Abstract
The Sea Slug Census program in Australia engages with citizen scientists to record the diversity and distribution of sea slugs across multiple locations. The program has consistently recorded shifts in distribution patterns but a recent, nine-day census in subtropical eastern Australia recorded unprecedented range extensions of tropical species. Seven species (six chromodorids and one polycerid) were found further south of their previously known distribution with Hypselodoris bertschi being recorded for the first time in Australia. These observations suggested the recent transport of larvae via the East Australian Current with recruitment to coastal sites possibly promoted by a protracted period of strong onshore winds associated with the 2021/22 La Niña in the western Pacific. With the increasing frequency of poleward range extensions of marine taxa, citizen science programs such as the Sea Slug Census provide the opportunity to substantially increase monitoring efforts. Linking with iNaturalist strengthens the value of the observations through online peer review to confirm species identities as well as the incorporation of substantiated (Research Grade) records into international biodiversity databases such as GBIF.
1. Introduction
Changes in the distribution patterns of species are occurring at unprecedented rates across the globe as anthropogenic effects, especially climate change, modify natural habitats and environmental conditions [1,2,3]. With these accelerating rates of change, arguably, there has never been a more important time to recruit members of the broader community to help quantify change. The importance of observations by community members as citizen scientists has long been recognized for terrestrial habitats but, possibly due to the comparative difficulty of observations and limited access for many would-be participants, marine-based citizen science programs lag behind their terrestrial counterparts. However, a range of recent programs has been implemented to encourage marine observations. The Sea Slug Census (SSC) is one such program. Commencing in Nelson Bay, New South Wales (NSW), Australia, in December 2013, participants (scuba divers, snorkelers, rock-pool ramblers) simply photograph each species of sea slug they encounter over nominated spatial and temporal scales (equivalent to a focused “bioblitz”) and submit them to the event organisers [4]. Species records are added to the program database and a report illustrating all species found is distributed to all participants and made more widely available through various web and social media sites (primarily the Sea Slug Census site on Facebook (Meta)). The popularity of the program, especially amongst scuba divers, has led to its expansion to 11 locations within Australia as well as sites in Indonesia and Vanuatu.
Motivations for participating stem not only from the fact that many species in the focal taxonomic group (Heterobranchia) are highly photogenic (e.g., the colourful nudibranchs) but also because participants are keen to monitor the health of their local marine habitats [4]. As most marine heterobranch species have relatively short lifespans and often highly specific food and habitat requirements [5,6], they have been hypothesised to be sensitive to environmental change, detectable through changes in species presence and distribution (e.g., [7,8,9]). This is supported through several of the earlier observations in the SSC program with citizen scientists providing a number of observations of range extensions across NSW [10,11,12,13,14].
There are a number of important considerations when establishing a citizen science program (reviewed by [15]). Providing a program that is appealing to potential participants is a key consideration and, for marine volunteers, additional motivations primarily relate to increasing their own knowledge whilst adding to the accumulation of scientific knowledge [16]. However, at the other end of the data collection process, in order for the observations to have value outside the specific and often geographically restricted project, it is essential that identifications are accurate and available to a wider audience. Until recently (2021), observations from the SSC program were identified by the program organisers with input from external experts where necessary and data were shared amongst the program participants. In order to make the program more globally relevant, from October 2021 (during the Great Southern Bioblitz of iNaturalist), participants were asked to register and submit photographic observations through iNaturalist (Available online: https://www.inaturalist.org/, Accessed on 1 March 2022). This platform is rapidly becoming one of the most important for collating observations of global biodiversity and provides not only a crowd-sourced review process for gaining consensus for identifications (termed Research Grade) but also open access to all observations that are also incorporated into the main global biodiversity databases (such as GBIF and, in Australia, Atlas of Living Australia) (e.g., [17]). This paper reports on specific observations submitted through iNaturalist as part of the January 2022 Coffs Coast Sea Slug Census within the Solitary Islands Marine Park (SIMP).
The SIMP lies on the subtropical east coast of Australia (Figure 1) and covers estuarine, shore and subtidal habitats. Marine communities comprise a mix of algal-dominated habitats close to shore [18] with increasing representation of more tropically affiliated species offshore [19,20]. The outer islands (North and South Solitary islands; Figure 1) are regularly influenced by the southward-flowing East Australian Current (EAC) and thus experience water temperatures that are 1–1.5 °C higher than nearshore locations [21]. The influence of the EAC is cited as a key reason for the dominance of hard corals around the mid-shelf and offshore islands with coral cover approaching that of more tropical locations at several sites [22]. Range extensions have been reported for a number of taxa over the past decade but, although there has been a progressive loss of macroalgal cover at a few mid-shelf sites [23], coral-dominated communities at the outer islands show no evidence of broadscale tropicalisation [24]. A thriving diving industry and a dedicated group of underwater volunteers [25] ensure that most main island sites are regularly visited with the consequent likelihood that novel or unusual species will be observed and reported. It is against this backdrop that we evaluated the list of taxa recorded during the recent Coffs Coast Sea Slug Census with a specific focus on species recorded for the first time and south of their previously documented range.
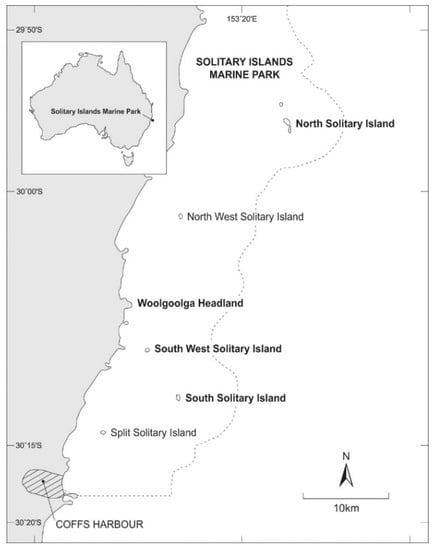
Figure 1.
The Solitary Islands Marine Park (boundary shown as a dotted line), subtropical eastern Australia. The geographical scope of the Coffs Coast Sea Slug Census included the entire marine park. Locations of observations for the seven species covered in this paper appear in bold font.
2. Materials and Methods
For the recent Coffs Coast Sea Slug Census, participants searched for and photographed sea slugs (Mollusca; Heterobranchia) from marine habitats over a 9-day period from 22–30 January 2022 with additional observations on 15 February 2022. There were no restrictions on the time of day for observations although only one search was conducted at night, on a coastal headland. The spatial scope of the study was the entire Solitary Islands Marine Park (Figure 1). Participants conducted searches of tidepools, snorkeled in shallow habitats and/or explored subtidal habitats via scuba and took pictures of sea slugs in situ. Species captured in images were collated through iNaturalist and identifications were crowd-sourced to reach consensus (Research Grade). These were also checked, along with distribution records, against a range of resources including reference books [26,27,28,29], websites covering sea slugs (e.g., Sea Slug Forum) and databases (GBIF) including those compiled from recent records in the SSC program [11,30,31].
A determination of an extension to a species range was made by reference to the databases of the authors as well as publications detailing sea slug distributions in NSW [10,11,12,13,14,30,31]. The taxonomic structure of this paper follows the World Register of Marine Species [32].
3. Results
SYSTEMATICS
Class: Gastropoda
Subclass: Heterobranchia
Order: Nudibranchia Cuvier, 1817
Family: Chromodorididae Bergh, 1891
Genus: Chromodoris Alder & Hancock, 1855
Chromodoris quagga Bonomo & Gosliner, 2020
(Figure 1)
Chromodoris quagga is similar to Chromodoris burni in colour and pattern; however, the presence of brown body pigment and the absence of white spots on the gills and rhinophores in C. quagga are diagnostic. Additionally, C. quagga is three times larger at ~35 mm compared with C. burni at 9–11 mm [33].
As a recently described species (in 2020), there is the potential for historic observations to remain unrecognised, potentially recorded as Chromodoris sp. in the literature and in online data repositories. Nevertheless, there have been several recent observations outside the Philippines (type location) and also in Indonesia, Papua New Guinea and New Caledonia [34].
In Australia, C. quagga has only been observed at South Solitary Island, NSW. It was first recorded by Steve Smith on 24 January 2019. Since then, two more animals have been recorded [34] (Figure 2). These observations from a single location made over a four-year period represent a poleward range extension of ~900 km from the nearest observation in New Caledonia (orthodromic distance between the observation latitudes 22°16′–30°6′) and the most southern global record of this species (Table 1).

Figure 2.
Chromodoris quagga, South Solitary Island, NSW, 29 January 2022. Photo: B. Touzell.

Table 1.
Distribution records of Chromodoris quagga in Oceania.
Genus: Hypselodoris Stimpson, 1855
Hypselodoris bertschi Gosliner & R. F. Johnson, 1999
(Figure 3)

Figure 3.
Hypselodoris bertschi, Woolgoolga Headland, NSW, 22 January 2022. The only observation of this species in Australia. Photo: S. D. A. Smith.
Hypselodoris bertschi is characterised by a translucent white body with rows of indistinct purple-blue elliptical spots alternating with opaque narrow white lines on the dorsum and a light blue foot margin. The rhinophores exhibit a median orange band and the gills have an orange rachis [35,36].
The recent (1999) description of H. bertschi helped to resolve the historic taxonomic instability associated with this species [37] by bringing into synonymy several confusing names dating back as far as 1860 [38]. However, the new species, H. bertschi, as currently accepted, and its synonymous taxa were considered to be restricted to the Hawaiian Islands, USA.
Since then, H. bertschi has been observed in Japan in 2001 and 2009 [39], French Polynesia in 2006 [40] and South Africa in 2007 and 2010 [40]. With only three records from the southern hemisphere across two locations, any observations may be regarded as noteworthy. An observation of a 15 mm individual in a coastal tidepool (depth 0.5 m) by Steve Smith at Woolgoolga Headland, NSW, on 22 January 2022 (Figure 3)—approximately 5900 km southwest of the nearest observation at Moorea, French Polynesia—represents not only the fourth record of this species in the southern hemisphere but also the first for Australian waters (Table 2).

Table 2.
South Pacific distribution records of Hypselodoris bertschi.
Hypselodoris imperialis (Pease, 1860)
(Figure 4)

Figure 4.
Hypselodoris imperialis, South Solitary Island, NSW, 24 January 2022. Photo: N. Fripp.
Hypselodoris imperialis is a large white nudibranch with an undulating dark blue mantle that occasionally broadens onto the dorsum into wide patches that contain yellow spots. Yellow spots are also scattered across the body. The gills are white and lined with blue [29,41].
This species, considered by several authors to be restricted to Hawaii and the Marshall Islands, USA and French Polynesia [29,41], has been frequently confused with Hypselodoris sp. 11 (Gosliner et al. [29]); however, the latter exhibits white gills lined with red [29].
Prior to January 2022, H. imperialis was observed only three times outside its Central Pacific range [42]: Papua New Guinea in 1998; Vanuatu in 2006; and Currimundi Reef, Sunshine Coast, QLD in 2019 (Table 3).

Table 3.
Selected Oceania distribution records of Hypselodoris imperialis.
Two observations of Hypselodoris imperialis were made in January 2022 at the Solitary Islands, NSW. The first was at South Solitary Island on 24 January 2022 of an 80 mm specimen photographed at a depth of 15 m by Nathan Fripp (Figure 4). The second, at North Solitary Island, NSW, was of a 60 mm specimen on 30 January 2022 photographed by Craig Lewis and Brett Touzell (Table 3).
These observations represent a 380 km southward range extension from the previous southernmost observation at Currimundi, QLD.
Hypselodoris sagamiensis (Baba, 1849)
(Figure 5)

Figure 5.
Hypselodoris sagamiensis, South Solitary Island, NSW, 25 January 2022. Photo: C. Lewis.
Hypselodoris sagamiensis has a translucent white body with opaque white patches on the mantle, occasionally raised into low pustules. Black spots may be distributed on the dorsum. The mantle exhibits a blue-purple margin that may be broken into lines or spots. There may also be a submarginal orange or yellow line, which may also be broken into spots. The rhinophore tips and gill edges are red or orange [44].
First described by Baba in 1949 (as Glossodoris sagamiensis) using type specimens collected by the Japanese Emperor at Sagami Bay, this species was considered to be restricted to Japanese waters until as recently as 2001 [44]. In 2006, Cobb and Willan [26] reported a putative first Australian observation of H. sagamiensis at Mooloolaba, QLD. However, 16 years earlier, in May 1990, H. sagamiensis had been photographed at Coffs Harbour, NSW, by Carol Buchanan but this image was only published in 2008 by Coleman [28] (p. 173) and therein mistakenly identified as Hypselodoris cf. bertschi. This observation was subsequently amended to H. sagamiensis in Coleman 2015 [27] (p. 149).
An observation of a 25 mm animal by Craig Lewis at a depth of 13 m at South Solitary Island on 25 January 2022 was the first observation of this species at its southern range limit for 32 years (Table 4).

Table 4.
Australian distribution records of Hypselodoris sagamiensis.
Genus: Goniobranchus Pease, 1866
Goniobranchus kuniei (Pruvot-Fol, 1930)
(Figure 6)

Figure 6.
Goniobranchus kuniei, South West Solitary Island, NSW, 23 January 2022. Photo: N. Fripp.
One of a group of similarly coloured mantle-flapping chromodorids, Goniobranchus kuniei was described from a specimen collected from the Isle of Pines, New Caledonia, from which it is named (kuni is the indigenous name for Île des Pins) [46]. This species is most similar in appearance to Goniobranchus geminus Rudman, 1987, which differs in the colour of the dorsal spots and marginal bands on the mantle. Goniobranchus kuniei has a broad Indo-West Pacific distribution from the Red Sea and Madagascar in the west to Tuamotu, French Polynesia, in the east and Okinawa, Japan, in the North.
In Australia, G. kuniei is known to occur on both the east and west coasts of Australia. In Western Australia it has been found as far south as Shark Bay [47,48]. In the east, it has been observed along much of the Queensland coast and offshore at Lord Howe Island, NSW [31,47]. However, the southernmost continental records are from the Sunshine Coast, QLD [43].
On 23 January 2022, a single 50 mm long specimen was observed at a depth of 12 m at South West Solitary Island, NSW, by Nathan Fripp (Figure 6). This observation represented a 400 km shift in the continental range from the Sunshine Coast, QLD, south into coastal NSW (Table 5).

Table 5.
Selected East Australian distribution records of Goniobranchus kuniei.
Goniobranchus rufomaculatus (Pease, 1871)
(Figure 7)

Figure 7.
Goniobranchus rufomaculatus, South Solitary Island, NSW, 26 January 2022. Photo: S. D. A. Smith.
Goniobranchus rufomaculatus has a white mantle with scattered yellow spots and three translucent patches of varying sizes between the gills and rhinophores. The gills are white and the mantle margin is edged with purple lines or spots [49]. It is very similar in appearance to Goniobranchus aureopurpureus Collingwood, 1881, but G. aureopurpureus lacks the translucent patches on the dorsum and the gills are a translucent purple or puce [50].
Pease described Goniobranchus rufomaculatus (as Chromodoris rufomaculata) using a specimen found under rocks in the intertidal zone at Huanine-iti in French Polynesia in 1871 [51]. It has an Indo-West Pacific distribution and has been recorded on both the east and west coasts of Australia. In Western Australia, it has been recorded at Dirk Hartog Island (26.15° S) and, in the east, at several location as far south as Lord Howe Island, NSW [31].
A specimen measuring 50 mm was observed at a depth of 8 m at South Solitary Island, NSW, on 24 January 2019 by Steve Smith. A specimen of the same size was also found and photographed by Steve Smith during the recent Coffs Coast Seas Slug Census at the same location (Figure 7). Similar to the observation of G. kuniei reported above, these observations represent a 400 km southward shift in the continental range from the Sunshine Coast, QLD (Table 6).

Table 6.
Selected distribution records of Goniobranchus rufomaculatus from Oceania.
Family: Polyceridae Alder & Hancock, 1845
Genus: Nembrotha Bergh, 1877
Nembrotha yonowae Goethel & Debelius, 1992
(Figure 8)

Figure 8.
Nembrotha yonowae, South Solitary Island, NSW, 15 February 2022. Photo: N. Fripp.
Nembrotha yonowae is a large polycerid with a dark brown or black body with orange pustules scattered across the mantle [53]. Described using specimens from the Maldives, it has an Indo-West Pacific distribution with records spanning east to Papua New Guinea and north to the Philippines [53].
In Australian waters, N. yonowae has been found in northern Western Australia at the remote Ashmore Reef in the Arafura Sea and on the east coast at Heron Island, GBR, QLD, as well as at Julian Rocks in northern NSW (Table 7).

Table 7.
Selected distribution records of Nembrotha yonowae from Oceania.
An observation of a 100 mm specimen by Nathan Fripp at a depth of 16 m at South Solitary Island on 15 February 2022 represented a 180 km southern range shift from its previous southernmost observation at Julian Rocks, NSW (Figure 8).
4. Discussion
The discovery of range extensions for seven species of tropical sea slug over a short survey period (9 days) during the Coffs Coast Sea Slug Census, and an additional dive 2 weeks later, is unprecedented within the SSC program. Although poleward range extensions have been sporadically recorded over the eight years of the program to date [13], the observations reported here were significant for a number of reasons. Firstly, Hypselodoris bertschi was recorded for the first time in Australia and at a nearshore, tidepool location (most previous records of range extensions have been at the offshore islands [13]). Secondly, the majority of new records were for species of Chromodorididae, a family with a predominantly tropical distribution comprising highly visible species that are unlikely to be overlooked by observers. Thus, most of these observations were highly likely to represent very recent additions to the local species pool. Two exceptions from the seven species reported here were Chromodoris quagga, which has now been recorded in three consecutive years at a single location, and Goniobranchus rufomaculatus, which has been recorded in three out the past four years and at two locations. Despite the repeated observations of these two species, there was no evidence of increased abundance and establishment of populations; all these records consequently fitted into the first stage of range extension, arrival (sensu [1]). As such, it is highly likely that the presence of these species was dependent on the transport of larvae from more northerly locations via the East Australian Current (EAC) [13,55]. To our knowledge, and from the records from the SSC program as well as historical datasets from various citizen scientists, we are unaware of any native tropically affiliated heterobranch species that have recently established populations in the Solitary Islands Marine Park (we note, however, the establishment of populations of the introduced and invasive aeolid nudibranch, Spurilla braziliana, throughout south-east Australia [56]).
The ability of a novel species to successfully recruit depends on a range of factors that include physico-chemical conditions, the presence of a suitable habitat (e.g., [57]) and food as well as biotic interactions with the local community (e.g., [2]). There is little doubt that the individuals observed here were not only surviving but also feeding sufficiently to reach sizes that were at, or greater than, the published size within their usual geographic range [29,58]. Indeed, the specimen of Hypselodoris imperialis recorded from South Solitary Island measured ~80 mm (extended crawl length), which was substantially greater than the published size (50 mm [58]). This suggests that suitable food resources were available for these taxa within the SIMP. Unfortunately, as so little is known about the feeding habits for many species of heterobranch sea slugs [5], we could not speculate on whether the survival and growth of these species were facilitated by the presence of a specific food source at the receiving sites, or the ability of the species to feed on a range of food sources. Clearly, species with catholic feeding requirements are more likely to be successful in recruiting to novel locations; information on feeding will, therefore, be useful for predicting the likely progression of range extensions from arrival to the establishment of populations.
The presence of Hypselodoris bertschi in a coastal tidepool, the first confirmed sighting in Australia, was perhaps the most interesting observation reported here. With a few exceptions [13], most previous novel records have come from observations at the offshore Solitary Islands, which are regularly influenced by the EAC, the likely source of tropical recruits to the region [55,59,60]. Incursions of the EAC across the continental shelf regularly occur but with considerable variations in terms of strength and duration [21]. These episodes are predicted to become more frequent with the progression of climate change [61,62]. Although speculative, the transport of larvae of H. bertschi to the coast, as well as the presence of the six other species at the islands, may have been facilitated by the strong onshore winds associated with the 2021/22 La Niña in the western Pacific (commencing in November 2021) [63]. The protracted period of onshore winds led to the stranding of a large number of plastic debris items that had clearly been at sea for a considerable period based on the extensive marine growth and patterns of degradation. These included items whose source could be traced to New Caledonia (based on embossing [64]). These ancillary observations confirmed the transport of surface waters to coastal waters in the months leading up to our observation period.
Although this study reports on a just a few observations of novel species in a geographically restricted area, it potentially has important implications for ongoing investigations of climate-driven range extensions and the role of citizen scientists. Subtropical regions have been suggested as being amongst the first to experience changes related to range-shifting species, potentially acting as refuges for taxa driven poleward by warming seas [65]. Although recent research has suggested that there has been little change in the biotic composition of key structural species such as corals over the past 25 years at the Solitary Islands [24], the observations reported here and previously [13] clearly show that novel species arrive regularly. However, monitoring species of all taxa that occur within the region is impractical. Our results suggest that heterobranch sea slugs, and especially nudibranchs from the family Chromodorididae, have the potential as a focus group, not only because observations of species in this taxon dominate our records of range extensions but also due to their popularity with recreational divers and naturalists, which can boost the search effort immensely [4]. One caveat is that a few species may be difficult to identify based solely on the external features captured in photographs [29] and mimicry is now known to occur in several species of chromodorid [66], necessitating circumspection in these cases. Programs such as the SSC can significantly contribute to documenting shifts in species distribution patterns, especially when linked with important databases. The recent association of the SSC program with iNaturalist has created a more powerful tool to monitor changing distributions and facilitated expert input to ensure that observations by participating citizen scientists effectively and accurately help update global species distribution patterns.
Author Contributions
Conceptualization, methodology, data curation, writing draft preparation, review and editing, S.D.A.S. and M.J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analysed in this study. These data can be found here: Available online. https://www.gbif.org/ (10 March 2022).
Acknowledgments
We thank all of the enthusiastic participants in the Sea Slug Census program for their passion and commitment to help document the diversity and distribution of sea slugs across the geographical range of the program. In particular, we would like to thank participants in the 2022 Coffs Coast Sea Slug Census with special mention to Nathan Fripp, Craig Lewis and Brett Touzell whose observations and photographs are featured in this paper. Determining the identity of species benefited from advice provided through the peer-review process on iNaturalist (especially from Hsini Lin, Erwin Koehler, Adrian Gale, Nick Lambert and Ben Travaglini), and advice from Marta Pola. The program was supported by members of the Solitary Islands Underwater Research Group and local businesses (Jetty Dive, Divequest Mullaway) who provided logistic support or small prizes as incentives for participants. Kathryn James prepared Figure 1. We are grateful to the three anonymous reviewers who helped improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bates, A.E.; Pecl, G.T.; Frusher, S.; Hobday, A.J.; Wernberg, T.; Smale, D.A.; Sunday, J.M.; Hill, N.A.; Dulvy, N.K.; Colwell, R.K. Defining and observing stages of climate-mediated range shifts in marine systems. Glob. Environ. Chang. 2014, 26, 27–38. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Gervais, C.R.; Champion, C.; Pecl, G.T. Species on the move around the Australian coastline: A continental-scale review of climate-driven species redistribution in marine systems. Glob. Chang. Biol. 2021, 27, 3200–3217. [Google Scholar] [CrossRef]
- Smith, S.D.A.; Davis, T.R. Slugging it out for science: Volunteers provide valuable data on the diversity and distribution of heterobranch sea slugs. Molluscan Res. 2019, 39, 214–223. [Google Scholar] [CrossRef]
- Rudman, W.B.; Willan, R.C. Opisthobranchia. The Southern Synthesis; Fauna of Australia; CSIRO: Melbourne, Australia, 1998. [Google Scholar]
- Smith, S.D.A.; Nimbs, M.J. Quantifying temporal variation in heterobranch (Mollusca: Gastropoda) sea slug assemblages: Tests of alternate models. Molluscan Res. 2017, 37, 140–147. [Google Scholar] [CrossRef]
- Goddard, J.H.R.; Gosliner, T.M.; Pearse, J.S. Impacts associated with the recent range shift of the aeolid nudibranch Phidiana hiltoni (Mollusca, Opisthobranchia) in California. Mar. Biol. 2011, 158, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.H.R.; Treneman, N.; Pence, W.E.; Mason, D.E.; Dobry, P.M.; Green, B.; Hoover, C. Nudibranch range shifts associated with the 2014 warm anomaly in the Northeast Pacific. Bull. South. Calif. Acad. Sci. 2016, 115, 15–40. [Google Scholar] [CrossRef]
- Schultz, S.T.; Goddard, J.H.R.; Gosliner, T.M.; Mason, D.E.; Pence, W.E.; McDonald, G.R.; Pearse, V.B.; Pearse, J.S. Climate-index response profiling indicates larval transport is driving population fluctuations in nudibranch gastropods from the northeast Pacific Ocean. Limnol. Oceanogr. 2011, 56, 749–763. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Smith, S.D.A. Welcome strangers: Southern range extensions for seven heterobranch sea slugs (Mollusca: Gastropoda) on the subtropical east Australian coast, a climate change hot spot. Reg. Stud. Mar. Sci. 2016, 8, 27–32. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Larkin, M.; Davis, T.R.; Harasti, D.; Willan, R.C.; Smith, S.D.A. Southern range extensions for twelve heterobranch sea slugs (Gastropoda: Heterobranchia) on the eastern coast of Australia. Mar. Biodivers. Rec. 2016, 9, 289. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Smith, S.D.A. Revision of the southern distribution limit for the tropical marine herbivore Syphonota geographica (A. Adams & Reeve, 1850) (Heterobranchia: Aplysiidae) in a global climate change hot-spot. Aust. Zool. 2017, 38, 582–589. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Smith, S.D.A. Beyond Capricornia: Tropical Sea Slugs (Gastropoda, Heterobranchia) Extend Their Distributions into the Tasman Sea. Diversity 2018, 10, 99. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Willan, R.C.; Smith, S.D.A. Range extensions for heterobranch sea slugs (formerly opisthobranch) belonging to the families Diaphanidae, Plakobranchidae and Facelinidae on the eastern coast of Australia. Mar. Biodivers. Rec. 2015, 8, e76. [Google Scholar] [CrossRef]
- Silvertown, J. A new dawn for citizen science. Trends Ecol. Evol. 2009, 24, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.Y.; Christidis, L.; Lloyd, D.J.; Pecl, G. Understanding drivers, barriers and information sources for public participation in marine citizen science. J. Sci. Commun. 2016, 15, A02. [Google Scholar] [CrossRef]
- Seltzer, C. Making Biodiversity Data Social, Shareable, and Scalable: Reflections on iNaturalist & citizen science. Biodivers. Inf. Sci. Stand. 2019, 3, e46670. [Google Scholar]
- Smith, S.D.A.; Simpson, R.D. Nearshore corals of the Coffs Harbour region, mid north coast, New South Wales. Wetl. Aust. 1991, 11, 1–9. [Google Scholar] [CrossRef]
- Malcolm, H.A.; Jordan, A.; Smith, S.D.A. Biogeographical and cross-shelf patterns of reef fish assemblages in a transition zone. Mar. Biodivers. 2010, 40, 181–193. [Google Scholar] [CrossRef]
- Harrison, M.A.; Smith, S.D.A. Cross-shelf variation in the structure of molluscan assemblages on shallow, rocky reefs in subtropical, eastern Australia. Mar. Biodivers. 2012, 42, 203–216. [Google Scholar] [CrossRef]
- Malcolm, H.A.; Davies, P.L.; Jordan, A.; Smith, S.D.A. Variation in sea temperature and the East Australian Current in the Solitary Islands region between 2001–2008. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 616–627. [Google Scholar] [CrossRef]
- Harriott, V.J.; Smith, S.D.A.; Harrison, P.L. Patterns of coral community structure of subtropical reefs in the Solitary-Islands Marine Reserve, Eastern Australia. Mar. Ecol. Prog. Ser. 1994, 109, 67–76. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L.; Booth, D.J.; Coleman, M.A.; Feary, D.A. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. In Proceedings of the Royal Society B: Biological Sciences; The Royal Society: London, UK, 2014; Volume 281, p. 20140846. [Google Scholar]
- Mizerek, T.L.; Madin, J.S.; Benzoni, F.; Huang, D.; Luiz, O.J.; Mera, H.; Schmidt-Roach, S.; Smith, S.D.A.; Sommer, B.; Baird, A.H. No evidence for tropicalization of coral assemblages in a subtropical climate change hot spot. Coral Reefs 2021, 40, 1451–1461. [Google Scholar] [CrossRef]
- Hammerton, Z.; Dimmock, K.; Hahn, C.; Dalton, S.J.; Smith, S.D.A. Scuba diving and marine conservation: Collaboration at two Australian subtropical destinations. Tour. Mar. Environ. 2012, 8, 77–90. [Google Scholar] [CrossRef][Green Version]
- Cobb, G.; Willan, R.C. Undersea Jewels: A Colour Guide to Nudibranchs; ABRS: Canberra, Australia, 2006.
- Coleman, N. Nudibranchs Encyclopedia-Catalogue of Asia/Indo Pacific Sea Slugs, 2nd ed.; Neville Coleman’s Underwater Geographic: Springwood, Australia, 2015; ISBN 9780947325411.
- Coleman, N. Nudibranchs Encyclopedia; Neville Coleman’s Underwater Geographic: Springwood, Australia, 2008; ISBN 9780947325411.
- Gosliner, T.M.; Valdés, Á.; Behrens, D.W. Nudibranch and Sea Slug Identification: Indo-Pacific, 2nd ed.; New World Publications: Jacksonville, FL, USA, 2018; ISBN 978-1-878348-67-8. [Google Scholar]
- Nimbs, M.J.; Smith, S.D.A. An illustrated inventory of the sea slugs of New South Wales, Australia (Gastropoda: Heterobranchia). Proc. R. Soc. Vic. 2017, 128, 44–113. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Hutton, I.; Davis, T.R.; Larkin, M.F.; Smith, S.D.A. The heterobranch sea slugs of Lord Howe Island, NSW, Australia (Mollusca: Gastropoda). Proc. R. Soc. Vic. 2020, 132, 12–41. [Google Scholar] [CrossRef]
- Horton, T.; Kroh, A.; Ahyong, S.; Bailly, N.; Boyko, C.B.; Brandão, S.N.; Gofas, S.; Hooper, J.N.A.; Hernandez, F.; Holovachov, O.; et al. World Register of Marine Species (WoRMS) 2020; World Register of Marine Species: Ostend, Belgium, 2020. [Google Scholar]
- Bonomo, L.J.; Gosliner, T.M. Adding stars to the Chromodoris (Nudibranchia, Chromodorididae) galaxy with the description of four new species. Zootaxa 2020, 4819, zootaxa-4819. [Google Scholar] [CrossRef] [PubMed]
- GBIF. Chromodoris quagga Bonomo & Gosliner, 2020. Available online: https://www.gbif.org/species/10730273 (accessed on 16 February 2022).
- Gosliner, T.M.; Johnson, R.F. Phylogeny of Hypselodoris (Nudibranchia: Chromodorididae) with a review of the monophyletic clade of Indo-Pacific species, including descriptions of twelve new species. Zool. J. Linn. Soc. 1997, 125, 1–114. [Google Scholar] [CrossRef]
- Sea Slug Forum: Hypselodoris bertschi. Available online: http://www.seaslugforum.net/showall/hypsbert (accessed on 16 February 2022).
- Bertsch, H.; Gosliner, T.M. Chromodorid nudibranchs from the Hawaiian Islands. Veliger 1989, 32, 247–265. [Google Scholar]
- Pease, W.H. Descriptions of new species of Mollusca from the Sandwich Islands. Proc. Zool. Soc. Lond. 1860, 28, 18–36. [Google Scholar]
- Sea Slug Forum: Imamoto, J., Hypselodoris bertschi? from Japan. Available online: http://www.seaslugforum.net/find/5490 (accessed on 16 February 2022).
- GBIF. Hypselodoris bertschi Gosliner & R.F. Johnson, 1999. Available online: https://www.gbif.org/species/4596896 (accessed on 16 February 2022).
- Sea Slug Forum: Risbecia imperialis (Pease, 1860). Available online: http://www.seaslugforum.net/showall/risbimpe (accessed on 16 February 2022).
- GBIF. Hypselodoris imperialis (Pease, 1860). Available online: https://www.gbif.org/species/6788184 (accessed on 16 February 2022).
- Mullins, D.; Schubert, J.; Farr, T. Nudibranch Domain. Available online: https://nudibranchdomain.org/ (accessed on 16 February 2022).
- Sea Slug Forum: Hypselodoris sagamiensis (Baba, 1949). Available online: http://www.seaslugforum.net/showall/hypssaga (accessed on 16 February 2022).
- GBIF. Hypselodoris sagamiensis (Baba, 1949). Available online: https://www.gbif.org/species/6126803 (accessed on 16 February 2022).
- Pruvot-Fol, A. Diagnose provisoirse (imcompètes) des espèces nouvelles et liste provisoire des mollusques nudibranches recueillis par Mme. A. Pruvot-Fol en nouvelle Cadédonie (Ile de Pins.). Bull. Muséum Natl. d’Histoire Nat. 1930, 2, 229–233. [Google Scholar]
- GBIF. Goniobranchus kuniei (Pruvot-Fol, 1930). Available online: https://www.gbif.org/species/6519733 (accessed on 16 February 2022).
- Sea Slug Forum: Chromodoris kuniei Pruvot-Fol, 1930. Available online: http://www.seaslugforum.net/find/chrokuni (accessed on 16 February 2022).
- Sea Slug Forum: Chromodoris rufomaculatus. Available online: http://www.seaslugforum.net/find/chrorufo (accessed on 16 February 2022).
- Rudman, W.B. The Chromodorididae (Opisthobranchia: Mollusca) of the Indo-West Pacific: Chromodoris epicuria, C. aureopurpurea, C. annulata, C. coi and Risbecia tryoni colour groups. Zool. J. Linn. Soc. 1986, 90, 305–407. [Google Scholar] [CrossRef]
- Pease, W.H. Descriptions of nudibranchiate Mollusca inhabiting Polynesia. Am. J. Conchol. 1871, 6, 299–305. [Google Scholar]
- GBIF. Goniobranchus rufomaculatus (Pease, 1871). Available online: https://www.gbif.org/species/6519729 (accessed on 16 February 2022).
- Pola, M.; Cervera, J.L.; Gosliner, T.M. Revision of the Indo-Pacific genus Nembrotha (Nudibranchia: Dorididae: Polyceridae), with a description of two new species. Sci. Mar. 2008, 72, 145–183. [Google Scholar] [CrossRef]
- GBIF. Nembrotha yonowae Goethel & Debelius, 1992. Available online: https://www.gbif.org/species/165495130 (accessed on 16 February 2022).
- Smith, S.D.A. Growth and population dynamics of the giant clam Tridacna maxima (Röding) at its southern limit of distribution in coastal, subtropical eastern Australia. Molluscan Res. 2011, 31, 37. [Google Scholar]
- Bridle, T. Spurilla braziliana-a new sea slug in South Australia. South Aust. Nat. 2017, 91, 29–33. [Google Scholar]
- Scott, A.; Harasti, D.; Davis, T.; Smith, S.D.A. Southernmost records of the host sea anemone, Stichodactyla haddoni, and associated commensal shrimps in a climate change hotspot. Mar. Biodivers. 2015, 45, 145–146. [Google Scholar] [CrossRef]
- Epstein, H.E.; Hallas, J.M.; Johnson, R.F.; Lopez, A.; Gosliner, T.M. Reading between the lines: Revealing cryptic species diversity and colour patterns in Hypselodoris nudibranchs (Mollusca: Heterobranchia: Chromodorididae). Zool. J. Linn. Soc. 2018, 186, 116–189. [Google Scholar] [CrossRef]
- Booth, D.J.; Figueira, W.F.; Gregson, M.A.; Brown, L.; Beretta, G. Occurrence of tropical fishes in temperate southeastern Australia: Role of the East Australian Current. Estuar. Coast. Shelf Sci. 2007, 72, 102–114. [Google Scholar] [CrossRef]
- Figueira, W.F.; Booth, D.J. Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Glob. Chang. Biol. 2010, 16, 506–516. [Google Scholar] [CrossRef]
- Suthers, I.M.; Young, J.W.; Baird, M.E.; Roughan, M.; Everett, J.D.; Brassington, G.B.; Byrne, M.; Condie, S.A.; Hartog, J.R.; Hassler, C.S. The strengthening East Australian Current, its eddies and biological effects—an introduction and overview. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 538–546. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Holbrook, N.J. Extending our understanding of South Pacific gyre “spin-up”: Modeling the East Australian Current in a future climate. J. Geophys. Res. Ocean. 2014, 119, 2788–2805. [Google Scholar] [CrossRef]
- Bureau of Metereology. Climate Driver Update. Available online: www.bom.gov.au/climate/enso/ (accessed on 16 March 2022).
- Smith, S.D.A.; Banister, K.; Fraser, N.; Edgar, R.J. Tracing the source of marine debris on the beaches of northern New South Wales, Australia: The bottles on beaches program. Mar. Pollut. Bull. 2018, 126, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Beger, M.; Sommer, B.; Harrison, P.L.; Smith, S.D.A.; Pandolfi, J.M. Conserving potential coral reef refuges at high latitudes. Divers. Distrib. 2014, 20, 245–257. [Google Scholar] [CrossRef]
- Layton, K.K.S.; Gosliner, T.M.; Wilson, N.G. Flexible colour patterns obscure identification and mimicry in Indo-Pacific Chromodoris nudibranchs (Gastropoda: Chromodorididae). Mol. Phylogenet. Evol. 2018, 124, 27–36. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).