with distinct, strongly raised plicae and distinct median carina; area between plicae smoothly rugulose; callus with 12 setae (Figure 4F). Legs slender with whitish setae; spur of hind tibia as long as breadth of basitarsus. Fore wing 2.4 times as long as broad; costal cell with row of setae on lower surface; speculum small, closed below; ST 0.2 times as long as MV, 1.2 times as long as PM (Figure 4D).
Metasoma 1.2 times as long as mesosoma, twice as long as broad (Figure 4A,B). Ovipositor sheaths projecting slightly behind top of metasoma in dorsal view.
Male. Unknown.
Comparative diagnosis. Cirrospilus ussuriensis sp. nov. is characterized by the pale yellowish mid lobe of mesoscutum with whitish lateral lobes and axillae, and the presence of strong propodeal plicae. It resembles C. diallus Walker, 1838 (Comparative material: 1♀, Vladimir Province, Vladimir, reared from Lithocolleis spinicolella Zl., 19.VII.1930 Skorikova coll.; Veromann det. 21.I.1987) in having distinct plicae on the propodeum (Figure 4G), as well as areoles of the sculpture of mesoscutum and scutellum formed by engraved lines (Figure 4E), but it differs from the latter in the following characteristics: mid lobe of mesoscutum pale yellowish (in C. diallus, nearly always metallic, but according to Bouček [47], at most, its narrow side parts, ranging with parapsidal furrows, or a cross-band anteriorly, may also be partly yellow in the south European form); axilla whitish (in C. diallus, usually green), scutellum quadrate (in C. diallus, elongated), propodeum with area between the plicae smoothly rugulose and about 1.6 times as long as broad (in C. diallus, punctuate-reticulate and about twice as long as broad); hind coxae pale yellow (in C. diallus, dark in the basal half.). In addition, the presence of strong plicae on the propodeum is characteristic in C. kumatai Kamijo, 1992 and C. dispersus Zhu, LaSalle and Huang, 2002, but both these species, according to descriptions of Kamijo [54] and Zhu et al. [76], have many scattered setae on the mid lobe of mesoscutum (in C. diallus and C. ussuriensis sp. nov., only two pairs of bristles on the mid lobe of mesoscutum). Cirrospilus ussuriensis sp. nov and C. diallus have distinct morphological differences.
Host. Phyllonorycter issikii on Tilia amurensis.
Distribution. Russia (Primorskiy Territory).
Etymology. The species’ name is a genitive noun referring to the territory in which the holotype was collected, the area of the Ussuri River.
Genus Elachertus Spinola, 1811
The specimens of the genus Elachertus were identified using the keys from different sources [12,49,55,64,74,75,77].
Elachertus fenestratus Nees, 1834
Figure 5A,B.
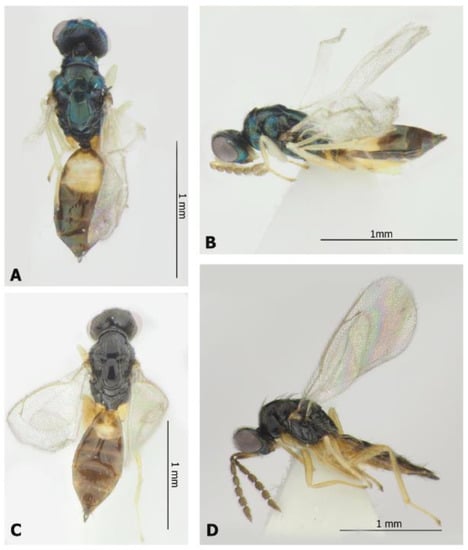
Figure 5.
Eulophinae (females): Elachertus fenestratus Nees, 1834 (A,B) and E. inunctus Nees, 1834 (C,D). Habitus, dorsal view (A,C) and lateral view (B,D).
Material examined. One female, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on Tilia platyphyllos, 26.VI.2020 (leaf mine coll.), 3.VII.2020 (par. em.), N. Kirichenko coll. “Tp-P-1-1, DNA barcoded: sample ID NK-20-28, process ID GPRU028-21”; one female, same label but from Ph. issikii mine on T. cordata, 2.VII.2021 (leaf mine coll.), 12.VII.2021 (par. em.), N. Kirichenko and M. Ryazanova coll. “Nov-21-Tc-5, DNA barcoded: sample ID NK908, process ID HYMRU089-21”; one female, same label, “Nov-21-Tc-3, DNA barcoded: sample ID NK906, process ID HYMRU087-21”; one male, same label but from Ph. issikii mine on T. platyphyllos, 2.VII.2021 (leaf mine coll.), 14.VII.2021 (par. em.), M. Ryazanova coll. and rearing, “SIB PD No. 14bis”.
Hosts. Ectoparasitoid of leaf mining lepidopterous larvae, mainly from the families Tortricidae, Gelechiidae and Coleophoridae [85].
Distribution. Russia: Ulyanovsk Province, Krasnodar Territory, Dagestan Republic, Novosibirsk Province (first record), Khabarovsk and Primorskiy Territories, Sakhalin (including Kuril Islands) Province, Kamchatka Territory, Chukotka Autonomous Area. Europe, Turkey, Yemen, Turkmenistan, Tajikistan, China, Korean Peninsula, Japan, North and South America [12,86,87].
Elachertus inunctus Nees, 1834
Figure 5C,D.
Material examined. One female, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on Tilia platyphyllos, 2.VII.2021 (leaf mine coll.) 14.VII.2021 (par. em.), M. Ryazanova coll. and rearing, “SIB-PD No. 14 bis”; one female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on T. amurensis, 07.VII.2021 (leaf mine coll.), 9–28.VII.2021 (par. em.), N. Kirichenko coll. “MTS-21-6-5, DNA barcoded: sample ID NK859, process ID HYMRU040-21”.
Hosts. Ectoparasitoid of leaf mining lepidopteran larvae from the families Elachistidae, Gracillariidae, Lyonetiidae, Oecophoridae, Nepticulidae and Tortricidae [85].
Distribution. Russia: Moscow and Ulyanovsk Provinces, Krasnodar and Stavropol Territories, Novosibirsk Province (first record), Khabarovsk and Primorskiy Territories, Sakhalin (including Kuril Islands) Province, Kamchatka Territory. Europe, Turkey, Turkmenistan, China, Korean Peninsula, Japan, southeast Asia [12,86,87].
Genus Pnigalio Schrank, 1802
The specimens of the genus Pnigalio were identified using the keys from different sources [12,42,44,46,56,63,64,85].
Pnigalio pectinicornis (Linnaeus, 1758)
Material examined. One female, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on Tilia cordata, 26.VI.2020 (leaf mine coll.), 5.VII.2021 (par em.), N. Kirichenko coll. and rearing, “Tc-P-10-1, DNA barcoded: sample ID NK-20-27, process ID GPRU027-21”.
Hosts. Widely polyphagous, primary or occasionally secondary, solitary ectoparasitoid leaf miner larvae belonging to the orders Lepidoptera (Gracillariidae, Lyonetiidae, Nepticulidae), Diptera (Agromyzidae, Tephritidae) and Coleoptera (Curculionidae) [69,85].
Distribution. Russia: Leningradskaya, Novgorod, Kaluga, Lipetsk, Kursk, Voronezh, Ulyanovsk Provinces, Udmurtia Republic, Krasnodar and Stavropol Territories, Crimea Republic, Sverdlovskkaya Province, Novosibirsk Province (first record), Primorskiy Territory. Europe, North Africa, Turkey, Israel, Iran, China, Australia [12,86,87], introduced to New Zealand [88].
Pnigalio soemius (Walker, 1839)
Figure 6F,G.
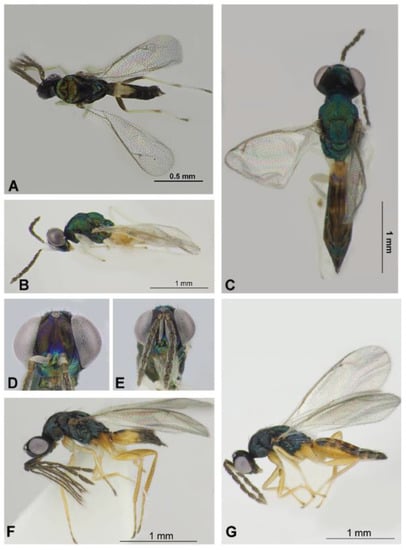
Figure 6.
Eulophinae (females, males): Sympiesis gordius (Walker, 1839) (male) (A), Sympiesis laevifrons Kamijo, 1965 (female) (B–E), Pnigalio soemius (Walker, 1839) (male and female) (F,G). Habitus in dorsal view (A,C) and lateral view (B,F,G), face and antennae in frontal view (D,E).
Material examined. One female, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on Tilia cordata, 2.VII.2021 (leaf mine coll.), 14.VII.2021 (par em.), M. Ryazanova coll. and rearing, SIB-PD No. 17 bis; one female, same label, SIB-PD No. 5 bis; one female, same label but 10-14.VII.2021 (par em.), M. Ryazanova coll. and rearing, SIB-PD No. 2 bis; one female, same label, SIB-PD No. 18 bis; one female, same label but 2.VII.2021, N. Kirichenko coll., “Nov-21-Tc-1, DNA barcoded: sample ID NK904, process ID HYMRU085-21”; one female, “Nov-21-Tc-6, DNA barcoded: sample ID NK909, process ID HYMRU090-21”; one male, same label but 8.VII.2021 (par em.), M. Ryazanova coll. and rearing, SIB-PD No. 10 bis; one male, same label but 12.VII.2021 (par em.), M. Ryazanova coll. and rearing, SIB-PD No. 8 bis.
Hosts. Solitary ectoparasitoid leaf miner larvae belonging to the orders Lepidoptera (Gracillariidae, Lyonetiidae, Nepticulidae, Yponomeutidae), Coleoptera (Curculionidae) and Diptera (Agromyzidae, Cecidomyiidae) [69,85].
Distribution. Russia: Leningradskaya, Moscow, Vladimir, Voronezh and Ulyanovsk Provinces, Krasnodar and Stavropol Territories, Crimea Republic, Novosibirsk Province (first record), Khabarovsk and Primorskiy Territories. Europe, Turkey, Syria, Iraq, Israel, Iran, Pakistan, China, Korean Peninsula, southeast Asia [12,86,87].
Pnigalio sp.
Material examined. One male, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on Tilia cordata, 2.VII.2021 (leaf mine coll.) 8.VII.2021 (par em.), M. Ryazanova coll. and rearing, SIB-PD No. 11 bis.
Remarks. This specimen is close to Pnigalio soemius but differs by the thick and short rami of the funicle, the second and third of which are with placoid sensilla (in P. soemius, rami are thin and without placoid sensilla); the specimen from Novosibirsk is also characterized by the absence of the costula of the propodeum.
Genus Sympiesis Foerster, 1856
The specimens of the genus Sympiesis were identified using the keys from different sources [12,31,43,46,49,55,64,73,85].
Sympiesis gordius (Walker, 1839)
Figure 6A.
Material examined. One male, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on Tilia cordata, 2.VII.2021 (leaf mine coll.) 8–12.VII.2021 (par. em.), M. Ryazanova coll. and rearing, SIB-PD No. 8 bis; one male, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on T. mandshurica, 8.VII.2021 coll. (leaf mines), 9–28.VII.2021, N. Kirichenko coll. “MTS-21-52-1, DNA barcoded: sample ID NK845, process ID HYMRU026-21”; one male, same label, “MTS-21-52-7, DNA barcoded: sample ID NK853, process ID HYMRU034-21”; one male, same label, “MTS-21-52-11, DNA barcoded: sample ID NK875, process ID HYMRU056-21”; one male, same label, “MTS-21-52-3, DNA barcoded: sample ID NK849, process ID HYMRU030-21”; one male, same label but from Ph. issikii mine on T. amurensis, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021. N. Kirichenko coll., “MTS-21-6-11, DNA barcoded: sample ID NK865, process ID HYMRU046-21”; one male, RFE-PD No. 20.
Hosts. Solitary primary or secondary ectoparasitoid of leaf mining larvae or (rarely) pupae, mainly on Phyllonorycter spp. (Lepidoptera: Gracillariidae) [85].
Distribution. Russia: Murmansk, Leningradskaya, Novgorod, Vladimir, Kaluga, Lipetsk, Tambov, Voronezh, Ulyanovsk, Rostov, Volgograd Provinces, Krasnodar and Stavropol Territories, Sverdlovskaya and Novosibirsk Provinces, Primorskiy Territory (first record). Europe, Armenia, Iran, Afghanistan, Kazakhstan, Mongolia, China, North America [12,86,87].
Sympiesis laevifrons Kamijo, 1965
Figure 6B–E.
Material examined. One female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on Tilia amurensis, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021 (par. em.), N. Kirichenko coll., “MTS-21-6-3, DNA barcoded: sample ID NK857, process ID HYMRU038-21”; one female, same label, “MTS-21-6-9, DNA barcoded: sample ID NK863, process ID HYMRU044-21”; one female, same label, “MTS-21-6-13, DNA barcoded: sample ID NK867, process ID HYMRU048-21”; one female, same label “MTS-21-6-14, DNA barcoded: sample ID NK868, process ID HYMRU049-21”; one female, same label, RFE-PD No. 10; one female, same label, RFE-PD No. 13; one female, same label, RFE-PD No. 15; one female, same label, RFE-PD No. 19; one female, same label, RFE-PD No. 20; one female, same label but reared from Ph. issikii mine on T. mandshurica, “MTS-21-52-15, DNA barcoded: specimen ID NK879, process ID HYMRU060-21”; one female, same label, “MTS-21-52-18; DNA barcoded: sample ID NK882, process ID HYMRU063-21”; one female, same label, “MTS-21-52-22, DNA barcoded: sample ID NK886, process ID HYMRU067-21”; two females, same label, RFE-PD No. 22; one female, same label, RFE-PD No. 23; one female, same label, RFE-PD No. 24; one female, same label, RFE-PD No. 27; one female, same label, RFE-PD No. 29; one female, same label, RFE-PD No. 31; one female, same label, “RFE-PD No. 32”; one female, same label, RFE-PD No. 34; one female, same label but from Ph. issikii mine on T. taquetii, RFE-PD No. 8.
Hosts. Parornix multimaculata (Matsumura, 1931), Phyllonorycter bicinctella (Matsumura, 1931), Ph. cretata (Kumata, 1957), Ph. issikii, Ph. pseudolautella (Kumata, 1963), Ph. ringoniella (Matsumura, 1931), Ph. sorbicola (Kumata, 1963), Ph. tristrigella (Haworth, 1828) (Lepidoptera: Gracillariidae) [31].
Distribution. Russia: Primorskiy Territory [12,86,87]. Japan [31].
Remarks. According to Kamijo [31], Sympiesis laevifrons is distinguishable by the prominent eyes, the short ocellocular line, the smooth face and vertex, the violet upper face and brownish yellow anteriorly first tergite (Figure 6D,E). Morphologically, the Far Eastern specimens belong to S. laevifrons; however, the DNA barcoding shows its similarity to S. gordius (see Section 3.2). More data would be needed to define the intra- and interspecific genetic diversity for these two species.
Subfamily Entedoninae Foerster, 1856
Achrysocharoides Girault, 1913
The specimens of the genus Achrysocharoides were identified using the keys from different sources [43,51,53,57,62,64,65,66,72].
Achrysocharoides cilla (Walker, 1839)
Figure 7A,B.
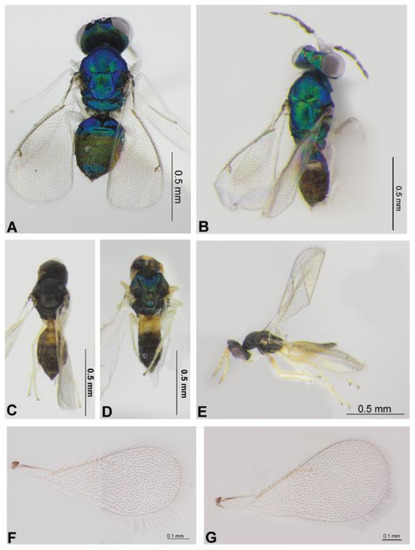
Figure 7.
Entedoninae and Tetrastichinae: Achrysocharoides cilla (Walker, 1839) (female and male) (A,B), Mischotetrastichus petiolatus (Erdös, 1961) (C,E,G), Minotetrastichus frontalis (Nees, 1834) (D) and Mischotetrastichus nadezhdae (Kostjukov, 1977) (F). Habitus in dorsal view (A–D) and lateral view (E), fore wing (G,F).
Material examined. One male, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on Tilia taquetii, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021, N. Kirichenko coll.”, “MTS-21-6a-1, DNA barcoded: sample ID NK856, process ID HYMRU037-21”; four females, same label, RFE-PD No. 8; one female, same label but reared from Ph. issikii mine on T. amurensis, “MTS-21-6-8, DNA barcoded: sample ID NK862, process ID HYMRU043-21”; three females, same label, RFE-PD No. 9; five females, same label, RFE-PD No. 10; four females, same label, RFE-PD No. 11; six females, same label, RFE-PD No. 12; two females, same label, RFE-PDNo. 14; one male, same label, RFE-PD No. 16; one female, same label, RFE-PD No. 19; one female, same label but reared from Ph. issikii on T. mandshurica, “MTS-21-52-12, DNA barcoded: sample ID NK876, process ID HYMRU057-21”; one female, same label, “MTS-21-52-13; DNA barcoded: sample ID NK877, process ID HYMRU058-21”; one male, same label, “MTS-21-52-14; DNA barcoded: sample ID NK878; process ID HYMRU059-21”; one female, same label, “MTS-21-52-17; DNA barcoded: sample ID NK881, process ID HYMRU062-21”; one female, same label, “MTS-21-52-20, DNA barcoded: sample ID NK884, process ID HYMRU065-21”; one female, same label, “MTS-21-52-29, DNA barcoded: sample ID NK893, process ID HYMRU074-21”; one female, same label, “MTS-21-52-30, DNA barcoded: sample ID NK894, process ID HYMRU075-21”; one female, same label, “MTS-21-52-33; DNA barcoded: sample ID NK897, process ID HYMRU078-21”; one female, same label, “MTS-21-52-10, DNA barcoded: sample ID NK874, process ID HYMRU055-21”; one female, same label, RFE-PD No. 21; one female, same label, RFE-PD No. 22; seven females, same label, RFE-PDNo. 23; two females, same label, RFE-PDNo. 24; two females, same label, RFE-PDNo. 26; five females, one male, same label, RFE-PDNo. 28; two females, same label, RFE-PDNo. 32; six female, one male, same label, RFE-PDNo. 33.
Hosts. Endoparasitoid of leaf miners from the family Gracillariidae (mainly Phyllonorycter spp.) [85].
Distribution. Russia: Astrakhan Province, Krasnodar and Stavropol Territories, Primorskiy Territory (first record). Europe [87].
Achrysocharoides nagasawi Kosheleva, sp. nov.
Figure 8A–G.
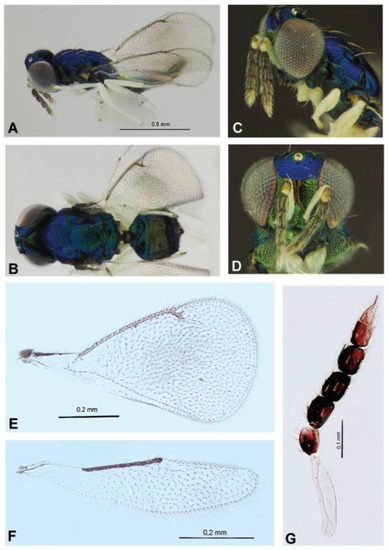
Figure 8.
Entedoninae: Achrysocharoides nagasawi sp. nov. (females), holotype (A–D) and paratypes (E–G). Habitus in lateral view (A) and dorsal view (B), head with antennae in lateral view (C), head in frontal view (D), fore wing (E), hind wing (F), antenna (G).
Type material. Holotype: one female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on Tilia mandshurica, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021 (par. em.), N. Kirichenko coll., RFE-PD No. 5.
Paratypes. Seven females with same label as in holotype; two females with same label but RFE-PD No. 22.
Description. Female. Body length 1.16–1.20 mm. Color. Frons below frontal suture bright green with coppery reflections; above the suture bright blue (Figure 8D). Mesoscutum, scutellum and propodeum metallic blue-green, most of mesoscutum dorsally blue, ventrally bright green; gaster green, first tergite blue-green with bronze reflections. Petiole dark brown with metallic tinges. Antennal scape white; pedicel pale yellow, brownish basally; flagellum brown with clava pale to whitish apically (Figure 8C,D,G). Legs white (Figure 8A), except hind coxae with metallic area restricted to proximal only about quarter. Tegulae metallic green. Wings hyaline or with a weak median infuscate spot, venation pale brownish (Figure 8E).
Sculpture. Genae smooth and shining. Frons below T-shaped frontal fork reticulate with meshes transverse; strongly reticulate and dull above fork (Figure 8D). Vertex behind ocellar triangle smooth, inside ocellar triangle with weak reticulation.
Head in dorsal view 1.8–1.9 times as long as broad; in frontal view 1.2–1.3 times as broad as high (Figure 8C); 1.14–1.20 times as broad as mesosoma. POL 2.00–2.25 times OOL, OOL 0.8–1.0 times POO. Occiput without sharp carina. Malar space well developed, 0.70–0.83 times as long as width of mouth and 0.30–0.39 times height of eye. Antenna with scape 5.0 times as long as broad, reaching to level of frontal fork; pedicellus plus flagellum 0.8–0.9 times breadth of head and as long as breadth of mesoscutum. Pedicel in lateral view 1.45–1.50 times as long as broad and 0.9–1.0 as long as F1; F2 as long as pedicellus and F3 1.1 times as long as F2. Clava (including stylus) 1.9–2.0 times as long as F3, C1 1.3 times as long as broad; C2 1.4 times as long as broad, at base slightly narrower than C1 and gradually tapering into apical stylus; the stylus about half as long as C2 (Figure 8G).
Mesosoma 1.46–1.50 times as long as broad. Mesoscutum 1.64–1.70 times as broad as long; its mid lobe with meshes of reticulation larger than reticulation of lateral lobes. Scutellum as long as broad, with smoother sculpture than mid lobe of mesoscutum. Scutellar pits absent. Dorsellum flat (Figure 8B). Propodeum medially about 0.4 times as long as scutellum, smooth, without longitudinal carinae or with barely visible traces; petiolar emargination shallow. Propodeal callus with three setae. Fore wing 1.9 times as long as broad, somewhat truncated apically, with a weak median infuscate spot. Speculum closed below. PM 0.6 times as long as ST (Figure 8E). Hind wing as in Figure 8F.
Metasoma. Petiole broadly expanded posteriorly into subrectangular portion with conspicuous shoulders in dorsal view (Figure 8B). Gaster oval-shaped, 1.5–1.6 times as long as broad, slightly narrower than mesosoma and 0.9 times as long as mesosoma.
Male. Unknown.
Comparative diagnosis. Achrysocharoides nagasawi sp. nov. belongs to the A. atys species group. According to Bryan [65], the species of this group have a petiole broadly expanded posteriorly into a subrectangular portion with conspicuous shoulders (dorsal view). A. nagasawi sp. nov. is clearly allied to A. carpini Bryan, 1980 in having the scape white, hind coxa white with metallic area restricted to proximal third or less, propodeum smooth, about 0.3 times as long as scutellum. However, the new species differs in the following characteristics: antenna with second claval segment white (antenna with brown flagellum in A. carpini), second claval segment not narrower than first and not tapering into apical stylus (as in A. carpini); clava (including stylus) 1.9–2.0 times as long as F3 (1.7 times in A. carpini), fore wing with a weak median infuscate spot (completely hyaline in A. carpini) and color of body evenly blue-green without purple reflection (as known in A. carpini).
Host. Phyllonorycter issikii (Gracillariidae) on Tilia mandshurica.
Distribution. Russian Far East (Primorskiy Territory).
Etymology. This species is named in honor of Dr Atsuhiko Nagasawa, a Japanese entomologist (Tohoku University, Sendai, Japan) who kindly helped us with the information about Achrysocharoides species described by K. Kamijo from Japan.
Achrysocharoides carinatus Kosheleva, sp. nov.
Figure 9A–F.
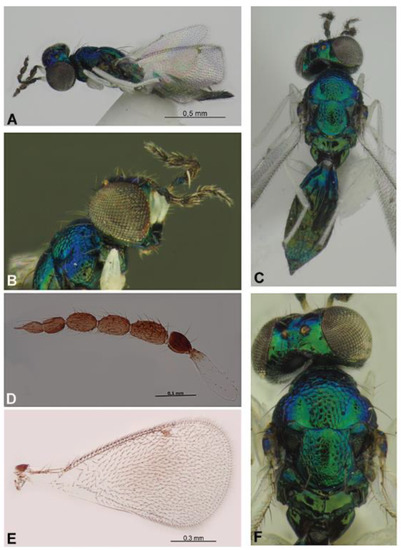
Figure 9.
Entedoninae: Achrysocharoides carinatus sp. nov. (female), holotype (A–E) and paratype (F). Habitus in lateral view (A) and dorsal view (C), head with antenna in lateral view (B), antenna (D), fore wing (E), head and mesosoma in dorsal view (F).
Material examined. Holotype: female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on Tilia mandshurica, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021, N. Kirichenko coll.”, RFE-PD No. 7.
Paratype. One female with the same label as in holotype.
Description. Female. Length 1.6 mm.
Color. Frons below frontal suture golden-green, above metallic bluish-purple. Vertex metallic bluish-purple, bright green in posterior part. Pronotum, mesoscutum and scutellum metallic bluish-green, dorsellum and propodeum without bluish reflection. Antennal scape white, brownish apically; flagellum brown (Figure 9B,D). Legs white (Figure 9A), hind coxa metallic green, with apex whitish. Fore wing hyaline with a weak median infuscate spot (Figure 9E). Petiole dark brown with metallic tinges. First and second tergites of gaster metallic bluish-green, remaining tergites metallic bright green with golden reflection.
Sculpture. Frons below frontal suture between scrobes and eyes with parts of raised reticulation, remaining parts smooth; above frontal suture smooth. Vertex smooth and shiny. Occipital margin with a sharp carina behind ocellar triangle (Figure 9C,F). Mid lobe of mesoscutum with raised and strong reticulation; notaular depressions smooth and shiny; lateral parts of scutellum with strong reticulation, its median and posterior parts with weak reticulation (Figure 9C,F). Axillae apical part smooth, its posterior part reticulate. Propodeum smooth and shiny with median carina and plicae.
Head in dorsal view 1.85–1.92 times as long as broad; in frontal view 1.38 times as broad as high; 1.0–1.2 times as broad as mesosoma. POL 1.0 times OOL, OOL 0.75 times POO. Malar space 0.58 times as long as width of mouth and 0.23 times height of eye. Antenna with scape 4.3 times as long as broad, not quite reaching to level of frontal fork (Figure 9B); pedicellus plus flagellum 0.9 times as long as breadth of head and 1.1 times as long as breadth of mesoscutum. Pedicel in lateral view 1.33 times as long as broad and 0.60 times as long as F1; third anellus thick, about 1/6 of dorsal length of F1; flagellum with two segmented clava, funiculus segments decreasing in length, 1.66–1.86 times as long as broad. Clava (including stylus) 1.8 times as long as F3, C1 1.3 times as long as broad; C2 1.5 times as long as broad, at base narrower than C1 and gradually tapering into apical stylus; the stylus about half as long as C2 (Figure 9D).
Mesosoma 1.4 times as long as broad (Figure 9C). Mesoscutum 1.67 times as broad as long. Scutellum about as long as broad (Figure 9F). Propodeum medially 0.38 times as long as scutellum, with complete median carina and plicae, and with strong posterior transverse carina along petiolar foramen (Figure 9C,F). Propodeal callus with three setae. Fore wing 1.88 times as long as broad, somewhat truncated apically, with a weak median infuscate spot, speculum small, closed below. ST ovate, as long as PM; speculum small (Figure 9E).
Metasoma. Petiole transverse, about 4.0 times as broad as long. Gaster elongated with subparallel sides and pointed apically (Figure 9C), 2.3–2.5 times as long as broad, 0.86 times as broad as mesosoma and 1.27–1.37 times as long as mesosoma.
Male. Unknown.
Comparative diagnosis. A. carinatus sp. nov. is close to A. crassinervis Kamijo, 1990, which belongs to the A. crassinervis species group, in the following characteristics, according to Kamijo [51]: occipital margin with sharp carina behind ocellar triangle; propodeum smooth, with strong, complete median carina and strong transverse posterior carina along petiolar foramen, plica present; mesoscutum and scutellum coarsely reticulate without pits; notaular depression broadly smooth. However, A. carinatus sp. nov. differs from the latter in the following characteristics: frontal fork not ridged, clava with a longer stylus, pronotum without collar and petiole transverse and not subconical.
Distribution. Russian Far East (Primorskiy Territory).
Etymology. Named after type of sculpture on the propodeum, from the Latin “carina” (=keel).
Chrysocharis Foerster, 1856
The specimens of the genus Chrysocharis were identified by keys from the following sources [43,61,67,68,70,71].
Chrysocharis laomedon (Walker, 1839)
Figure 10A,B.

Figure 10.
Entedoninae (females): Chrysocharis laomedon (Walker, 1839) (A,B) and Pleurotroppopsis japonica (Kamijo, 1977) (C,D). Habitus in dorsal view (A,D) and lateral view (B,C).
Material examined. One male, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on Tilia cordata, 2.VII.2021 (leaf mine coll.) 18–28.VII.2021 (par. em.), M. Ryazanova coll. and rearing, “Nov-21-Tc-2, DNA barcoded: sample ID NK905, process ID HYMRU086-21”; one female, same label, “Nov-21-Tc-4, DNA barcoded: sample ID NK907, process ID HYMRU088-21”; one female, same label, “Nov-21-Tc-7, DNA barcoded: sample ID NK910, process ID HYMRU091-21”; one female, same label but 2.VII.2021 (leaf mine coll.) 16.VII.2021 (par. em.), M. Ryazanova coll. and rearing, SIB-PD No. 15bis; two females, same label but 2.VII.2021 (leaf mine coll.) 18.VII.2021 (par. em.), SIB-PD No. 9bis; one female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on T. mandshurica, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021 (par. em.), N. Kirichenko coll., “MTS-21-52-2, DNA barcoded: sample ID NK846, process ID HYMRU027-21”; two females, six males, same label, RFE-PD No. 1; four females, same label, RFE-PD No. 2; four females, same label, RFE-PD No. 3; one female, same label, “MTS-21-52-4, DNA barcoded: sample ID NK850, process ID HYMRU031-21”; six females, two males, same label, RFE-PD No. 4; one female, same label, “MTS-21-52-5, DNA barcoded: sample ID NK851, process ID HYMRU032-21”; three females, one male, same label, RFE-PD No. 5; six females, same label, RFE-PDNo. 7; five females, one male, same label, RFE-PD No. 21; one female, same label, “MTS-21-52-8; DNA barcoded: sample ID NK872, process ID HYMRU053-21”; four females, two males, same label, RFE-PD No. 22; two females, same label, RFE-PD No. 24; three females, same label, RFE-PD No. 25; one female, one male, same label, RFE-PD No. 26; one female, same label, RFE-PD No. 27; one female, same label, “MTS-21-52-19; DNA barcoded: sample ID NK883, process ID HYMRU064-21”; one female, same label, RFE-PD No. 28; one male, same label, “MTS-21-52-23, DNA barcoded: sample ID NK887, process ID HYMRU068-21”; two females, one male, same label, RFE-PDNo. 31; one female, same label, “MTS-21-52-26, DNA barcoded: sample ID NK890, process ID HYMRU071-21“; one female, same label, “MTS-21-52-27, DNA barcoded: sample ID NK891, process ID HYMRU071-21”; one female, one male, same label, RFE-PD No. 32; one female, same label, “MTS-21-52-28, DNA barcoded: sample ID NK892, process ID HYMRU073-21”; three females, same label, RFE-PD No. 34; one female, same label, “MTS-21-52-31, DNA barcoded: sample ID NK895, process ID HYMRU076-21”; seven females, one male, some label but from Ph. issikii mine on T. amurensis, RFE-PDNo. 6; six females, one male, same label, RFE-PDNo. 9; seven females, one male, same label, RFE-PDNo. 10; four females, two males, same label, RFE-PDNo. 11; two females, three males, same label, RFE-PDNo. 12; two females, RFE-PDNo. 13; six females, one male, same label, RFE-PDNo. 14; one female, same label, “MTS-21-6-7, DNA barcoded: sample ID NK861, process ID HYMRU042-21”; three females, two males, same label, RFE-PDNo. 15; three females, two males, same label, RFE-PDNo. 16; one female, same label, “MTS-21-6-10, DNA barcoded: sample ID NK864, process ID HYMRU045-21”; four females, same label, RFE-PDNo. 17; three females, one male, same label, RFE-PDNo. 18; one female, same label, “MTS-21-6-15, DNA barcoded: sample ID NK869, process ID HYMRU050-21”; one male, same label, “MTS-21-6-16; DNA barcoded: sample ID NK870, process ID HYMRU051-21”; two females, two males, same label, RFE-PDNo. 19; nine females, same label, RFE-PDNo. 20; one male, same label, “MTS-21-6-17; DNA barcoded: sample ID NK871, process ID HYMRU052-21”; two males, same label but from Ph. issikii mine on T. taquetii, RFE-PD No. 8.
Hosts. Solitary endoparasitoid in larvae or pupae of Phyllonorycter spp. (Lepidoptera: Gracillariidae) [68].
Distribution. Russia: Moscow and Ulyanovsk Provinces, Dagestan Republic, Novosibirsk Province (first record), Primorskiy Territory. Europe, Turkey, Israel, Iran, Japan, North America [87].
Pediobius Walker, 1846
The specimens of the genus Pediobius were identified using the keys from different sources [48,61,64,78].
Pediobius cassidae Erdős, 1958
Material examined. One female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, arboretum, from Ph. issikii mine on T. mandshurica, 15.VII.2021 coll. (leaf mine), 21.VII.2021, N. Kirichenko coll. “RFE-14, DNA barcoded: sample ID NK-20-2, process ID GPRU002-21”.
Hosts. Primary, often secondary, endoparasite of eggs, larvae and pupae of Coleoptera (Chrysomelidae) and Lepidoptera (Pyralidae, Erebidae, Tortricidae) [85].
Distribution. Russia: Moscow, Ulyanovsk and Rostov Provinces, Krasnodar Territory, Tomsk Province, Primorskiy Territory. Europe, Ukraine, Turkey, Yemen, Iran, China [78,87].
Pleurotroppopsis Girault, 1913
The specimens of the genus Pleurotroppopsis were identified using the keys from different sources [32,50,52].
Pleurotroppopsis japonica (Kamijo, 1977)
Figure 10C,D.
Material examined. One female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on Tilia amurensis, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021 (par. em.), N. Kirichenko coll., “D-3, MTS-21-6-4, DNA barcoded: sample ID NK858, process ID HYMRU039-21”; one female, same label, RFE-PD No. 11.
Hosts. Parasitoid of various leaf miners in Japan: lepidopteran Aristaea asteris Kumata, 1977, Chrysaster hagicola Kumata 1961, Phyllonorycter issikii (Kumata, 1963), Ph. leucocorona (Kumata, 1957), Ph. lyoniae (Kumata, 1963), Ph. ringoniella (Matsumura, 1931), Ph. similis Kumata, 1982 (Gracillariidae) and Tischeria quercifolia Kuroko, 1982 (Tischeriidae); coleopteran Rhynchaenus takabayashii (Kôno, 1928) (Curculionidae) [32,52].
Distribution. Russia (first record): Russian Far East (Primorskiy Territory). Korean Peninsula, Japan [32,52].
Remarks. Morphologically, the Far Eastern specimens correspond to the description in Kamijo [32] but weakly differ by pale yellow spur of hind tibia (in Japanese specimens, it is brownish-yellow); however, both have the apices infuscate.
Subfamily Tetrastichinae Foerster, 1856
Minotetrastichus Kostjukov, 1977
The specimen of the genus Minotetrastichus was identified by keys from the following sources [12,45,58].
Minotetrastichus frontalis (Nees, 1834)
Figure 7D.
Material examined. One female, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on T. cordata, 2.VII.2021 (leaf mine coll.) 14.VII.2021 (par. em.), M. Ryazanova coll. and rearing, SIB-PD No. 18bis.
Hosts. Many species of leaf mining Lepidoptera (especially Phyllonorycter spp.), Coleoptera (Rhynchaenus spp.) and Hymenoptera (Tenthredinidae), as gregarious ectoparasite of their larvae; sometimes, as a facultative secondary or tertiary parasite of Braconidae and Chalcidoidea [45].
Distribution. Russia: Nizhny Novgorod, Moscow and Ulyanovsk Provinces, Stavropol Territory, Crimea Republic, the Urals, Altai Territory, Novosibirsk Province (first record), Amur Province, Khabarovsk and Primorskiy Territories. Europe, Ukraine, Pakistan, North America [45,87].
Mischotetrastichus Graham, 1987
The specimens of the genus Mischotetrastichus were identified by keys from the following sources [12,34,45,59,60,61,89].
Mischotetrastichus nadezhdae (Kostjukov, 1977)
Figure 7F.
Material examined. One female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on T. mandshurica, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021 (par. em.), N. Kirichenko coll., “MTS-21-52-6, DNA barcoded: sample ID NK852, process ID HYMRU033-21”.
Hosts. Phyllonorycter issikii (Kumata) (first record).
Distribution (according to the authors [12,60]). Russian Far East (Primorskiy Territory, Sakhalin Province).
Mischotetrastichus petiolatus (Erdős, 1961)
Figure 7C,E,G.
Material examined. One female, Novosibirsk Province, Novosibirsk, Central Siberian botanical garden, from Ph. issikii mine on T. platyphyllos, 2.VII.2021 (leaf mine coll.) 18.VII.2021 (par. em.), M. Ryazanova coll. and rearing, SIB-PD No. 7bis.
Hosts. Phyllonorycter rajella (Linnaeus, 1758), Ph. carpini (Kumata, 1963), Ph. hancola (Kumata, 1958), Ph.issikii [34,45].
Distribution. Russia: Leningradskaya, Pskov, Moscow, Ulyanovsk and Novosibirsk (first record) Provinces [12,87]. Europe, Japan [31].
Remarks. The Novosibirsk specimens of M. petiolatus morphologically appear to be the same as the European specimens according to descriptions given in Erdős [89] and Kostjukov [59,60] but closer to the specimens in the diagnosis by Kamijo and Ikeda [34] in the following features: antenna honey yellow with clava slightly darker and legs yellowish brown with fore coxae blackish, fore femora infuscate basally.
Superfamily Ichneumonoidea Letreille, 1802
Family Braconidae Nees, 1811
Subfamily Microgastrinae Foerster, 1863
Pholetesor Mason, 1981
The specimens of the genus Pholetesor were identified using the keys from different sources [81,90,91].
This medium-sized genus in a very large subfamily includes more than 35 species distributed in almost all zoogeographic regions (except the Afrotropics). The members of this genus are known as endoparasitoids of the leaf mining moths, mainly from the families Bucculatricidae, Coleophoridae, Elachistidae, Gracillariidae (especially), Lyonetiidae and Tischeriidae.
Pholetesor nataliae Belokobylskij, sp. nov.
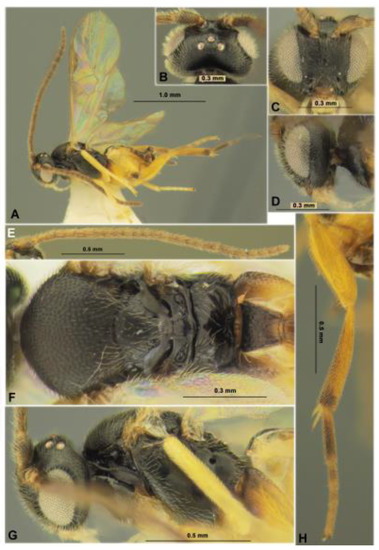
Figure 11.
Microgastrinae: Pholetesor nataliae sp. nov. (holotype, female). Habitus in lateral view (A), head in dorsal (B), frontal (C) and lateral (D) views, antenna (E), mesosoma in dorsal view (F), mesosoma in lateral view (G), hind leg (H).

Figure 12.
Microgastrinae: Pholetesor nataliae sp. nov. (holotype, female). Fore wing (A), hind wing (B), metasoma in dorsal (C) and lateral (D) views.
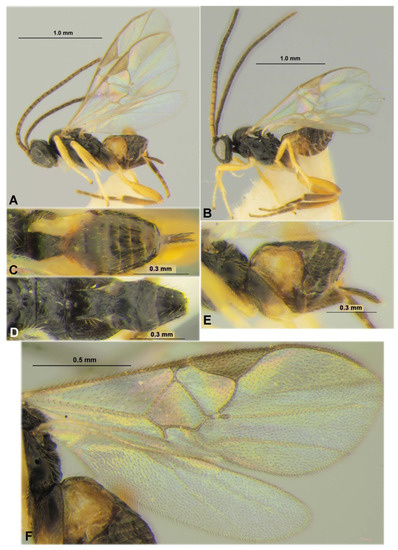
Figure 13.
Microgastrinae: Pholetesor nataliae sp. nov. (paratypes), female (A,C,E,F) and male (B,D). Habitus in lateral view (A,B), metasoma in dorsal view (C,D), metasoma in lateral view (E), fore and hind wings (F).
Type material. Holotype: female, Primorskiy Territory, Ussuriysk District, Gornotaezhnoe, forested area around village, from Ph. issikii mine on Tilia mandshurica, 8.VII.2021 (leaf mine coll.), 9–28.VII.2021, N. Kirichenko coll., “MTS-21-52-25, DNA barcoded: sample ID NK889, process ID HYMRU070-21”.
Paratypes. Same label as in holotype: two females, “MTS-21-6-1, DNA barcoded: sample ID NK854, process HYMRU035-21” and “MTS-21-6-2, DNA barcoded: sample ID NK855, process ID HYMRU036-21”; four females, three males, under one number RFE-PD No. 22, including one male “MTS-21-52-9, DNA barcoded: sample ID NK873, process ID HYMRU054-21; two females, two males, RFE-PD No. 23; two females, RFE-PD No. 24; one male, RFE-PD No. 25; one female “MTS-21-52-16, DNA barcoded: sample ID NK880, process ID HYMRU061-21); one female, two males, RFE-PD No. 26; one female, three males, RFE-PD No. 27; three females, two males, RFE-PD No. 28, including one female (MTS-21-52-21, DNA barcoded: sample ID NK885, process ID HYMRU066-21); one female, RFE-PD No. 29; three females, three males, RFE-PD No. 30, including one male “MTS-21-52-24; DNA barcoded: sample ID NK888, process ID HYMRU069-21”; one male, RFE-PD No. 31; six females, RFE-PD No. 32; three females, one male, RFE-PD No. 33; one female, one male, RFE-PD No. 8; one male, “MTS-21-52-34, DNA barcoded: sample ID NK898, process ID HYMRU079-21”; same locality but reared from Ph. issikii on T. taquetii; two females, RFE-PD No. 10; same locality but reared from Ph. issikii mine on T. amurensis; one female, RFE-PD No. 11, two males, RFE-PD No. 12; two females, RFE-PD No. 13; two males, “RFE-PD No. 14” and “MTS-21-6-6, DNA barcoded: sample ID: NK860, process ID HYMRU041-21”; one female, RFE-PD No. 15; one female, RFE-PD No. 18.
Additional material examined. One female, Gornotaezhnoe, forest around Ussuriysk Astrophysical Observatory, 11.VII.2021 (leaf mine coll.), 25.VII.2021 (par. em.), “RFE-21-1, DNA barcoded: sample ID NK-20-31, process ID GPRU031-21”; one male, Gornotaezhnoe, arboretum, 15.VII.2021 (leaf mine coll.), 23.VII.2021 (par. em.) “RFE-17-2, DNA barcoded: sample ID NK-20-4, process ID GPRU004-21”.
Description. Female. Body length 1.8 mm; fore wing length (from tegula) 1.9 mm.
Head. In dorsal view 1.9 times wider than median length, 2.0 times width across eyes and temple, 1.1 times as wide as mesoscutum. Temple in dorsal view 0.6 times as long as eye (measurement on straight line), behind eyes distinctly roundly narrowed. Ocelli in low triangle, its base 1.3 times sides; POL 1.5 times Od, 0.75 times OOL; OOL 2.0 times Od. Eye 1.7 times higher than wide. Minimum width of face 1.2 times its median height (from toruli to middle of supraclypeal groove), width just below toruli, 1.4 times its median height. Clypeus short, separated by distinct and narrow groove, distinctly concave ventrally, distinctly separated below from closed mandible forming relatively wide-open area covered from within by enlarged labium. Malar space almost equal to basal width of mandible. The malar suture is distinct and curved.
Antenna 18-segmented, about 1.2 times longer than body. First flagellar segment 3.3 times longer than maximum width, almost as long as second segment; second segment 2.7 times longer than maximum width. Penultimate segment 1.9 times longer than wide, 0.9 times as long as apical segment; apical segment weakly acuminated.
Mesosoma. In lateral view, 1.3 times longer than maximum height. Mesoscutum 1.1 times wider than median length. Prescutellar sulcus (depression) rather deep, very short, with several very small fovea. Scutellum weakly convex (lateral view). Metanotum medio-dorsally with two low lateral-curved carinae separated suboval medial area and fused with posterior small tubercle. Lateral pronotal lobe with distinct, wide and partly finely crenulate curved upper longitudinal sulcus, with distinct, narrow and at least partly weakly crenulate median oblique sulcus. Propodeal spiracle distinct, circular, situated before middle of propodeum. Propodeum without median carina and areola, but posteriorly with distinct and relatively short carinae divergented towards apex.
Wings. Fore wing 2.5 times longer than maximum width. Metacarp (1-R1) 1.1 times longer than pterostigma, 4.3 times longer than distance from apex of metacarp to apex of radial (marginal) cell. Pterostigma 3.1 times longer than maximum width. Radius (r) arising almost from middle of pterostigma. First radial abscissa (r) 0.8 times as long as width of pterostigma, as long as first radiomedial vein (2-SR) and curvedly connected with it. Discoidal (first discal) cell 1.2 times wider than height. Distance (1-CU1) between basal (1-M) vein and nervulus (cu-a) 0.7 times as long as distance (2-CU1) between nervulus (cu-a) and recurrent vein (m-cu). Setae on median (basal) cells mainly rather sparse and evenly distributed, on submedian (subbasal) cells setae sparse, and mainly absent in basal half. Hind wing 4.0 times longer than maximum width. Plical (vannal) lobe with dense short setae beyond its widest part.
Legs. Foreleg without subapical curved spine on apical tarsal segment. Middle leg with inner (longest) tibial spur about as long as middle basitarsus. Hind femur 3.4 times longer than wide. Hind tibial inner spur 2.0 times longer than outer spur, inner spur 0.55 times as long as hind basitarsus. Outer side of hind tibia without spines.
Metasoma 0.7 times as long as head and mesosoma combined. First tergite distinctly, more or less evenly and almost linearly narrowed toward apex (dorsal view), distinctly humped subcentrally (lateral view), without median longitudinal sulcus. Length of first tergite 1.4 times maximum subbasal width, 2.0 times its minimum apical width, 1.4 times median length of propodeum, 1.8 times length of second tergite. Second tergite 1.8 times as wide apically as median length; median field trapezoid, distinctly delineated by weakly crenulate and rather shallow and narrow lateral furrows divergent posteriorly; basal width of this area about 0.5 times its apical width, 0.7 times its length. Third tergite without longitudinal or transverse sulci, as long as second tergite. Hypopygium robust, acuminated distally, medio-ventrally without folds, not projecting beyond apex of metasoma. Ovipositor relatively short, but distinctly projected behind top of hypopygium, curved down; its sheath weakly widened toward apex and apically acuminated at short distance, with relatively short but rather dense setae, 0.6 times as long as hind tibia, 1.6 times longer than first tergite.
Sculpture. Vertex weakly matte, with sparse and weak sculpture of setiferous punctures. Frons shiny smooth. Face rather densely and relatively weakly punctate, almost matt, but more shiny laterally and below. Mesoscutum dull, with dense and shallow small punctures and matt interspaces; trace of notauli not different from sculpture. Scutellum almost smooth, rather shiny and with very fine and sparse punctation. Propodeum mostly smooth and shiny, with short and divergent forward carinae started from postero-median area. Mesopleuron mainly smooth and shiny, dull with rather weak and dense reticulation with rugosity below, precoxal sulcus almost smooth. Metapleuron entirely smooth and dull. Hind coxa with outer face almost entirely smooth. Hind femur entirely weakly and very densely punctulate with a satiny appearance. First tergite distinctly and densely reticulate-rugose in posterior 0.5–0.6, smooth in wide basal area and in relatively narrow and long posterior median stripe. Second tergite mainly smooth, only with fine but distinct longitudinal striae along oblique sulci. Third and following tergites entirely smooth, with evenly distributed, moderately long and rather dense setae.
Color. Body mainly black, second metasomal tergite mainly brown, paler laterally, metasoma ventro-laterally yellow and partly infuscate. Palpi pale yellow. Tegula and humeral plate light brown. First and middle legs yellow, brownish yellow basally; hind leg mainly brownish yellow, coxa almost black in basal 0.2–0.3, hind tibia in apical 0.2 and most part of hind tarsus almost black. Wings hyaline, pterostigma light brown, partly distally infuscate.
Variability. Body length 1.5–1.8 mm; fore wing length 1.9–2.0 mm. Head. In dorsal view 1.8–2.0 times wider than median length, 1.9–2.0 times width across eyes and temple, 1.1–1.2 times as wide as mesoscutum. Temple in dorsal view 0.5–0.7 times as long as eye (measurement on straight line). Ocellar triangle base 1.3–1.4 times sides; POL 1.3–1.7 times Od, 0.7–0.9 times OOL; OOL 1.8–2.3 times Od. Minimum width of face 1.1–1.2 times median height, width just below toruli 1.4–1.5 times its median height. Malar space 0.7–0.9 times basal width of mandible. First flagellar segment of antenna 3.3–3.6 times longer than maximum width, 0.95–1.05 times as long as second segment; second segment 2.7–3.5 times longer than maximum width. Penultimate segment 1.9–2.3 times longer than wide, 0.8–0.9 times as long as apical segment; apical segment weakly acuminated. Mesosoma. In lateral view, 1.3–1.5 times longer than maximum height. Mesoscutum 1.1–1.2 times wider than median length. Wings. Fore wing 2.5–2.8 times longer than maximum width. Metacarp (1-R1) 1.1–1.3 times longer than pterostigma, 4.2–5.3 times longer than distance from apex of metacarp to apex of radial (marginal) cell. Pterostigma 2.5–3.1 times longer than maximum width. First radial abscissa (r) 0.7–0.9 times as long as width of pterostigma, about as long as first radiomedial vein (2-SR). Discoidal (first discal) cell 1.2–1.3 times wider than height. Distance (1-CU1) between basal (1-M) vein and nervulus (cu-a) 0.7 times as long as distance (2-CU1) between nervulus (cu-a) and recurrent vein (m-cu). Hind wing 3.5–4.0 times longer than maximum width. Legs. Middle leg with inner (longest) tibial spur 0.7–0.9 times as long as middle basitarsus. Hind femur 3.2–3.5 times longer than wide. Hind tibial inner spur 0.55–0.70 times as long as hind basitarsus. Metasoma 0.7–0.8 times as long as head and mesosoma combined. First tergite almost linearly narrowed toward apex. Length of first tergite 1.3–1.4 times maximum subbasal width, 2.0–2.3 times its minimum apical width, 1.3–1.4 times median length of propodeum, 1.8–2.0 times length of second tergite. Second tergite mainly smooth medially and laterally, usually with fine but rather distinct and dense (especially posteriorly) longitudinal striae along oblique sulci; sometimes, lateral sulci very shallow; 1.8–2.6 times as wide apically as median length; basal width of medial field 0.4–0.5 times its apical width, 0.7–0.8 times its length. Third tergite 0.9–1.0 times as long as second tergite. Ovipositor sheath 0.5–0.6 times as long as hind tibia, 1.2–1.6 times longer than first tergite. Color. Second metasomal tergite black with brownish margins or mainly brown but paler laterally. Tegula and humeral plate light brown to infuscate, gray. Hind coxa almost black in basal 0.3–0.8, hind tibia often (but not always) in apical 0.2 and hind tarsus entirely or only at most part almost black or dark brown; sometimes, fifth tarsal segment brown. Pterostigma of fore wing light brown to pale gray, sometimes distally or marginally infuscate.
Male. Body length 1.4–1.9 mm; fore wing length 1.7–1.9 mm. Antenna thicker than in female; its first flagellar segment 2.4–3.5 times longer than maximum width; second segment 2.2–3.3 times longer than maximum width. First metasomal tergite smooth or almost smooth in basal 0.5–0.7. Median area of second tergite often mainly smooth. Often hind legs darkened. Hind coxa usually entirely black; sometimes, hind femur predominantly dark brown and with small light brown spots; hind tibia widely or almost entirely infuscate, but usually paler basally. Otherwise, similar to female, except sexual characters.
Hosts. Phyllonorycter issikii (Kumata, 1963) (Lepidoptera: Gracillariidae).
Distribution. Russian Far East (Primorskiy Territory).
Etymology. The name refers to the collector of this new parasitoid species, Dr. Natalia I. Kirichenko (Russia, Krasnoyarsk) who studied Ph. issikii in Asian Russia and published several important papers on its invasion in the Palaearctic.
Comparative diagnosis. The new species is difficult to distinguish morphologically from Pholetesor circumscriptus (Nees, 1834). Their external diagnostic characteristics, in particular, the shape and sculpture of the first and second metasomal tergites, sculptural pattern on the mesoscutum, distribution of sculpture on the propodeum, etc., vary at the intraspecific level and somewhat overlap at the interspecific level. However, these two sibling species are allopatric; Ph. nataliae occurs in the Russian Far East (so far found only in the south of the Primorskiy Territory) and perhaps presents in Korea [92], whereas Ph. circumscriptus is distributed in Europe, the Caucasus, Israel, Iran, USA (Alaska) and was introduced in New Zealand [91]. The DNA barcoding shows large genetic interspecific divergence (~8%) (see Section 3.1).
Remarks. It is very likely that the specimens of Pholetesor circumscriptus previously recorded in North Korea [92] and the Russian Far East [81,91] belong to the newly described Ph. nataliae.
Subfamily Exothecinae
Colastes braconius Haliday, 1833
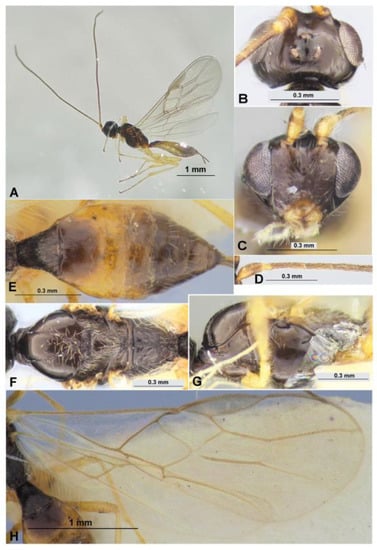
Figure 14.
Exothecinae: Colastes braconius Haliday, 1833 (female). Habitus in lateral view (A), head in dorsal (B) and frontal (C) views, basal segments of antenna (D), metasoma in dorsal view (E), mesosoma in dorsal (F) and lateral (G) views, fore and hind wings (H).
Material examined. One female, Novosibirsk Province, Central Siberian botanical garden, from Ph. issikii mine on Tilia platyphyllos, 26.VI.2020 (leaf mine coll.), 8.VII.2020 (par. em.), N. Kirichenko coll. “Tp-P-4-1, DNA barcoded: sample ID NK-20-30, process ID GPRU030-21”.
Distribution. Russia: European part (overall), Urals, Novosibirsk, Irkutsk Provinces, Zabaikalskiy, Khabarovsk, Primorskiy and Kamchatka Territories, Sakhalin (including Kuriles) Province. Tunisia, western, central and eastern Europe, Georgia, Armenia, Azerbaijan, Turkey, Iran, Kazakhstan, Mongolia, Korea, Japan [87,91].
Hosts. Polyphagous ectoparasitoid usually of leaf mining lepidopteran caterpillars from the families Coleophoridae, Cosmopterigidae, Elachistidae, Eriocraniidae, Gracillariidae, Heliozelidae, Lycaenidae, Lyonetiidae, Momphidae, Nepticulidae, Pyralidae, Tischeriidae, Tortricidae and Ypsolophidae; dipteran larvae from the family Agromyzidae; coleopteran larvae from the family Curculionidae; and hymenopteran larvae from the family Tenthredinidae [87,91].
Remarks. This species was already recorded as the parasitoid of Ph. issikii in Slovakia [25] and Bulgaria [30].
4. Discussion
Morphological and/or molecular genetic characterization allowed us to identify, overall, 19 hymenopteran parasitoids developing on Phyllonorycter issikii: 12 species in the Russian Far East (native range) and 10 species in western Siberia (invaded range of the moth), with only 3 shared species, indicating the divergence of parasitoids complexes in these two distant regions. The research conducted in Asian Russia made it possible to extend the checklist of parasitoids of Ph. issikii from 73 to 79 species and provide new records on their distribution and trophic associations in the Eastern Palaearctic (see Table S2).
All parasitoids we identified in western Siberia (except one Pnigalio sp.) are known to have trophic relation with the lime leaf miner in the invaded regions: the European part of Russia, western and central European countries (Table S2). Pnigalio sp. reared from Ph. issikii mines sampled in the Central Siberian Botanical Garden (Novosibirsk) could be a new species to science. We chose to avoid describing it here based on a single male specimen. More specimens would be needed to confirm the novelty of this species as well as obtain the DNA barcoding data for the genetic characterization (which was beyond the scope of the present study).
In contrast to western Siberia, the exploration of Ph. issikii parasitoids in the Russian Far East (Primorskiy Territory, Ussuriysk District) provided a number of novel records. As a result, four new species were described from the moth’s native region: Achrysocharoides nagasawi sp. nov., A. carinatus sp. nov., Cirrospilus ussuriensis sp. nov. and Pholetesor nataliae sp. nov. The eulophid Mischotetrastichus nadezhdae was reared from premature stages of Ph. issikii. for the first time. Furthermore, the eulophid Achrysocharoides cilla and the braconid Colastes braconius were documented in Russia as parasitoids of Ph. issikii for the first time. Unlike in the early studies exploring parasitoid complexes of the moth in the European part of Russia, these two parasitoids were never reared from the moth’s immature stages [19,20,23,28]. Prior to our research, the relationship of A. cilla and C. braconius with Ph. issikii was initially identified in Hungary [29,30]. Moreover, the eulophid Pleurotroppopsis japonica known to parasitize on Ph. issikii in Japan [32] is a novel record for Russia. The eulophids Sympiesis gordius and Achrysocharoides cilla are novel to the Russian Far East (Primorskiy Territory), whereas eight species, Elachertus fenestratus, E. inunctus, Pnigalio soemius, P. pectinicornis, Sympiesis gordius, Chrysocharis laomedon, Minotetrastichus frontalis and Mischotetrastichus petiolatus, are novel to the Novosibirsk Province.
It is important to stress that the parasitoids of the family Braconidae were hardly reared from Phyllonorycter issikii. In the following sources [9,21,23,25,29,30], a few braconids were indicated to be trophically linked with Ph. issikii: Colastes braconius Haliday, 1833 (subfamily Exothecinae) [25,30], Dolichogenidea dilecta (Haliday, 1834), Pholetesor circumscriptus (Nees, 1834), Ph. exiguus (Haliday, 1834), Pholetesor sp. and Apanteles sp. (all from subfamily Microgastrinae) [9,21,23,24,25,29].
DNA barcoding allowed us to identify the majority of parasitoids reared from Ph. issikii in Asian Russia and detect problematic taxonomic issues within some species complexes. In particular, the specimens morphologically identified here as Sympiesis gordius and S. laevifrons share one BIN (BOLD:AAE2642) and show tiny minimal pairwise distances compared to maximal intraspecific distances within each species (i.e., over 2% in each case). In fact, these two species were identified from Ph. issikii by several authors: S. gordius was documented in the invaded regions in the European part of Russia and European countries [18,23,29]; S. laevifrons was documented as a parasitoid of Ph. issikii in Japan [31]. We documented both species in the Primorskiy Territory in the Russian Far East in sympatry. Taking into account that these two species are morphologically similar, and their DNA barcodes show a significant degree of similarity, collecting additional specimens across species ranges would be needed for the revision of this complex. In our restudy, however, this task was not covered.
DNA barcoding also allowed us to differentiate the species which are new to science. The specimens of Pholetesor reared from Ph. issikii in the Russian Far East (Gornotayozhnoe) is morphologically highly similar to Pholetesor circumscriptus (Nees, 1834). However, DNA barcoding data for the Far Eastern specimens showed distinct genetic differences from the European specimens of Ph. circumscriptus. Thus, as a result, we described here a new sibling species, Pholetesor nataliae, currently recorded only in the south of the continental Far East. It is very likely that Pholetesor circumscriptus recorded in Korea [92] and Pholetesor sp. from Japan (Hokkaido) [9] also belong to the new species, Pholetesor nataliae. However, for such a conclusion, more samplings for morphological and molecular genetic analysis would be desirable.
To conclude, our study provides the first insight into the hymenopteran parasitoids of Ph. issikii in Asian Russia. More in-depth studies would be needed to explore the complexes of Ph. issikii parasitoids in this part of Russia using the integrative approach combining morphological and molecular genetic analyses for species identification and for obtaining data on their effectiveness in controlling Ph. issikii in its primary vs. secondary range.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14090707/s1, Table S1: The specimens of Phyllonorycter issikii parasitoids involved into molecular genetic analysis and the related sequences borrowed from BOLD for comparison. For each specimen, sample ID and process ID are provided, linking the records in the BOLD database with the voucher specimen data; Table S2: The checklist of Phyllonorycter issikii parasitoids in the Palaearctic.
Author Contributions
Conceptualization and methodology, O.V.K., N.I.K. and S.A.B.; software, N.I.K.; validation, N.I.K. and S.A.B.; formal analysis and investigation, O.V.K., N.I.K. and S.A.B.; resources, N.I.K.; data curation, N.I.K. and S.A.B.; writing—original draft preparation, review and editing, visualization, O.V.K., N.I.K. and S.A.B.; supervision, project administration, funding acquisition, N.I.K. and S.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Research Project, No. 122031100272-3, for S.A.B. (new species description), the basic project of Sukachev Institute of Forest SB RAS, project No. 0287-2021-0011 (field sampling), and the Russian Science Foundation, project No. 22-16-00075 (molecular genetic data analysis), for N.I.K.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The genetic data used in the study are publicly accessible in BOLD using the link dx.doi.org/10.5883/DS-ISSIKPAR.
Acknowledgments
We thank Maria Ryazanova (Krasnoyarsk) for helping with the collection of the leaf mines of Ph. issikii in Novosibirsk in 2021, Maria Tomoshevich (Novosibirsk) for allowing us to use the resources of collections of living plants in the open and closed grounds (USU_440534, arboretum exposition) in the Central Siberian Botanical Garden (Novosibirsk, Russia), Svetlana Gorokhova (Gornotaezhnoe) for assistance in the field in the Russian Far East, Margarita Ponomarenko (Vladivostok) for her support and joint research, and Olga Kuznetsova (Siberian Federal University, Krasnoyarsk, Russia) for checking the English language in the manuscript. We are grateful to two anonymous reviewers for their useful comments on the early version of the manuscript. We also thank the team at the Biodiversity Institute of Ontario, University of Guelph (Ontario, Canada), for their great assistance in producing the DNA barcodes for our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Šefrová, H. Phyllonorycter issikii (Kumata, 1963)–bionomics, ecological impact and spread in Europe (Lepidoptera, Gracillariidae). Acta Univ. Agric. Silvic. Mendel. Brun 2002, 50, 99–104. [Google Scholar]
- Ermolaev, I.V.; Rubleva, E.A. History, rate and factors of invasion of lime leafminer Phyllonorycter issikii (Kumata, 1963) (Lepidoptera, Gracillariidae) in Eurasia. Russ. J. Biol. Invasions 2017, 1, 2–19. [Google Scholar] [CrossRef]
- Kirichenko, N.; Triberti, P.; Ohshima, I.; Haran, J.; Byun, B.-K.; Li, H.; Augustin, S.; Roques, A.; Lopez-Vaamonde, C. From east to west across the Palearctic: Phylogeography of the invasive lime leaf miner Phyllonorycter issikii (Lepidoptera: Gracillariidae) and discovery of a putative new cryptic species in East Asia. PLoS ONE 2017, 12, e0171104. [Google Scholar] [CrossRef]
- Kirichenko, N.I.; Zakharov, E.V.; Lopez-Vaamonde, C. Tracing the invasion of a leaf mining moth in the Palearctic through DNA barcoding of historical herbaria. Sci. Rep. 2022, 12, 5065. [Google Scholar] [CrossRef]
- Kirichenko, N.I.; Triberti, P.; Akulov, E.N.; Ponomarenko, M.G.; Lopez-Vaamonde, C. Novel data on the taxonomic diversity, distribution and host plants of leafmining moths of the family Gracillariidae (Lepidoptera) in Siberia based on the DNA-barcoding. Entomol. Rev. 2019, 90, 769–819. [Google Scholar] [CrossRef]
- Kirichenko, N. The lime leafminer Phyllonorycter issikii in Western Siberia: Some ecological characteristics of the population of the recent invader. Contemp. Probl. Ecol. 2014, 7, 114–121. [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Zorin, D.A. Ecological consequences of invasion of Phyllonorycter issikii (Lepidoptera, Gracillariidae) in lime forests in Udmurtia. Entomol. Rev. 2011, 91, 592–598. [Google Scholar] [CrossRef]
- De Prins, J.; De Prins, W. Global Taxonomic Database of Gracillariidae. Available online: http://www.gracillariidae.net/ (accessed on 7 June 2022).
- Hirao, T.; Murakami, M. Quantitative food webs of lepidopteran leafminers and their parasitoids in a Japanese deciduous forest. Ecol. Res. 2008, 23, 159–168. [Google Scholar] [CrossRef]
- Mey, W. Über die Bedeutung autochthoner Parasitoidenkomplexe bei der rezenten Arealexpansion von vier Phyllonorycter-Arten in Europa (Insecta, Lepidoptera, Hymenoptera). Mitt. Zool. Mus. Berl. 1991, 67, 177–194, (In Germany with English Abstract). [Google Scholar] [CrossRef]
- Osipova, A.S. A complex of Invertebrates-Phylophages of Prioksko-Terrasnyi Nature Reserve and Its Use for Forest Monitoring. Avtoreferat Dissertatsii … Kandata Biologicheskikh Nauk, Moscow 1995. (In Russian)
- Storozheva, N.A.; Kostjukov, V.V.; Yefremova, Z.A. Fam. Eulophidae. In Key to the Insects of the Russian Far East. Neuropteroidea, Mecoptera, Hymenoptera; Lehr, P.A., Ed.; Dal’Nauka: Vladivostok, Russia, 1995; Volume 4, Part 2; pp. 291–505. (In Russian) [Google Scholar]
- Matošević, D. Prvi nalaz vrste Phyllonorycter issikii i rasprostranjenost invazivnih vrsta lisnih minera iz porodice Gracillariidae u Hrvatskoj. Rad. Sumar Inst. Jastrebarko 2007, 42, 127–142, (In Croatian with English Summary). [Google Scholar]
- Yegorenkova, E.N.; Yefremova, Z.A.; Kostjukov, V.V. Contributions to the knowledge of Tetrastichine wasps (Hymenoptera, Eulophidae, Tetrastichinae) of the Middle Volga region. Entomol. Rev. 2007, 87, 1180–1192. [Google Scholar] [CrossRef]
- Yefremova, Z.A.; Mishchenko, A.V. A complex of parasitoids (Hymenoptera, Eulophidae) of Phyllonorycter issikii (Lepidoptera, Gracillariidae) from the Middle Volga river Basin. Zool. Zhurnal 2008, 87, 189–196. (In Russian) [Google Scholar] [CrossRef]
- Yefremova, Z.A.; Mishchenko, A.V. New data on trophic relations between eulophid parasitic wasps (Hymenoptera, Eulophidae) and lepidopterans in Ul’yanovsk Province. Entomol. Obozr. 2009, 88, 29–37. (In Russian) [Google Scholar] [CrossRef]
- Yefremova, Z.A.; Mishchenko, A.V. The dynamics of the populations of dominant parasitoids (Hymenoptera: Eulophidae) of Phyllonorycter issikii (Kumata) (Lepidoptera: Gracillariidae) in the Middle Volga Basin. Russ. Entomol. J. 2010, 80, 64–75. (In Russian) [Google Scholar]
- Yefremova, Z.; Mishchenko, A. The preimaginal stages of Minotetrastichus frontalis (Nees) and Chrysocharis laomedon (Walker) (Hymenoptera: Eulophidae), parasitoids associated with Phyllonorycter issikii (Kumata, 1963) (Lepidoptera: Gracillariidae). J. Nat. Hist. 2012, 46, 1283–1305. [Google Scholar] [CrossRef]
- Yefremova, Z.A.; Krayushkina, A.V.; Mishchenko, A.V. Parasitoid complexes (Hymenoptera, Eulophidae) of species of the genus Phyllonorycter (Lepidoptera, Gracillariidae) in the Middle Volga Basin. Zool. Zhurnal 2009, 88, 1213–1221. (In Russian) [Google Scholar] [CrossRef]
- Tomov, R. A review of mortality factors of three invasive leafminer moths (Lepidoptera) in Bulgaria. In Proceedings of the VI Congress of Plant Protection (Book II), Zlatibor, Serbia, 23–27 November 2009; pp. 83–85. [Google Scholar]
- Ermolaev, I.V.; Yefremova, Z.A.; Izhboldina, N.V. Parasitoids as a mortality factor for the lime leafminer (Phyllonorycter issikii, Lepidoptera, Gracillariidae). Zool. Zhurnal 2011, 20, 24–32. (In Russian) [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Yefremova, Z.A.; Domrachev, T.B. The influence of parasitoids (Hymenoptera, Eulophidae) on survival of the lime leafminer Phyllonorycter issikii (Lepidoptera, Gracillariidae) in Udmurtia. Entomol. Rev. 2018, 98, 407–413. [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Yefremova, Z.A.; Gerasimova, N.A.; Korolyeva, E.A.; Lushnikov, N.N.; Petrov, A.I.; Pchel’nikov, A.A. Parasitoids (Hymenoptera) of the lime leafminer Phyllonorycter issikii (Lepidoptera, Gracillariidae) in different cities of the Russian Federation and their role in the mortality of the invasive pest. Entomol. Rev. 2019, 99, 485–493. [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Yefremova, Z.A. Parasitoids (Hymenoptera, Eulophidae, Braconidae) of the lime leafminer Phyllonorycter issikii (Kumata) (Lepidoptera, Gracillariidae). Eur. J. Entomol. 2015, 14, 217–223. (In Russian) [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Aimbetova, S.I. Parasitoids (Hymenoptera, Eulophidae, Ichneumonidae, Braconidae) of the lime leafminer Phyllonorycter issikii (Kumata) (Lepidoptera, Gracillariidae) in Bratislava. Bull. Udmurtia Univ. Ser. Biol. Earth Sci. 2016, 26, 118–125. (In Russian) [Google Scholar]
- Meshkova, V.L.; Mikulina, I.N. Entomophages of adventive leafminers in green plantations of Kharkov. In Sovremennoe Sostoyanie i Perspektivy Okhrany i Zashchity Lesov v Sisteme Ustoichivogo Razvitiya, Materialy Mezhdunarodnoi Nauchno-Prakticheskoi Konferentsii; Institut Lesa NAN Belarusi: Gomel’, Belarus, 2013; pp. 92–96. (In Russian) [Google Scholar]
- Gokhman, V.E.; Yefremova, Z.A.; Yegorenkova, E.N. Karyotypes of parasitic wasps of the family Eulophidae (Hymenoptera) attacking leaf–mining Lepidoptera (Gracillariidae, Gelechiidae). Comp. Cytogenet. 2014, 8, 31–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishchenko, A.V. New data on parasitism of eulophid wasps (Hymenoptera: Eulophidae) on Phyllonorycter issikii (Kumata, 1963) (Lepidoptera: Gracillariidae), a pest of limetree in the Middle Volga Region (Russia). Cauc. Entomol. Bull. 2014, 10, 131–136. (In Russian) [Google Scholar] [CrossRef]
- Szőcs, L.; Melika, G.; Thuroczy, C.; Csoka, G. Parasitoids of lime leaf miner Phyllonorycter issikii (Lepidoptera: Gracillariidae) recorded throughout the area it recently colonized. Eur. J. Entomol. 2015, 112, 591–598. [Google Scholar] [CrossRef]
- Toshova, T.B.; Boyadzhiev, P.; Todorov, I.; Draganova, S. Parasitoids and fungal pathogens of Phyllonorycter issikii (Kumata, 1963) from Bulgaria. Biologia 2018, 73, 1237–1245. [Google Scholar] [CrossRef]
- Kamijo, K. Description of five new species of Eulophinae from Japan and other notes (Hymenoptera: Chalcidoidea). Insecta Matsumurana 1965, 28, 69–78. [Google Scholar]
- Kamijo, K. Five new species of Cotterellia (Hymenoptera, Eulophidae) from Japan. Kontyû 1977, 45, 253–261. [Google Scholar]
- Kamijo, K. Notes on Pediobius Walker (Hymenoptera, Eulophidae) from Japan, with description of a new species. Kontyû 1986, 54, 70–78. [Google Scholar]
- Kamijo, K.; Ikeda, E. A revision of Citrostichus and Mischotetrastichus (Hymenoptera: Eulophidae), with descriptions of a new genus and new species. Jpn. J. Entomol. 1997, 65, 562–582. [Google Scholar]
- Ikeda, E. Revision of the Japanese species of Chrysocharis (Hymenoptera, Eulophidae), II. Jpn. J. Entomol. 1996, 64, 275–287. [Google Scholar]
- Ermolaev, V.P. A review of the fauna and ecology of miner-moths (Lepidoptera, Gracillariidae) of the Primorye Territory. The Fauna of Insects of the Far East. Tr. Zool. Inst. Proc. Zool. Inst. 1977, 70, 98–116. (In Russian) [Google Scholar]
- De Waard, J.R.; Ivanova, N.V.; Hajibabaei, M.; Hebert, P.D.N. Assembling DNA barcodes: Analytical methods. In Methods in Molecular Biology: Environmental Genetics; Cristopher, M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 275–293. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Jeanmougin, F.; Thompson, J.D.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal, X. Trends Biochem. Sci. 1998, 23, 403–405. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molec. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Erdős, J. Eulophidae hungaricae indescriptae. Ann. Hist-Nat. Mus. Natl. Hung. 1954, 5, 323–366. [Google Scholar]
- Graham, M.W.R. de V. Keys to the British genera and species of Elachertinae, Eulophinae, Entedontinae and Euderinae (Hym., Chalcidoidea). Trans. Soc. Br. Entomol. 1959, 13, 169–204. [Google Scholar]
- Graham, M.W.R. de V. Additions and corrections to the British list of Eulophidae (Hym., Chalcidoidea), with descriptions of some new species. Trans. Soc. Br. Entomol. 1963, 15, 167–275. [Google Scholar]
- Graham, M.D.V. A reclassification of the European Tetrastichinae (Hymenoptera: Eulophidae), with a revision of certain genera. Bull. Br. Mus. Nat. 1987, 55, 1–392. [Google Scholar]
- Bouček, Z. A study of central European Eulophidae, I: Eulophinae (Hymenoptera). Acta Entomol. Mus. Natl. Pragae 1959, 33, 117–170. [Google Scholar]
- Bouček, Z. A study of central European Eulophidae, II: Diaulinopsis and Cirrospilus (Hymenoptera). Bull. Br. Mus. Nat. 1959, 33, 171–194. [Google Scholar]
- Bouček, Z. Studies of European Eulophidae, IV: Pediobius Walk. and two allied genera (Hymenoptera). Acta Entomol. Mus. Natl. Pragae 1965, 36, 5–90. [Google Scholar]
- Bouček, Z. Descriptive and taxonomic notes on ten mainly new species of West Palaearctic Eulophidae (Hym.). Acta Entomol. Mus. Natl. Pragae 1969, 38, 525–543. [Google Scholar]
- Bouček, Z. Australasian Chalcidoidea (Hymenoptera). A Biosystematic Revision of Genera of Fourteen Families, with a Reclassification of Species; CAB International: Wallingford, Oxon, UK; Cambrian News Ltd.: Aberystwyth, Wales, 1988; pp. 584–832. [Google Scholar]
- Kamijo, K. Descriptions of five new species of Achrysocharoides (Hymenoptera: Eulophidae) from Japan, with notes on species-groups. Akitu 1990, 119, 1–15. [Google Scholar]
- Kamijo, K. Notes on Pleurotropposis (Hymenoptera, Eulophidae) and its allied genera, with descriptions of four new species from Japan. Jpn. J. Entomol. 1990, 58, 816–826. [Google Scholar]
- Kamijo, K. Revision of North American Achrysocharoides (Hymenoptera: Eulophidae). Akitu 1991, 124, 1–34. [Google Scholar]
- Kamijo, K. Two new species of Cirrospilus (Hymenoptera, Eulophidae) from Japan. Jpn. J. Entomol. 1992, 60, 391–395. [Google Scholar]
- Askew, R.R. Hymenoptera 2. Chalcidoidea Section (b). In Handbooks for the Identification of British Insects; Royal Entomological Society: London, UK, 1968; Volume 8, Part 2, Section (b); pp. 1–39. [Google Scholar]
- Askew, R.R. Species of Pnigalio and Chrysocharis (Hymenoptera: Eulophidae) parasitic on Tischeriidae (Lepidoptera), with the description of a new species. Entomol. Gaz. 1984, 25, 103–109. [Google Scholar]
- Askew, R.R.; Ruse, M. Biology and taxonomy of the genus Enaysma Delucchi (Hym., Eulophidae, Entedontinae) with special reference to the British fauna. Trans. R. Entomol. Soc. Lond. 1974, 125, 257–294. [Google Scholar] [CrossRef]
- Kostjukov, V.V. Comparative morphology of chalcids of the subfamily Tetrastichinae and the systematics of the genus Tetrastichus Haliday 1844 (Hymenoptera, Eulophidae). Entomol. Obozr. 1977, 56, 177–194. [Google Scholar]
- Kostjukov, V.V. On the occurrence of the genus Ceratoneura Ashmead (Hymenoptera, Chalcidoidea, Eulophidae) in Europe. In New and Little Known Species of Insects of the European Part of the USSR, 2nd ed.; Tobias, V.I., Richter, V.A., Eds.; Zoological Institute Academy of sciences of the USSR: Leningrad, Russia, 1977; pp. 93–96. (In Russian) [Google Scholar]
- Kostjukov, V.V. A new chalcid species of the genus Ceratoneura Ashmead (Hymenoptera, Eulophidae, Tetrastichinae) from the Primorye Territory. Tr. Zool. Inst. Proc. Zool. Inst 1977, 70, 125–127. (In Russian) [Google Scholar]
- Kostjukov, V.V. Superfam. Chalcidoidea. 46. Sem. Eulophidae. In Keys to Insects of the Russian Far East. Neuropteroidea, Mecoptera, Hymenoptera; Ler, P.A., Ed.; Dalnauka: Vladivostok, Russia, 2000; Volume 4, Part 4; pp. 578–581. ISBN 5-8044-0031-2. (In Russian) [Google Scholar]
- Yoshimoto, C.M. The North American species of the genus Achrysocharoides (Hymenoptera: Eulophidae). Can. Entomol. 1977, 109, 907–930. [Google Scholar] [CrossRef]
- Yoshimoto, C.M. Review of the North American Pnigalio Schrank (Hymenoptera: Eulophidae). Can. Entomol. 1983, 115, 971–1000. [Google Scholar] [CrossRef]
- Trjapitzin, V.A. Family Eulophidae. In Key to Insects of the European Part of the USSR. Hymenoptera; Medvedev, G.S., Ed.; Nauka: Leningrad, Russia, 1978; Volume 3, Part 2; pp. 381–467. (In Russian) [Google Scholar]
- Bryan, G. The British species of Achrysocharoides (Hymenoptera, Eulophidae). Syst. Entomol. 1980, 5, 245–262. [Google Scholar] [CrossRef]
- Hansson, C. Taxonomic notes on the genus Achrysocharoides Girault, 1913 (Hymenoptera: Eulophidae), with a redescription and a description of a new species. Entomol. Scand. 1983, 14, 281–291. [Google Scholar] [CrossRef]
- Hansson, C. The Entedontine Genera Achrysocharoides Girault, Chrysocharis Förster and Kratoysma Bouček (Hymenoptera: Eulophidae) in the Oriental Region. Entomol. Scand. 1985, 16, 217–226. [Google Scholar] [CrossRef]
- Hansson, C. Taxonomy and biology of the Palaearctic species of Chrysocharis Forster, 1856 (Hymenoptera: Eulophidae). Entomol. Scand. Suppl. 1985, 26, 1–130. [Google Scholar]
- Hansson, C. New records of Swedish Eulophidae and Pteromalidae (Hymenoptera: Chalcidoidea), with data on host species. Entomol. Tidskr. 1987, 108, 167–173. [Google Scholar]
- Hansson, C. Revision of the New World species of Chrysocharis Forster (Hymenoptera: Eulophidae). Entomol. Scand. Suppl. 1987, 31, 3–86. [Google Scholar]
- Hansson, C. Revised key to the Nearctic species of Chrysocharis Förster (Hymenoptera: Eulophidae) including three new species. J. Hymenopt. Res. 1995, 4, 80–98. [Google Scholar]
- Hansson, C. Achrysocharoides Girault (Hymenoptera, Eulophidae) new to tropical America, with eight new species. ZooKeys 2012, 173, 79–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Storozheva, N.A. A key to Palaearctic species of the chalcid genus Sympiesis Förster, 1856 (Hymenoptera, Eulophidae). Entomol. Obozr. 1982, 61, 164–176. (In Russian) [Google Scholar]
- Schauff, M.E. Taxonomic study of the Nearctic species of Elacherus Spinola (Hymenoptera: Eulophidae). Proc. Entomol. Soc. Wash. 1985, 87, 843–858. [Google Scholar]
- Zhu, C.D.; Huang, D.W. A study of Chinese Elachertus Spinola (Hymenoptera: Eulophidae). Zool. Stud. 2001, 40, 317–354. [Google Scholar]
- Zhu, C.D.; LaSalle, J.; Huang, D.W. A study of Chinese Cirrospilus Westwood (Hymenoptera: Eulophidae). Zool. Stud. 2002, 41, 23–46. [Google Scholar]
- Yefremova, Z.A.; Myartseva, S.N. Eulophidae (Hymenoptera: Chalcidoidea) of Turkmenistan, with emphasis on the genera Elachertus Spinola, 1811 and Hyssopus Girault. Entomol. Mon. Mag. 2004, 140, 113–122. [Google Scholar]
- Cao, H.; La Salle, J.; Zhu, C. Chinese species of Pediobius Walker (Hymenoptera: Eulophidae). Zootaxa 2017, 4240, 1–71. [Google Scholar] [CrossRef]
- Papp, J. A survey of the European species of Apanteles Först. (Hymenoptera, Braconidae: Microgastrinae), VII. The carbonarius-, circumscriptus-, fraternus-, pallipes-, parasitellae-, vitripennis-, liparidis-, octonarius- and thompsoni-group. Ann. Hist-Nat. Mus. Natl. Hung. 1983, 75, 247–283. [Google Scholar]
- Tobias, V.I.; Belokobylskij, S.A.; Kotenko, A.G. Family Braconidae. In Key to Insects of the USSR European Part. Hymenoptera; Medvedev, G.S., Ed.; Nauka: Leningrad, Russia, 1986; Volume 3, Part 4; pp. 3–500. (In Russian) [Google Scholar]
- Kotenko, A.G. Subfam. Microgastrinae. In Key to Insects of the Russian Far East. Neuropteroidea, Mecoptera, Hymenoptera; Lelej, A.S., Ed.; Dalnauka: Vladivostok, Russia, 2007; Volume 4, Part 5; pp. 134–192. (In Russian) [Google Scholar]
- Gibson, G.A.P. Morphology and Terminology. In Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera), 3rd ed.; Gibson, G.A.P., Huber, J.T., Woolley, J.B., Eds.; National Research Council Research Press: Ottawa, ON, Canada, 1997; pp. 16–44. [Google Scholar]
- Belokobylskij, S.A.; Maeto, K. Doryctinae (Hymenoptera, Braconidae) of Japan; Warshawska Drukarnia Naukowa: Warszawa, Poland, 2009; pp. 1–806. [Google Scholar]
- Van Achterberg, C. Illustrated key to the subfamilies of the Braconidae (Hymenoptera: Ichneumonoidea). Zool. Verh. 1993, 283, 1–189. [Google Scholar]
- Bouček, Z.; Askew, R.R. Hym. Chalcidoidea. Palaearctic Eulophidae (excl. Tetrastichinae). In Index of Entomophagous Insects, 2nd ed.; Delucchi, V., Remaudière, G., Eds.; Le François: Paris, France, 1968; pp. 11–254. [Google Scholar]
- Yefremova, Z.A. Catalogue of the Eulophidae (Hymenoptera: Chalcidoidea) of Russia. Linz. Biol. Beitr. 2002, 34, 563–618. [Google Scholar]
- Belokobylskij, S.A.; Samartsev, K.G.; Il’inskaya, A.S. Annotated Catalogue of the Hymenoptera of Russia. Volume II. Apocrita: Parasitica; Zoological Institute RAS: St. Petersburg, Russia, 2019; 594p. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Erdős, J. Fauna Eulophidarum Hungariae generibus speciebusque novis aucta (Hymenoptera). Ann. Hist-Nat. Mus. Natl. Hung. 1961, 53, 471–489. [Google Scholar]
- Mason, W.R.M. The polyphyletic nature of Apanteles Foerster (Hymenoptera: Braconidae): A phylogeny and reclassification of Microgastrinae. Mem. Entomol. Soc. Can. 1981, 115, 1–147. [Google Scholar] [CrossRef]
- Yu, D.S.K.; van Achterberg, C.; Horstmann, K. Taxapad 2016, Ichneumonoidea 2015. Database on Flash-Drive (CD).
- Papp, J. Braconidae (Hymenoptera) from Korea. XII. Acta Zool. Hung. 1990, 36, 87–119. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).