Linking Leaf N:P Stoichiometry to Species Richness and Composition along a Slope Aspect Gradient in the Eastern Tibetan Meadows
Abstract
:1. Introduction
2. Materials and Methods
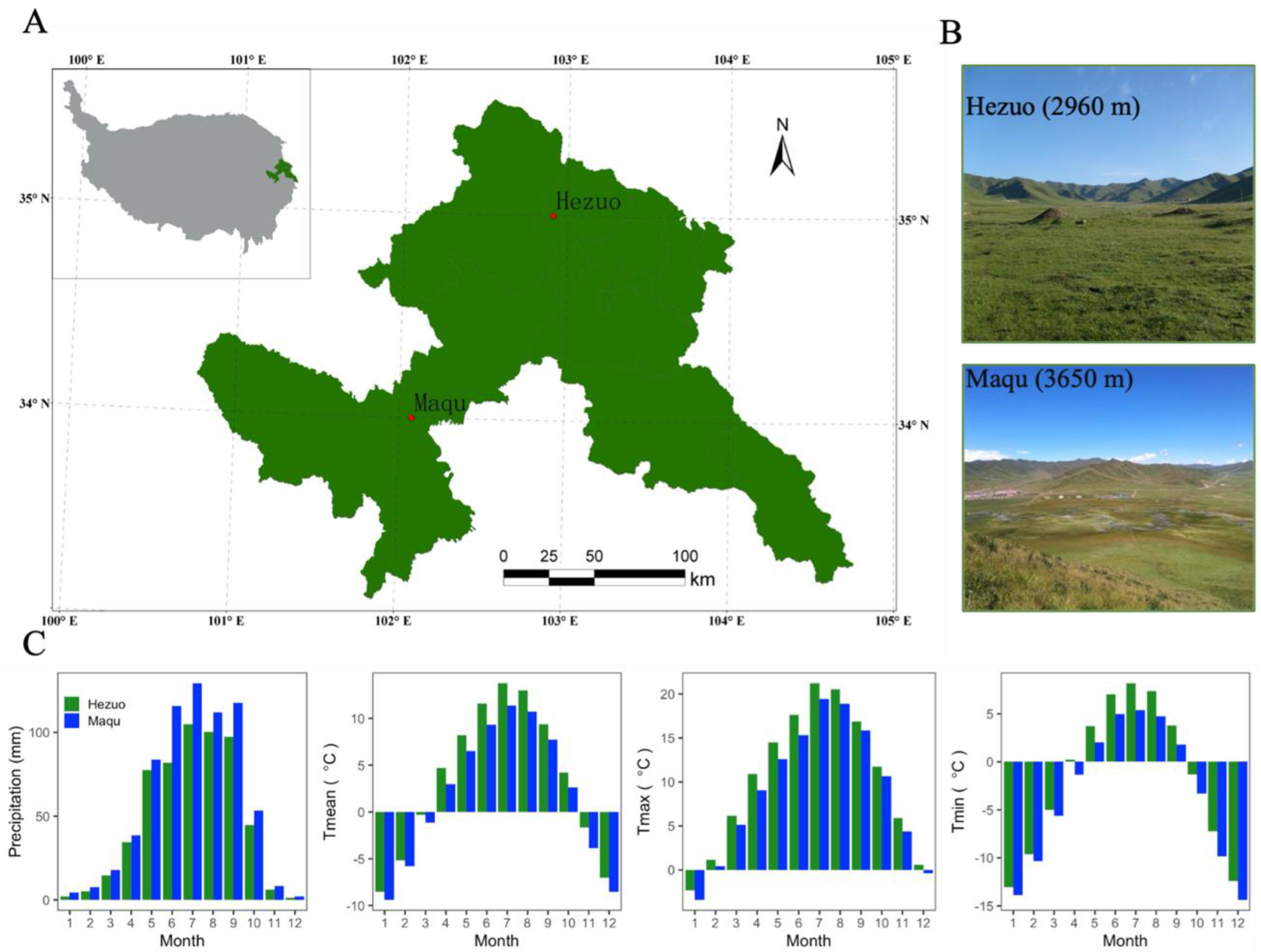
2.1. Study Sites
2.2. Leaf N and P Concentration Measurements
2.3. Species Composition Measurement
2.4. Data Analysis
3. Results
3.1. Variations in Leaf [N], [P], and N:P Ratio across the Slope Aspects
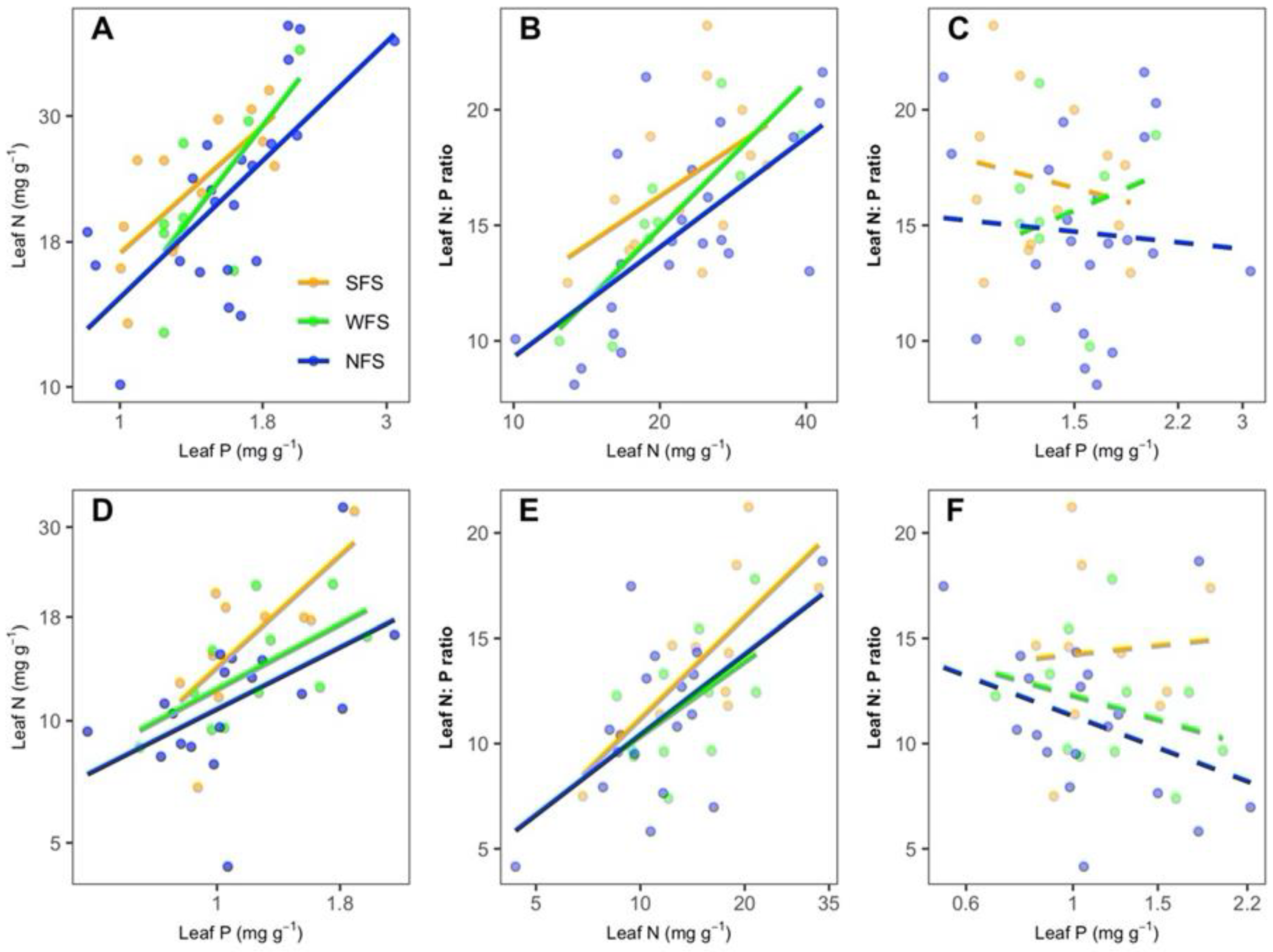
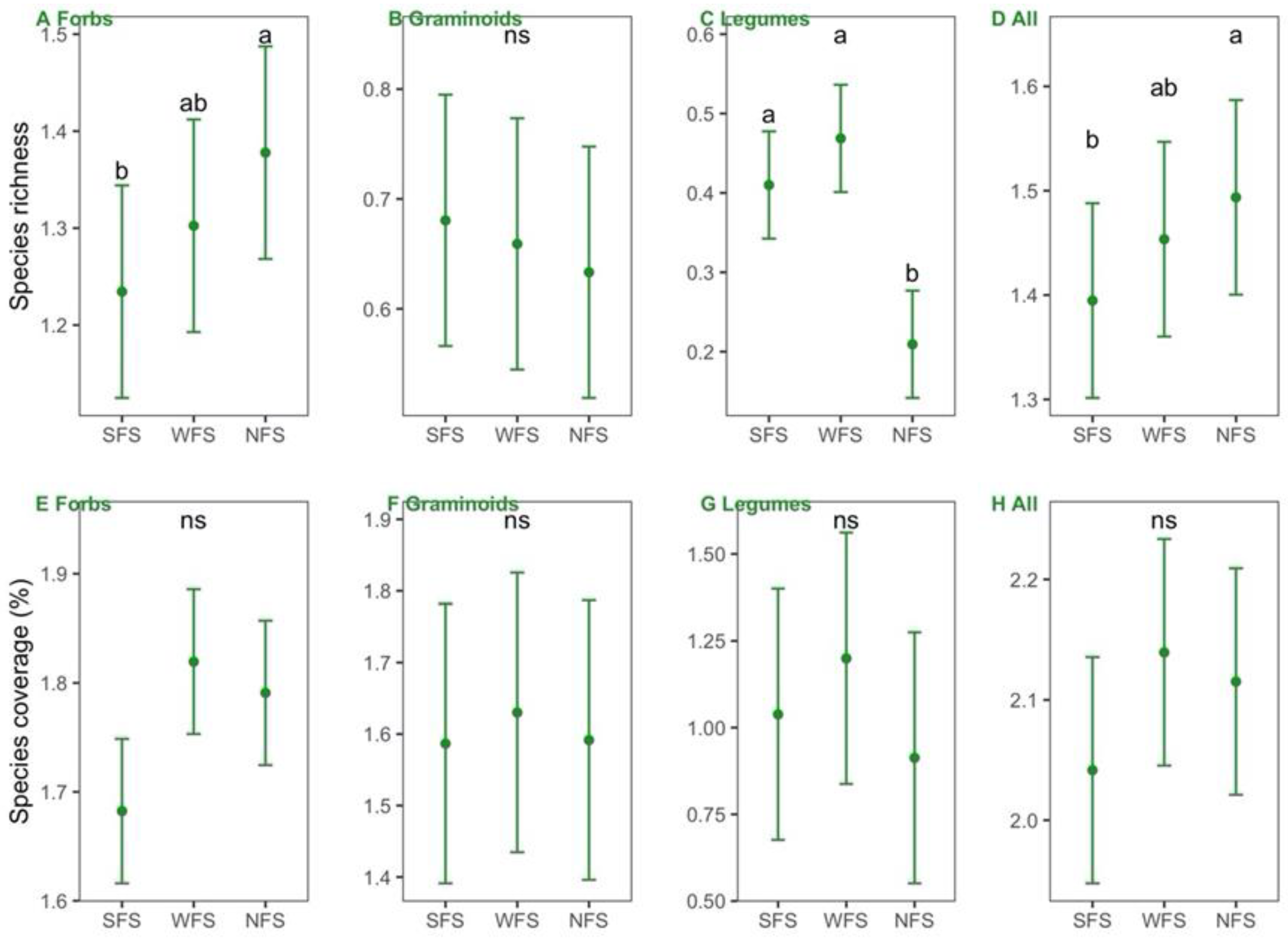
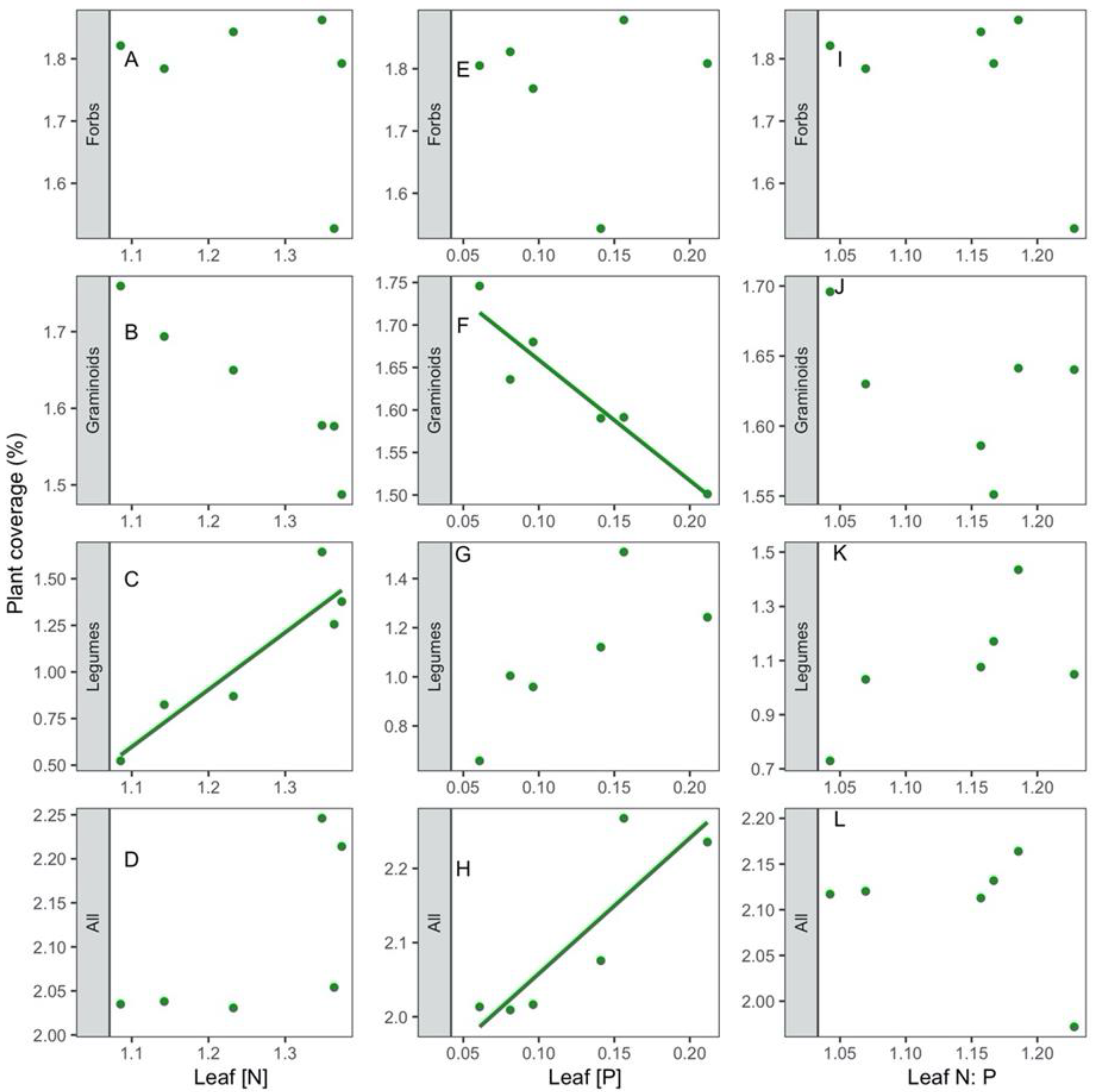
3.2. Correlations of Leaf Traits with Species Richness and Coverage
4. Discussion
4.1. Variations in Leaf [N], [P], and N:P Ratio across Different Slope Aspects
4.2. Correlations of Leaf N:P Stoichiometry with Species Richness and Coverage across the Slope Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Elser, J.; Sterner, R.; Gorokhova, E.a.; Fagan, W.; Markow, T.; Cotner, J.; Harrison, J.; Hobbie, S.; Odell, G.; Weider, L. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Photosynthesis: Physiological and ecological considerations. Plant Physiol 2002, 9, 172–174. [Google Scholar]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Schade, J.D.; Espeleta, J.F.; Klausmeier, C.A.; McGroddy, M.E.; Thomas, S.A.; Zhang, L. A conceptual framework for ecosystem stoichiometry: Balancing resource supply and demand. Oikos 2005, 109, 40–51. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to Phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Chen, Y.; Han, W.; Tang, L.; Tang, Z.; Fang, J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 2013, 36, 178–184. [Google Scholar] [CrossRef]
- He, J.-S.; Wang, L.; Flynn, D.F.; Wang, X.; Ma, W.; Fang, J. Leaf nitrogen: Phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2008, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; He, W.; Zhang, X.; An, L.; Xu, S. Patterns of Leaf N:P Stoichiometry along Climatic Gradients in Sandy Region, North of China. J. Plant Ecol. 2016, 11, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Zhuang, H.; Wu, L.; Liu, Q.; Shen, G.; Berg, B.; Man, R.; Liu, C. Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: An analysis based on local observations. For. Ecol. Manag. 2011, 261, 195–202. [Google Scholar] [CrossRef]
- Waigwa, A.N.; Mwangi, B.N.; Wahiti, G.R.; Omengo, F.; Zhou, Y.; Wang, Q.; Schmid, B. Variation of morphological and leaf stoichiometric traits of two endemic species along the elevation gradient of Mount Kenya, East Africa. J. Plant Ecol. 2020, 13, 785–792. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.; He, N.; Xia, F.; Wang, Q.; Wang, R.; Xu, Z.; Jia, Y. Invariant allometric scaling of nitrogen and phosphorus in leaves, stems, and fine roots of woody plants along an altitudinal gradient. J. Plant Res. 2016, 129, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güsewell, S.; Bailey, K.M.; Roem, W.J.; Bedford, B.L. Nutrient limitation and botanical diversity in wetlands: Can fertilisation raise species richness? Oikos 2005, 109, 71–80. [Google Scholar] [CrossRef]
- Blanck, Y.-L.; Gowda, J.; Mårtensson, L.-M.; Sandberg, J.; Fransson, A.-M. Plant species richness in a natural Argentinian matorral shrub-land correlates negatively with levels of plant phosphorus. Plant Soil 2011, 345, 11–21. [Google Scholar] [CrossRef]
- Sasaki, T.; Yoshihara, Y.; Jamsran, U.; Ohkuro, T. Ecological stoichiometry explains larger-scale facilitation processes by shrubs on species coexistence among understory plants. Ecol. Eng. 2010, 36, 1070–1075. [Google Scholar] [CrossRef]
- Huston, M.A.; DeAngelis, D.L. Competition and coexistence: The effects of resource transport and supply rates. Am. Nat. 1994, 144, 954–977. [Google Scholar] [CrossRef]
- Bracken, M.E.; Hillebrand, H.; Borer, E.T.; Seabloom, E.W.; Cebrian, J.; Cleland, E.E.; Elser, J.J.; Gruner, D.S.; Harpole, W.S.; Ngai, J.T. Signatures of nutrient limitation and co-limitation: Responses of autotroph internal nutrient concentrations to nitrogen and phosphorus additions. Oikos 2015, 124, 113–121. [Google Scholar] [CrossRef]
- Braakhekke, W.G.; Hooftman, D.A. The resource balance hypothesis of plant species diversity in grassland. J. Veg. Sci. 1999, 10, 187–200. [Google Scholar] [CrossRef]
- Theodose, T.A.; Roths, J.B. Variation in nutrient availability and plant species diversity across forb and graminoid zones of a Northern New England high salt marsh. Plant Ecol. 1999, 143, 219–228. [Google Scholar] [CrossRef]
- Pekin, B.K.; Boer, M.M.; Wittkuhn, R.S.; Macfarlane, C.; Grierson, P.F. Plant diversity is linked to nutrient limitation of dominant species in a world biodiversity hotspot. J. Veg. Sci. 2012, 23, 745–754. [Google Scholar] [CrossRef]
- Harpole, W.S.; Sullivan, L.L.; Lind, E.M.; Firn, J.; Adler, P.B.; Borer, E.T.; Chase, J.; Fay, P.A.; Hautier, Y.; Hillebrand, H. Addition of multiple limiting resources reduces grassland diversity. Nature 2016, 537, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Olde Venterink, H. Does phosphorus limitation promote species-rich plant communities? Plant Soil 2011, 345, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Armesto, J.J.; Martίnez, J.A. Relations between vegetation structure and slope aspect in the mediterranean region of Chile. J. Ecol. 1978, 881–889. [Google Scholar] [CrossRef]
- Bennie, J.; Hill, M.O.; Baxter, R.; Huntley, B. Influence of slope and aspect on long-term vegetation change in British chalk grasslands. J. Ecol. 2006, 94, 355–368. [Google Scholar] [CrossRef]
- Gallardo-Cruz, J.A.; Pérez-García, E.A.; Meave, J.A. β-Diversity and vegetation structure as influenced by slope aspect and altitude in a seasonally dry tropical landscape. Landsc. Ecol. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Kutiel, P. Slope aspect effect on soil and vegetation in a Mediterranean ecosystem. Isr. J. Plant Sci. 1992, 41, 243–250. [Google Scholar]
- Kutiel, P.; Lavee, H. Effect of slope aspect on soil and vegetation properties along an aridity transect. Isr. J. Plant Sci. 1999, 47, 169–178. [Google Scholar] [CrossRef]
- Yang, J.; El-Kassaby, Y.A.; Guan, W. The effect of slope aspect on vegetation attributes in a mountainous dry valley, Southwest China. Sci. Rep. 2020, 10, 16465. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Miller, P.C. Soil moisture relations in the southern California chaparral. Ecology 1980, 61, 98–107. [Google Scholar] [CrossRef]
- Sidari, M.; Ronzello, G.; Vecchio, G.; Muscolo, A. Influence of slope aspects on soil chemical and biochemical properties in a Pinus laricio forest ecosystem of Aspromonte (Southern Italy). Eur. J. Soil Biol. 2008, 44, 364–372. [Google Scholar] [CrossRef]
- Singh, S. Understanding the role of slope aspect in shaping the vegetation attributes and soil properties in Montane ecosystems. Trop. Ecol. 2018, 59, 417–430. [Google Scholar]
- Li, X.; Nie, Y.; Song, X.; Zhang, R.; Wang, G. Patterns of species diversity and functional diversity along a south- to north-facing slope in a subalpine meadow. Community Ecol. 2011, 12, 179–187. [Google Scholar] [CrossRef]
- Li, X.; Song, X.; Zhao, J.; Lu, H.; Qian, C.; Zhao, X. Shifts and plasticity of plant leaf mass per area and leaf size among slope aspects in a subalpine meadow. Ecol. Evol. 2021, 11, 14042–14055. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington DC, USA, 1954.
- Qin, Y.; Feng, Q.; Adamowski, J.F.; Zhu, M.; Zhang, X. Community level response of leaf stoichiometry to slope aspect in a montane environment: A case study from the Central Qilian Mountains, China. Glob. Ecol. Conserv. 2021, 28, e01703. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M. Linkages of C: N:P stoichiometry between soil and leaf and their response to climatic factors along altitudinal gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Petrlic, K.; Verachtert, E.; Bochet, E.; Poesen, J. Effects of slope angle and aspect on plant cover and species richness in a humid Mediterranean badland. Earth Surf. Processes Landf. 2014, 39, 1705–1716. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, B.; Li, M.; Xiao, R.; Rao, K.; Wang, J.; Zhang, T.; Guo, J. Community composition, structure and productivity in response to nitrogen and phosphorus additions in a temperate meadow. Sci. Total Environ. 2019, 654, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benítez-López, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 398–413. [Google Scholar] [CrossRef] [Green Version]
- Soons, M.B.; Hefting, M.M.; Dorland, E.; Lamers, L.P.; Versteeg, C.; Bobbink, R. Nitrogen effects on plant species richness in herbaceous communities are more widespread and stronger than those of phosphorus. Biol. Conserv. 2017, 212, 390–397. [Google Scholar] [CrossRef]
- De Schrijver, A.; De Frenne, P.; Ampoorter, E.; Van Nevel, L.; Demey, A.; Wuyts, K.; Verheyen, K. Cumulative nitrogen input drives species loss in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2011, 20, 803–816. [Google Scholar] [CrossRef]
- Wilson, S.D.; Tilman, D. Quadratic variation in old-field species richness along gradients of disturbance and nitrogen. Ecology 2002, 83, 492–504. [Google Scholar] [CrossRef]
- De Kroon, H.; Bobbink, R. Clonal plant dominance under elevated nitrogen deposition, with special reference to Brachypodium pinnatum in chalk grassland. In The Ecology and Evolution of Clonal Plants; Backhuys Publishers: Leiden, The Netherlands, 1997; pp. 359–379. [Google Scholar]


| SPECIES | FAMILY | PFGs | Hezuo | Maqu | ||
|---|---|---|---|---|---|---|
| N (mg g−1) | P (mg g−1) | N (mg g−1) | P (mg g−1) | |||
| Allium beesianum | Amaryllidaceae | Forbs | 16.69 | 1.43 | ||
| Allium condensatum | Amaryllidaceae | Forbs | 16.28 | 1.14 | ||
| Anaphalis hancockii | Asteraceae | Forbs | 17.86 | 1.28 | ||
| Anaphalis lactea | Asteraceae | Forbs | 26.02 | 1.66 | 10.45 | 0.78 |
| Anemone trullifolia var. linearis | Ranunculaceae | Forbs | 13.16 | 1.04 | ||
| Aster tataricus | Asteraceae | Forbs | 11.02 | 0.78 | ||
| Astragalus membranaceus var.membranaceus | Fabaceae | Legumes | 24.52 | 1.73 | 29.29 | 1.83 |
| Bupleurum sp | Apiaceae | Forbs | 14.01 | 1.05 | ||
| Cyperaceae sp | Cyperaceae | Graminoids | 17.30 | 1.24 | ||
| Daucus carota | Apiaceae | Forbs | 7.80 | 0.99 | ||
| Elephantopus scaber | Asteraceae | Forbs | 20.89 | 1.60 | ||
| Fragaria ananassa | Rosaceae | Forbs | 13.33 | 1.65 | ||
| Gentiana macrophylla | Gentianaceae | Forbs | 22.42 | 1.25 | ||
| Gentianopsis barbata | Gentianaceae | Forbs | 14.10 | 1.25 | ||
| Gueldenstaedtia verna | Fabaceae | Legumes | 26.17 | 1.27 | 21.44 | 1.21 |
| Hamamelis mollis | Hamamelidaceae | Shrubs | 17.83 | 1.29 | ||
| Kobresia humilis | Cyperaceae | Graminoids | 14.61 | 1.01 | ||
| Lancea tibetica | Mazaceae | Forbs | 19.10 | 1.53 | ||
| Leontopodium leontopodioides | Asteraceae | Forbs | 13.61 | 0.93 | ||
| Ligularia virgaurea | Asteraceae | Forbs | 11.69 | 1.10 | ||
| Medicago falcata | Fabaceae | Legumes | 4.36 | 1.05 | ||
| Medicago lupulina | Fabaceae | Legumes | 32.35 | 1.90 | ||
| Nardostachys jatamansi | Caprifoliaceae | Forbs | 14.26 | 1.07 | ||
| Nardostachys jatamansi | Caprifoliaceae | Forbs | 8.15 | 0.88 | ||
| Nepeta cataria | Lamiaceae | Forbs | 25.12 | 1.65 | ||
| Oxytropis sp | Fabaceae | Legumes | 36.79 | 2.00 | ||
| Pedicularis szetschuanica | Orobanchaceae | Forbs | 13.38 | 1.94 | ||
| Plantago asiatica | Plantaginaceae | Forbs | 16.07 | 1.56 | ||
| Polygonum macrophyllum | Polygonaceae | Forbs | 9.63 | 1.01 | ||
| Polygonum viviparum | Polygonaceae | Forbs | 25.53 | 1.59 | 15.41 | 1.83 |
| Potentilla anserina | Rosaceae | Forbs | 13.79 | 1.57 | ||
| Potentilla bifurca | Rosaceae | Forbs | 17.76 | 1.25 | ||
| Potentilla fragarioides | Rosaceae | Forbs | 12.92 | 1.03 | ||
| Roegneria kamoji | Poaceae | Graminoids | 18.00 | 1.41 | ||
| Saussurea graminea | Asteraceae | Forbs | 9.40 | 0.54 | ||
| Saussurea graminifolia | Asteraceae | Forbs | 15.92 | 1.39 | ||
| Saussurea sp | Asteraceae | Forbs | 18.62 | 1.20 | 8.77 | 0.84 |
| Scirpus triqueter | Cyperaceae | Graminoids | 17.55 | 0.89 | ||
| Stellera chamaejasme | Thymelaeaceae | Forbs | 36.87 | 2.31 | ||
| Taraxacum sp | Asteraceae | Forbs | 33.27 | 1.85 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Hu, Y.; Zhang, R.; Zhao, X.; Qian, C. Linking Leaf N:P Stoichiometry to Species Richness and Composition along a Slope Aspect Gradient in the Eastern Tibetan Meadows. Diversity 2022, 14, 245. https://doi.org/10.3390/d14040245
Li X, Hu Y, Zhang R, Zhao X, Qian C. Linking Leaf N:P Stoichiometry to Species Richness and Composition along a Slope Aspect Gradient in the Eastern Tibetan Meadows. Diversity. 2022; 14(4):245. https://doi.org/10.3390/d14040245
Chicago/Turabian StyleLi, Xin’e, Yafei Hu, Renyi Zhang, Xin Zhao, and Cheng Qian. 2022. "Linking Leaf N:P Stoichiometry to Species Richness and Composition along a Slope Aspect Gradient in the Eastern Tibetan Meadows" Diversity 14, no. 4: 245. https://doi.org/10.3390/d14040245
APA StyleLi, X., Hu, Y., Zhang, R., Zhao, X., & Qian, C. (2022). Linking Leaf N:P Stoichiometry to Species Richness and Composition along a Slope Aspect Gradient in the Eastern Tibetan Meadows. Diversity, 14(4), 245. https://doi.org/10.3390/d14040245






