Evaluation of the “Bottleneck” Effect in an Isolated Population of Microtus hartingi (Rodentia, Arvicolinae) from the Eastern Rhodopes (Bulgaria) by Methods of Integrative Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Morphology
2.1.1. Morphology and Morphometry
2.1.2. Statistical Analysis
2.1.3. High-Resolution X-ray Computed Microtomography
2.2. Molecular Analysis
2.2.1. Sampling and Laboratory Techniques
2.2.2. Phylogenetic Analysis
2.3. Hybridization
3. Results
3.1. Morphology and Morphometric Analysis
3.1.1. Exterior
3.1.2. Morphometric Analysis
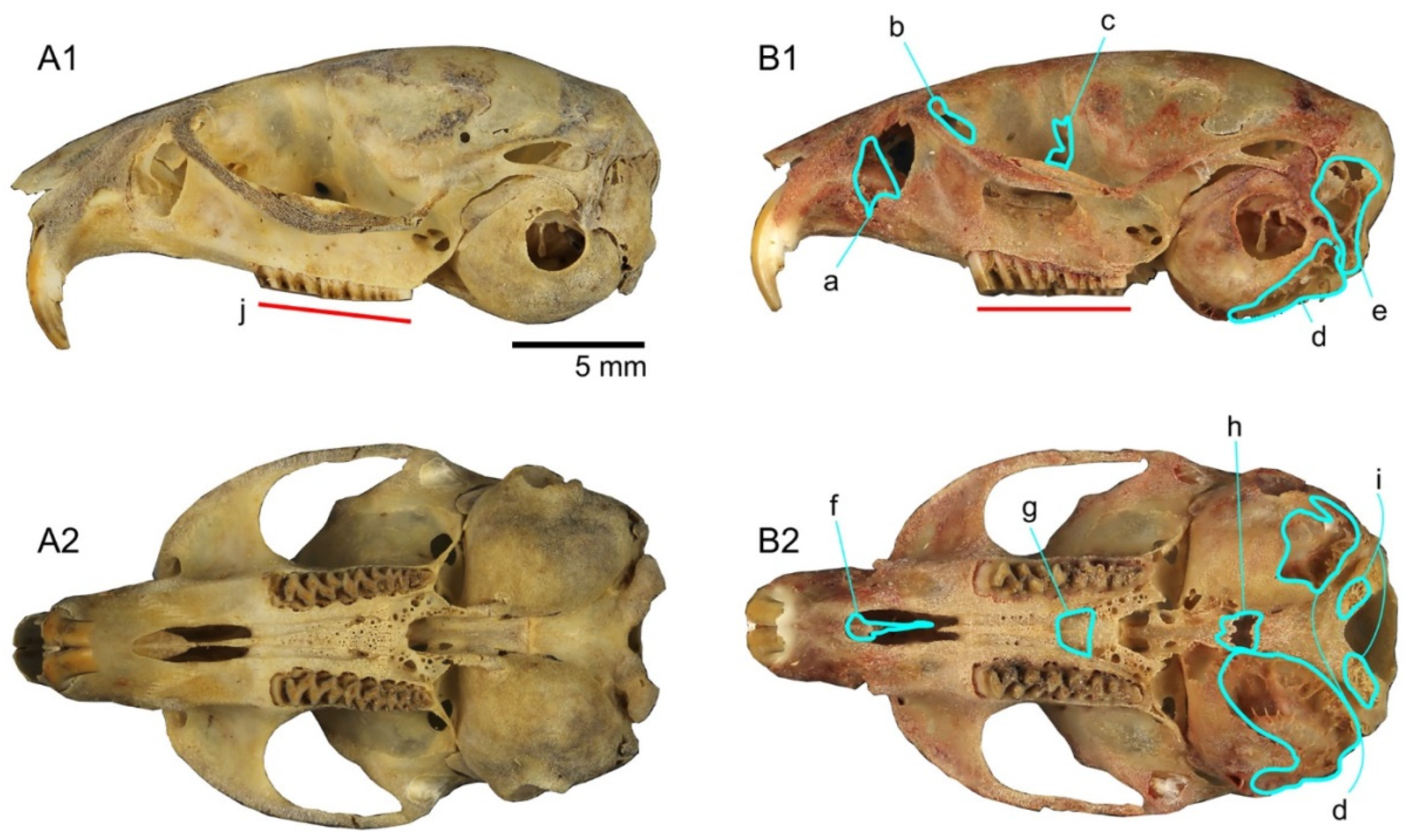
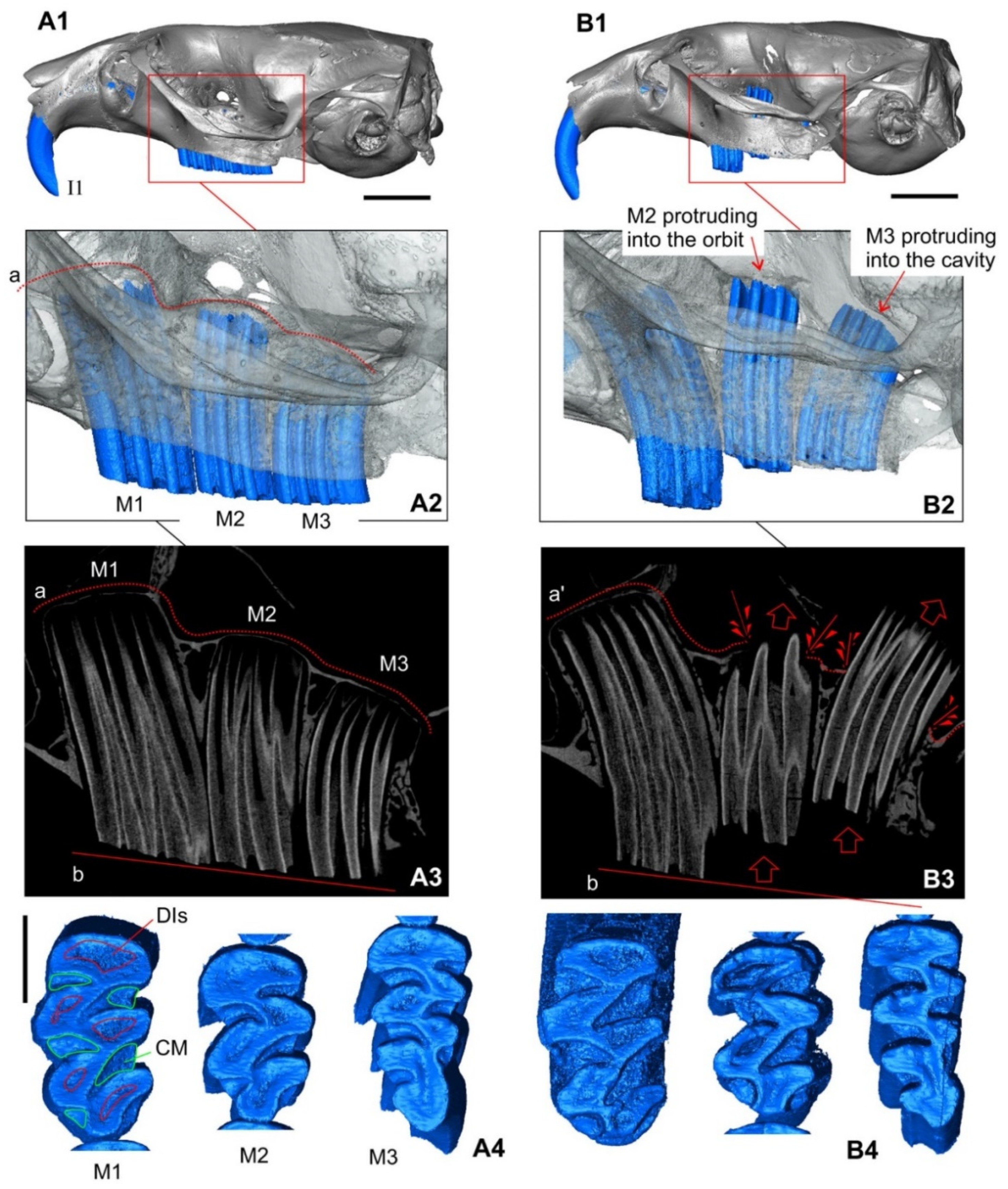
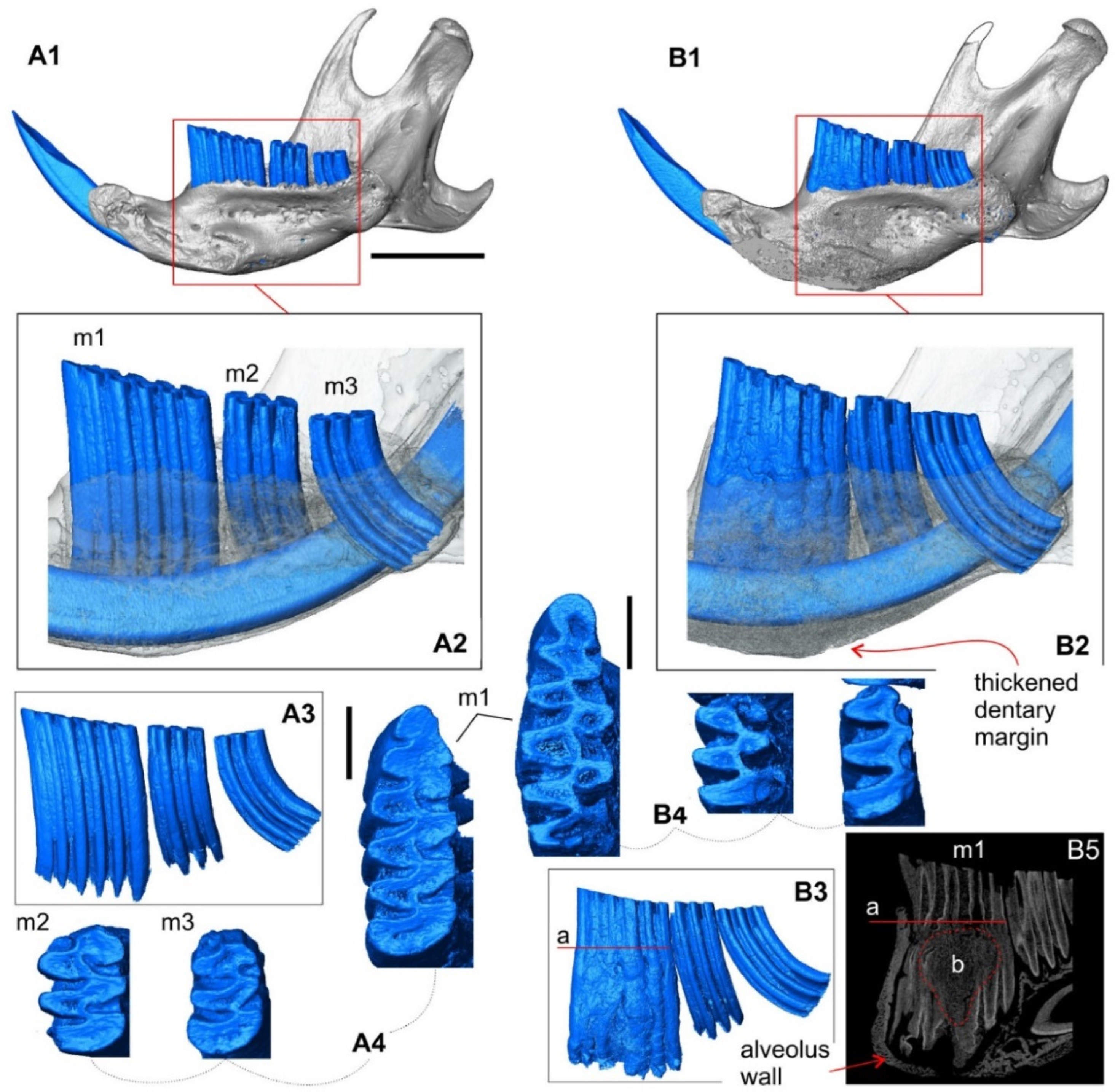
3.1.3. The Skull: Visual Comparisons and Computed Microtomographic Analysis
3.1.4. The Baculum
3.2. Analysis of Cytb Gene Variation
3.3. Experimental Hybridization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kryštufek, B.; Bužan, E.V.; Vohralík, V.; Zareie, R.; Özkan, B. Mitochondrial cytochrome b sequence yields new insight into the speciation of social voles in south-west Asia. Biol. J. Linn. Soc. 2009, 98, 121–128. [Google Scholar] [CrossRef]
- Yiğit, N.; Çolak, E. On the distribution and taxonomic status of Microtus guentheri (Danford and Alston; 1880) and Microtus lydius Blackler; 1916 (Mammalia: Rodentia) in Turkey. Turk. J. Zool. 2002, 26, 197–204. [Google Scholar]
- Golenishchev, F.N.; Abramson, N.I. New data on phylogeography of voles’ subgenus Sumeriomys (Arvicolinae; Rodentia). In Proceedings of the International Conference “Mammals of Russia and Adjacent Territories” IX Congress of Theriological Society RAS, Moscow, Russia, 1–4 February 2011; KMK Scientific Press: Moscow, Russia, 2011; p. 117. (In Russian). [Google Scholar]
- Golenishchev, F.N.; Malikov, V.G. The systematics and distribution of the grey voles of the tribe Microtini (Rodentia; Arvicolinae) in the Caucasus and Asia Minor. In Proceedings of the International Conference “Biological Diversity and Conservation Problems of the Fauna of the Caucasus”, Yerevan, Armenia, 26–29 September 2011; LLC Spika: Yerevan, Armenia, 2011; pp. 101–105. (In Russian). [Google Scholar]
- Zorenko, T.A.; Atanasov, N.; Golenishchev, F.N. Behavioral differentiation and hybridization of the European and Asian forms of Harting’ vole Microtus hartingi (Rodentia; Arvicolinae). Russian J. Theriol. 2016, 15, 133–150. [Google Scholar] [CrossRef]
- Chassovnikarova, T.G.; Markov, G.G.; Atanasov, N.I.; Dimitrov, H.A. Sex chromosome polymorphism in Bulgarian populations of Microtus guentheri (Danford & Alston; 1880). J. Nat. Hist. 2008, 42, 261–267. [Google Scholar] [CrossRef]
- Zorenko, A.; Kagainis, U.; Baraškova, Ļ. Does the geometric and linear morphometry of the brain reflect the divergence in the “guentheri” group (Arvicolinae; Sumeriomys). Russian J. Theriol. 2020, 19, 45–57. [Google Scholar] [CrossRef]
- Zorenko, T.A.; Kagainis, U. Discrimination of the two subspecies of Microtus hartingi (Rodentia: Arvicolinae) by shape and linear features of the spermatozoon. Russian J. Theriol. 2021, 21, 143–157. [Google Scholar] [CrossRef]
- Zorenko, T.A. Communal reproduction of females of two subspecies of Harting’s vole; Microtus (Sumeriomys) hartingi (Rodentia; Arvicolinae); under experimental conditions. Zool. J. 2022, 101, 1048–1060. (In Russian) [Google Scholar]
- Kryštufek, B.; Shenbrot, G.I. Voles and Lemmings (Arvicolinae) of the Palaearctic Region; Maribor University Press: Maribor, Slovenia, 2022; p. 436. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Abramson, N.I.; Lebedev, V.S.; Tesakov, A.S.; Bannikova, A.A. Supraspecies relationships in the subfamily Arvicolinae (Rodentia, Cricetidae): An unexpected result of nuclear gene analysis. Mol. Biol. 2009, 4, 834–846. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor andanalysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Yiğit, N.; Çetintürk, D.; Çolak, E. Phylogenetic assessment of voles of the Guentheri Group (Mammalia: Microtus) in Turkish Thrace and Western Anatolia. Eur. Zool. J. 2017, 84, 252–260. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Golenishchev, F.N.; Sablina, O.V.; Borodin, P.M.; Gerasimov, S. Taxonomy of voles of the subgenus Sumeriomys Argyropulo; 1933 (Rodentia; Arvicolinae; Microtus). Russ. J. Theriol. 2002, 1, 43–55. [Google Scholar] [CrossRef]
- Tabur, M.A.; Uğur, E.D.; Albayrak, I. Taxonomical and Karyological Features of Microtus hartingi (Barrett-Hamilton; 1903) (Mammalia: Rodentia) with Some Biological and Ecological Features. Pakistan J. Zool. 2015, 47, 691–698. Available online: http://acikerisim.sdu.edu.tr/xmlui/handle/123456789/62813 (accessed on 26 November 2021).
- Yiğit, N.; Markov, G.; Colak, E.; Kocheva, M.; Saygili, F.; Yuce, D.; Cam, P. Phenotypic features of the “guentheri” group Vole (Mammalia: Rodentia) in Turkey and Southeast Bulgaria: Evidence for its taxonomic detachment. Acta Zool. Bulg. 2012, 64, 23–32. [Google Scholar]
- Abramson, N.I.; Bodrov, S.Y.; Bondareva, O.V.; Genelt-Yanovskiy, E.A.; Petrova, T.V. A mitochondrial genome phylogeny of voles and lemmings (Rodentia: Arvicolinae): Evolutionary and taxonomic implications. PLoS ONE 2021, 16, e0248198. [Google Scholar] [CrossRef]
- Thanou, E.; Paragamian, K.; Lymberakis, P. Social but lonely: Species delimitation of social voles and the evolutionary history of the only Microtus species living in Africa. J. Zoolog. Syst. Evol. 2019, 58, 475–498. [Google Scholar] [CrossRef]
- Jeannet, M.; Fontana, L. Microtus (Sumeriomys) bifrons nov. sp. (Rodentia; Mammalia); a new vole in the French Upper Pleistocene identified at the Petits Guinards site (Creuzier-le-Vieux; Allier; France). Paleo Rev. D’archéologie Préhistorique 2015, 26, 59–77. [Google Scholar] [CrossRef]
- Tchernov, E. Succession of Rodent Faunas during the Upper Pleistocene of Israel. In Mammalia Depicta; Parey: Berlin, Germany, 1968; p. 152. [Google Scholar]
- Alpaslan, F.S. Some small mammal fossils of Üçağızlı Cave (Hatay; Turkey). Turk. J Zool. 2011, 35, 755–768. [Google Scholar] [CrossRef]
- Storch, G. Eine mittelpleistozäne Nager-Fauna von der Insel Chios, Ägäis (Mammalia: Rodentia). Senckenbergiana Biol. 1975, 56, 165–189. [Google Scholar]
- Santel, W.; Von Koenigswald, W. Preliminary report on the middle Pleistocene small mammal fauna from Yarimburgaz Cave in Turkish Thrace. EG Quat. Sci. J. 1998, 48, 162–169. [Google Scholar] [CrossRef]
- Masseti, M. Mammals of the Mediterranean islands: Homogenisationand the loss of biodiversity. Mammalia 2009, 73, 169–202. [Google Scholar] [CrossRef]
- Algan, O.; Çaǧatay, N.; Tchepalyga, A.; Ongan, D.; Eastoe, C.; Gökaşan, E. Stratigraphy of the Sediment Infil in Bosporus Strait: Water Exchange between the Black and Mediterranean Seas during the Last Glacial Holocene. J. Geophys. Res. 2001, 20, 209–218. [Google Scholar] [CrossRef]
- Çağatay, M.N.; Görür, N.; Algan, O.; Eastoe, C.; Tchapalyga, A.; Ongan, D.; Kuhn, T.; Kuşçu, I. Late Glacial-Holocene paleoceanography of the Sea of Marmara: Timing of connections with the Mediterranean and the Black seas. Mar. Geol. 2000, 167, 191–206. [Google Scholar] [CrossRef]
- Genov, I. Model of palaeoenvironmental evolution of the Black Sea region during the last glacial maximum—Holocene. Oceanology 2009, 49, 540–557. [Google Scholar] [CrossRef]
- Krijgsman, W.; Tesakov, A.; Yanina, T.; Lazarev, S.; Danukalova, G.; Van Baak, C.G.C.; Agustí, J.; Alçiçek, M.C.; Aliyeva, E.; Bista, D.; et al. Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution. Earth-Sci. Rev. 2019, 188, 1–40. [Google Scholar] [CrossRef]
- McHugh, C.M.G.; Gurung, D.; Giosan, L.; Ryan, W.B.F.; Mart, Y.; Sancar, U.; Buckles, L.; Çagatay, M.N. The last reconnection of the Marmara Sea (Turkey) to the World Ocean: A paleoceanographic and paleoclimatic perspective. Mar. Geol. 2008, 255, 64–82. [Google Scholar] [CrossRef]
- Markova, A.K.; van Kolfschoten, T.; Bohncke, S.J.P.; Kosintsev, P.A.; Mol, J.; Puzachenko, A.Y.; Simakova, A.N.; Smirnov, N.G.; Verpoorte, A.; Golovachev, I.B. Evolution of the European Ecosystems during Pleistocene—Holocene Transition (24–8 kyr BP); GEOS: Moscow, Russia, 2019; p. 279. [Google Scholar]
- Okay, N.; Okay, A. Tectonically induced Quaternary drainage diversion in the northeastern Aegean. J. Geol. Soc. 2002, 159, 393–399. [Google Scholar] [CrossRef]
- Markov, G.; Dimitrov, H. Habitat fragmentation and its implications for abundance of Guenther’s vole in southeastern Bulgaria (Strandzha mountain region). Biotechnolology Biotechnol. Equip. 2010, 24, 679–682. [Google Scholar] [CrossRef]
- Kryštufek, B.; Zorenko, T.; Bontzorlos, V.; Mahmoudi, A.; Atanasov, N.; Ivajnšič, D. Incipient road to extinction of a keystone herbivore in south-eastern Europe: Harting’s vole (Microtus hartingi) under climate change. Clim. Chang. 2018, 149, 443–456. [Google Scholar] [CrossRef]
- Lacey, E.A.; Sherman, P.W. Cooperative breeding in naked mole-rats: Implications for vertebrate and invertebrate sociality. In Cooperative Breeding in Mammals; Solomon, N.G., French, J.A., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 267–301. [Google Scholar] [CrossRef]
- Joly, E. Essay: On the close relationship between speciation; inbreeding and recessive mutations. Nat. Preced. 2010. [Google Scholar] [CrossRef]
- Jheon, A.H.; Prochazkova, M.; Sherman, M.; Manoli, D.S.; Shah, N.M.; Carbone, L.; Klein, O. Spontaneous emergence of overgrown molar teeth in a colony of Prairie voles (Microtus ochrogaster). Int. J. Oral Sci. 2015, 7, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Imai, D.M.; Pesapane, R.; Conroy, C.J.; Alarcon, C.N.; Allan, N.; Okino, R.A.; Fung, J.; Murphy, B.G.; Verstraete, F.J.M.; Foley, J.E. Apical elongation of molar teeth in captive Microtus voles. Vet. Pathol. 2018, 55, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.B.; Alworth, L.C.; Blas-Machado, U. Molar malocclusions in pine voles (Microtus pinetorum). Am. Assoc. Lab. Anim. Sci. 2009, 48, 412–415. [Google Scholar]
- Imbschweiler, I.; Schauerte, N.; Henjes, C.; Fehr, M.; Baumgärtner, W. Odontogenic dysplasia in the molar teeth of steppe lemmings (Lagurus lagurus). Vet. J. 2011, 188, 365–368. [Google Scholar] [CrossRef]
- Sugita, S.; Uchiumi, O.; Fujiwara, K.; Shumpei, N.S.; Katsuhiro, F.K. Brain deformation caused by hyperplasia molar teeth (macrodonts) in the Japanese field vole (Microtus montebelli). Exp. Anim. 1995, 43, 769–772. [Google Scholar] [CrossRef][Green Version]
- Fraguedakis-Tsolis, S.E.; Chondropoulos, B.P.; Stamatopoulos, C.V.; Giokas, S. Morphological variation of the five vole species of the genus Microtus (Mammalia; Rodentia; Arvicolinae) occurring in Greece. Acta Zool. 2009, 90, 254–264. [Google Scholar] [CrossRef]
- Aksenova, T.G. Comparative morphological analysis of the baculum structure of the voles from tribe Microtini (Rodentia, Cricetidae). Communication 1. Genus Lasiopodomys, Chionomys, Microtus (subgenus Microtus). Tr. Zool. Inst. RAN 1980, 99, 62–77. (In Russian) [Google Scholar]
- Zhabin, S.G.; Trechenkov, E.A.; Artifeksov, S.B.; Pavlenko, I.I.; Polukarov, A.N. Comparative evaluation of sperm DNA fragmentation and other sperm parameters. Probl. Reproduktsii 2015, 21, 121–124. [Google Scholar] [CrossRef]
- Bikchurina, T.I.; Golenishchev, F.N.; Kizilova, E.A.; Mahmoudi, A.; Borodin, P.M. Reproductive isolation between taxonomically controversial forms of the gray voles (Microtus, Rodentia; Arvicolinae): Cytological mechanisms and taxonomical implications. Front. Genet. 2021, 12, 653837. [Google Scholar] [CrossRef] [PubMed]
- Thanou, E.; Tryfonopoulos, G.; Chondropoulos, B.; Fraguedakis-Tsolis, S. Comparative phylogeography of the five Greek vole species infers the existence of multiple South Balkan subrefugia. Ital. J. Mammal. 2012, 79, 363–376. [Google Scholar] [CrossRef]
- Golenishchev, F.N.; Malikov, V.G.; Nazari, F.; Vaziri, S.; Sablina, O.V.; Polyakov, A.V. New species of vole of “guentheri” group (Rodentia; Arvicolinae; Microtus) from Iran. Russ. J. Theriol. 2003, 1, 117–123. [Google Scholar] [CrossRef]
- Zima, J.; Arslan, A.; Benda, P.; Macholán, M.; Kryštufek, B. Chromosomal variation in social voles: A robertsonian fusion in Günther’s vole. Acta Theriol. 2013, 58, 255–265. [Google Scholar] [CrossRef]
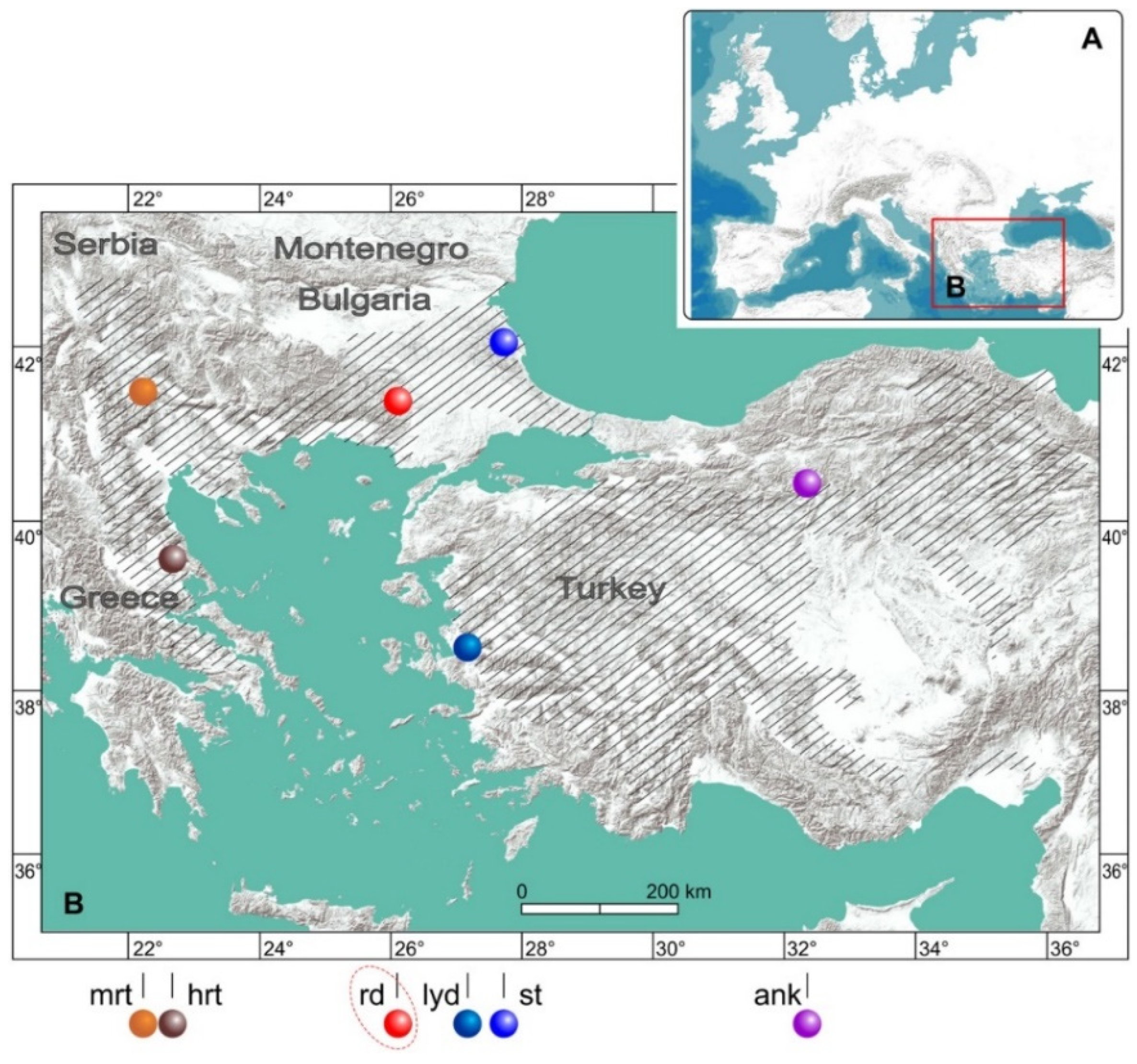



| Species/Subspecies | Locality | Genbank Accession Numbers |
|---|---|---|
| Microtus h. hartingi | Greece | FJ641110, FJ641116, FJ641119–FJ641121, FJ641127–FJ641130, FJ641132, FJ641137 |
| Microtus h. hartingi | Greece (south and Lesbos) | MK631990–MK631993 |
| M. h. ankaraensis | Turkey, Anatolia | FJ767745–FJ767747, FJ767751, FJ767752, ON324051 |
| M. h. martinoi | North Greece, northern Macedonia | AY513804, FJ767744 |
| M. h. strandzensis | Turkey, Thrace | 6488* |
| M. hartingi ssp.? | Bulgaria, the Rhodopes | ON324049, ON324050 |
| Northeastern Greece | FJ641135, FJ641136, FJ641141 | |
| M. dogramacii | Turkey | AY513793–AY513795, MK631997, MK631998 |
| M. qazvinensis | Iran | KM390973, KM390974, KM390979 |
| M. guentheri philistinus | Syria and Israel | AY513805–AY513807, FJ767743, KC953620, KC953621 MT381935, ON324052 |
| M. mustersi | Libya | MK631994–MK631996 |
| M. socialis | Georgia and Iran | AY513830, AY513831 |
| M. anatolicus | Turkey | FJ767740, FJ767741, FJ767742 |
| M. irani | Turkey and Iran | FJ767739, FJ767749, KM269337 |
| M. paradoxus | Turkmenistan | KC953622-KC953624 |
| M. schelkovnikovi | Azerbaijan | AM910619, KY754043 |
| M. arvalis | AM991041, AY220760, AY220762 | |
| M. rossiaemeridionalis | KU957996, AY513821, MRU54495 |
| M. hartingi (Rhodopy) (n = 13) m ± SE (Min–Max) | M. g. hartingi * | M. h. hartingi (n = 2) | M. h. strandzensis (n = 41) m ± SE (Min–Max) | M. h. martinoi (n = 2) | M. h. lydius * | M. h. lydius (n = 17) m ± SE (Min–Max) | M. h. ankaraensis (n = 28) m ± SE (Min–Max) | |
|---|---|---|---|---|---|---|---|---|
| L | 118 ± 1.34 (111–125) | 107 | 100–117 | 120.0 ± 1.13 (108–135) | 105–124 | 115 | 152 ± 3.1 (140–171) | 146 ± 3.5 (131–154) |
| C | 28.7 ± 1.32 (19–34) | 26 | 23–27 | 27.4 ± 0.47 (23–33) | 23.5–28 | 26 | 29.3 ± 1.1 (24–36) | 29.7 ± 1.7 (23–35) |
| PL | 20.0 ± 0.20 (19–21) | 18 | 19 | 18.6 ± 0.12 (17.5–20) | 2–20.4 | 18 | 21.3 ± 0.3 (20–23) | 22.2 ± 0.6 (20–24) |
| Au | 12.7 ± 0.18 (12–14) | - | 12–13 | 11.4 ± 0.12 (10–14) | 10.5–10.6 | 11 | 14.0 ± 0.4 (12–17) | 13.7 ± 0.9 (10–16) |
| CBL | 30.1 ± 0.54 (26.2–32.1) | 29.5 | 28,5 | 29.3 ± 0.16 (27.4–32.4) | 26.3–29.2 | 26.6 | 29.0 ± 0.31 (26.6–31.1) | 29.3 ± 0.22 (27.0–31.3) |
| ZB | 17.5 ± 0.58 (14.9–19.0) | 17.1 | 15–17 | 16.7 ± 0.11 (15.3–18.5) | 15.0–16.1 | 15.2 | 16.8 ± 0.27 (14.5–18.5) | 17.1 ± 0.17 (15.2–18.7) |
| DL | 9.3 ± 0.13 (8.2–9.8) | 9.0 | 7.8–8.2 | 9.2 ± 0.07 (8.4–9.2) | 7.8–8.1 | 8.7 | 8.8 ± 0.13 (7.9–9.7) | 8.6 ± 0.09 (7.9–9.8) |
| OB | 14.4 ± 0.24 (12.4–15.2) | 13.7 | 13.7–14.9 | 13.8 ± 0.08 (12.4–15.4) | 13.1–14.2 | - | 14.7 ± 0.14 (13.5–15.6) | 14.8 ± 0.11 (13.7–15.8) |
| OH | 9.3 ± 0.09 (8.9–9.9) | 8.9 | 7.8–7.9 | 8.1 ± 0.05 (7.6–8.7) | 8.7 | 8.1 | 8.2 ± 0.09 (7.5–8.9) | 8.6 ± 0.09 (7.6–9.8) |
| TOH | 11.2 ± 0.14 (10.1–11.7) | 10.1 | 10.3–10.8 | 10.4 ± 0.05 (9.6–11.2) | 10.3–11.2 | 9.2 | 10.8 ± 0.12 (10.0–11.8) | 10.9 ± 0.07 (10.3–11.9) |
| BB | 7.0 ± 0.12 (6.3–7.4) | 6.6 | 6.6–7.2 | 6.4 ± 0.04 (5.8–7.2) | 6.3–6.9 | 6.6 | 6.9 ± 0.07 (6.4–7.4) | 7.1 ± 0.05 (6.5–7.6) |
| BL | 8.5 ± 0.24 (6.8–9.7) | - | 8.5–9.2 | 8.1 ± 0.06 (7.4–8.8) | 7.7–9.0 | - | 8.4 ± 0.13 (7.8–9.5) | 8.6 ± 0.11 (7.4–9.6) |
| MBL | 9.7 ± 0.20 (8.4–10.7) | 9.0 | 9.5–9.8 | 9.4 ± 0.07 (8.4–10.4) | 8.7–9.3 | 8.9 | 9.6 ± 0.09 (8.8–10.0) | 9.8 ± 0.10 (8.7–11.0) |
| IB | 3.9 ± 0.04 (3.5–4.1) | 3.6 | 3.6 | 3.5 ± 0.013 (3.4–3.7) | 3.6–4.0 | 3.9 | 3.8 ± 0.05 (3.4–4.2) | 3.8 ± 0.03 (3.3–4.1) |
| RH | 7.1 ± 0.13 (6.2–7.9) | 6.9 | 6.4–7.0 | 7.0 ± 0.05 (6.4–7.7) | 5.9–7.5 | 6.2 | 7.0 ± 0.11 (6.1–7.7) | 7.1 ± 0.11 (6.2–8.4) |
| RB | 6.0 ± 0.08 (5.5–6.4) | 5.1 | 4.9–5.2 | 5.9 ± 0.04 (5.2–6.7) | 5.2–5.6 | 5.0 | 5.6 ± 0.10 (5.0–6.8) | 5.6 ± 0.05 (5.1–6.0) |
| M(1–3) | 7.2 ± 0.09 (6.5–7.7) | 6.7 | 7.1–7.3 | 6.8 ± 0.05 (6.2–7.6) | 6.3–6.7 | 6.4 | 7.0 ± 0.12 (6.2–8.1) | 7.0 ± 0.08 (6.0–7.9) |
| m(1–3) | 7.1 ± 0.13 (6.1–7.8) | 7.0 | 6.8–7.1 | 6.7 ± 0.04 (6.2–7.2) | 6.7–7.1 | 6.3 | 6.9 ± 0.12 (6.2–8.2) | 6.9 ± 0.08 (6.1–7.7) |
| ML | 18.1 ± 0.33 (15.4–19.5) | 17.9 | 17.2–17.3 | 17.7 ± 0.11 (16.5–19.7) | 16.5–18.3 | 16.7 | 18.4 ± 0.23 (16.7–19.9) | 18.5 ± 0.17 (16.6–20.1) |
| Taxon and Locality | n | Total Length | Shaft’s Length | Width of the Shaft’s Base | Length of the Trident’s Medial Ossicle (=Process) | Length of the Trident’s Lateral Ossicle (=Process) |
|---|---|---|---|---|---|---|
| M. hartingi (Rhodopes) | 2 | 5.30–5.32 | 3.52–3.72 | 1.63–2.21 | 1.58–1.74 | 1.20–1.25 |
| M. h. strandzensis (Gramatikovo, Bulgaria) * | 5 | 4.43 (4.15–4.75) | 3.15 (2.95–3.50) | 1.92 (1.55–2.30) | 1.24 (1.15–1.35) | 1.08 (1.05–1.15) |
| M. h. ankaraensis (Ankara, Turkey) ** | 14 | 2.9 ± 0.3 | 1.5 ± 0.3 | |||
| M. h. lydius (İzmir, Aydın, Turkey) ** | 7 | 2.3 ± 0.5 | 1.0 ± 0.4 | |||
| M. h. lydius (Isparta, Turkey) *** | 11–12 | 4.18 ± 0.26 (3.58–4.68) | 2.82 ± 0.14 (2.54–3.15) | 1.33 ± 0.17 (1.05–1.55) |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1 M. hartingi hartingi | 0.015 | |||||
| 2 M. h. ankaraensis | 0.019 | 0.008 | ||||
| 3 M. hartingi Rodopes and N-E Greece | 0.018 | 0.018 | 0.012 | |||
| 4 M. dogramacii | 0.048 | 0.045 | 0.046 | 0.004 | ||
| 5 M. qazvinensis | 0.052 | 0.051 | 0.050 | 0.028 | 0.008 | |
| 6 M. guentheri | 0.065 | 0.057 | 0.069 | 0.049 | 0.056 | 0.004 |
| Crossbreeding Types | Number of Pairs | Breeding Pairs (%) | Days Before Birth of Offspring | Number of Newborn Pups | Average Number of Newborns per Litter | Average Number of Surviving Pups | Mortality Through the 45th Day |
|---|---|---|---|---|---|---|---|
| ♂ A × ♀ A | 35 | 100 | 31.2 ± 1.29 | 158 | 4.5 ± 0.26 | 4.2 ± 0.23 | 7.6 |
| ♂ H × ♀ H | 35 | 100 | 26.2 ± 0.58 | 189 | 5.4 ± 0.21 | 5.2 ± 0.23 | 2.7 |
| ♂ H × ♀ A | 11 | 100 | 31.3 ± 4.40 | 42 | 3.8 ± 0.63 | 2.7 ± 0.51 | 25.6 |
| ♂ A × ♀ H | 13 | 100 | 29.3 ± 2.27 | 65 | 4.6 ± 0.34 | 3.9 ± 0.52 | 33.1 |
| ♂ A × ♀ F1 (♂ A × ♀ H) | 9 | 77.8 | 38.3 ± 4.54 | 32 | 4.6 ± 0.57 | 4.1 ± 0.40 | 6.1 |
| ♂ A × ♀ F1 (♂ H × ♀ A) | 3 | 100 | 24.3 ± 0.33 | 11 | 3.7 ± 0.88 | 2.7 ± 0.67 | 23.3 |
| ♂ F1 (♂ A × ♀H) × ♀ A | 5 | 20 | 27.0 | 8 | 8 | 8 | 0 |
| ♂ F1 (♂ H × ♀ A) × ♀ A | 6 | 33.3 | 32.0 ± 6.0 | 7 | 3.5 ± 0.50 | 0 | 100 |
| ♂ H × ♀ F1 (♂ H × ♀ A) | 7 | 83.3 | 32.0 ± 5.38 | 24 | 4.8 ± 0.37 | 4.6 ± 0.40 | 4 |
| ♂ H × ♀ F1 (♂ A × ♀ H) | 5 | 40 | 25.5 ± 0.50 | 9 | 4.5 ± 0.50 | 4.5 ± 0.37 | 0 |
| ♂ F1 (♂ H × ♀ A) × ♀ H | 10 | 50 | 38.2 ± 7.79 | 25 | 5.0 ± 0.71 | 2.8 ± 1.39 | 53.4 |
| ♂ F1 × ♀ H (♂A × ♀H) | 8 | 50 | 32.2 ± 4.07 | 19 | 4.8 ± 0.75 | 2.5 ± 1.19 | 54.2 |
| ♂ F1 × ♀ F1 (♂ A × ♀ H) | 17 | 58.8 | 42.5 ± 5.38 | 46 | 4.6 ± 0.27 | 3.6 ± 0.50 | 22.5 |
| ♂ F1 × ♀ F1 (♂ H × ♀ A) | 11 | 45.5 | 31.0 ± 3.75 | 24 | 4.8 ± 0.80 | 4.4 ± 0.75 | 3.3 |
| ♂ F2 × ♀ F2 (♂ H × ♀ A) | 10 | 60 | 41.8 ± 3.41 | 29 | 4.8 ± 0.31 | 4.2 ± 0.40 | 14.5 |
| ♂ B1 × ♀ B1 | 15 | 46.7 | 42.0 ± 4.71 | 31 | 4.4 ± 0.48 | 3.7 ± 0.64 | 16.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golenishchev, F.N.; Zorenko, T.A.; Petrova, T.V.; Voyta, L.L.; Kryuchkova, L.Y.; Atanasov, N. Evaluation of the “Bottleneck” Effect in an Isolated Population of Microtus hartingi (Rodentia, Arvicolinae) from the Eastern Rhodopes (Bulgaria) by Methods of Integrative Analysis. Diversity 2022, 14, 709. https://doi.org/10.3390/d14090709
Golenishchev FN, Zorenko TA, Petrova TV, Voyta LL, Kryuchkova LY, Atanasov N. Evaluation of the “Bottleneck” Effect in an Isolated Population of Microtus hartingi (Rodentia, Arvicolinae) from the Eastern Rhodopes (Bulgaria) by Methods of Integrative Analysis. Diversity. 2022; 14(9):709. https://doi.org/10.3390/d14090709
Chicago/Turabian StyleGolenishchev, Fedor N., Tanya A. Zorenko, Tatyana V. Petrova, Leonid L. Voyta, Lyudmila Yu. Kryuchkova, and Nasko Atanasov. 2022. "Evaluation of the “Bottleneck” Effect in an Isolated Population of Microtus hartingi (Rodentia, Arvicolinae) from the Eastern Rhodopes (Bulgaria) by Methods of Integrative Analysis" Diversity 14, no. 9: 709. https://doi.org/10.3390/d14090709
APA StyleGolenishchev, F. N., Zorenko, T. A., Petrova, T. V., Voyta, L. L., Kryuchkova, L. Y., & Atanasov, N. (2022). Evaluation of the “Bottleneck” Effect in an Isolated Population of Microtus hartingi (Rodentia, Arvicolinae) from the Eastern Rhodopes (Bulgaria) by Methods of Integrative Analysis. Diversity, 14(9), 709. https://doi.org/10.3390/d14090709







