Abstract
Owing to the wide variation in their morphological characteristics across diverse geographies, the identification and classification of plants in the Nymphaea genus are challenging. Therefore, the present study investigated the pollen morphological characteristics of hardy water lilies (N. ‘Rose Arey’, N. ‘Perry’s Fire Opal’, and N. ‘Peter Slocum’), their relationship with species classification and system evolution, and their cross-compatibility with three strains of Nymphaea hybrid (NH-1, NH-2, and NH-3), a tropical waterlily. Pollen of the hardy water lilies was single-grained, oblate, and 18.31–20.47 × 32.51–37.64 μm. The pollen apertures were of the ring-groove type, and the pollen exine ornamentation was rod- and tumour-shaped. Pollen grains of different species differed in size, the obviousness of tumour ornamentation, and the size and density of rod-like ornamentation; their germination rates also differed significantly. Viabilities of N. ‘Rose Arey’ and N. ‘Peter Slocum’ pollen were the highest and lowest, respectively. According to the artificial pollination results, all hybrid combinations except three (NH-1 × N. ‘Peter Slocum’, NH-2 × N. ‘Peter Slocum’, and NH-3 × N. ‘Peter Slocum’) bore seeds; combinations with NH-2 as the female parent and N. ‘Rose Arey’ as the male parent had the highest seed-setting rates.
1. Introduction
The water lily is a perennial aquatic plant of the genus Nymphaea in the family Nymphaeaceae; it has an early evolutionary origin and represents a rich plant resource. There are more than 50 species of water lilies in the world, mainly distributed in tropical, subtropical, and temperate regions [1]. The water lily is an indispensable and important material in designing garden waterscapes. Furthermore, it contains important active ingredients that can be used in food and beverages [2] and possesses antibacterial [3], anti-anxiety [4], and anti-hepatotoxicity [5] activities. Thus, this plant is widely used in the fields of horticulture, ornamental botany, food science, and medicine. Although plants from the genus Nymphaea have been cultivated and researched both domestically and abroad, there are still some disagreements pertaining to their classification, phylogenetic relationship, and the systematic evolution of some species. At present, plants from the genus Nymphaea are mainly identified according to the morphology of external parts, such as stems, leaves, flowers, and seeds, given that these are easy to observe. These plants can be divided into five subgenera: Nymphaea (northern temperatures), Brachyceras (pantropical), Anecphya (Australia), Hydrocallis (neotropics), and Lotos (palaeotropics) [6]. According to its demand for survival temperature, the water lily can also be divided into two ecological types: the hardy water lily and the tropical water lily [6]. The former is mainly naturally distributed in subtropical and temperate areas, and the latter is mainly distributed in tropical areas. However, some studies have found that, with changes in climate and environment, the size of the leaves and flowers and some qualitative characteristics of the flowers of water lilies will gradually change [7,8], and the morphological characteristics of water lilies in different distribution areas will also vary greatly. Therefore, the identification and classification of plants from this genus are difficult.
Pollen is a unique plant organ. After long-term evolution, it often forms a specific morphology, including with regards to features such as size, shape, aperture type, and exine ornamentation. The morphological characteristics of pollen are relatively stable and easy to observe; thus, pollen can provide evidence regarding the systematic classification, origin, and evolution of species [9,10], which is also an important basis for exploring the evolution of various species and varieties [11,12]. In the discussion regarding the pollen of primitive angiosperms, many researchers believe that the pollen characteristics of water lily play an important role in the evolutionary history of several plants [13,14,15]; this is an important part of the phylogenetic analysis of angiosperms and seed plants [16,17,18,19]. However, the current research on plants from the genus Nymphaea has mainly focused on their reproductive biology [20,21], physiology, biochemistry [22], and functional activity [23]. Although many researchers have studied the pollen morphology of water lilies [21,24,25,26], most of them have focused on tropical water lily species, and there are only a few studies on the pollen morphology of hardy water lilies.
N. ‘Rose Arey’, N. ‘Perry’s Fire Opal’, and N. ‘Peter Slocum’ are three hardy water lily varieties from the genus Nymphaea; they are important for the breeding of hardy water lilies. Nymphaea hybrid is a precious tropical water lily with large and colourful flowers and a rich aroma, especially the rare blue strain of the genus Nymphaea. It has a very high ornamental value and is an important breeding material for water lilies [27]. Additionally, it has important practical value in the fields of medicine, industry, food, and cosmetics [28]. However, N. hybrid plants cannot survive winter naturally in northern China, which has become the main limiting factor affecting their introduction and cultivation in temperate regions.
Therefore, to the best of our knowledge, this study, for the first time, examined the pollen morphology, including the exine ultrastructure, of these three hardy water lilies, using scanning electron microscopy (SEM), and the viability of their pollen was measured using an in vitro germination experiment. Using these three hardy water lilies as the male parents and three widely used N. hybrid strains with different colours as the female parents, a crossbreeding experiment was performed, and the hybrid compatibility between N. hybrid and hardy water lilies was preliminarily explored. This study provides palynological evidence for the taxonomy of Nymphaea plants and serves as a basis for the crossbreeding of water lilies, innovation of germplasm resources, and the identification of different varieties of Nymphaea plants.
2. Materials and Methods
2.1. Plant Materials
From July to September 2019, pollen viability measurements and pollen microstructure observations of hardy water lilies were performed in the laboratory of the Nanjing Forestry University (118°82′ E, 32°08′ N), Nanjing, Jiangsu Province, China. The crossbreeding experiment was performed in the Hangzhou Longmen ancient town scenic area (29°54′ N, 119°56′ E), Hangzhou, Zhejiang Province, China.
The aim of the crossbreeding experiment was to cultivate new varieties of water lily with a long flowering period; large, fragrant, and colourful flowers; and cold-resistant seeds. Based on the previous literature review and investigation, three widely known different coloured strains of N. hybrid, a precious tropical water lily: NH-1 (yellow type), NH-2 (pink type), and NH-3 (blue–purple type), were used as female parents, and three widely used hardy water lily varieties: N. ‘Rose Arey’, N. ‘Perry’s Fire Opal’, and N. ‘Peter Slocum’ were used as male parents (Figure 1, Table 1).

Figure 1.
Morphology of the inflorescence of three hardy water lily varieties. (a) N. ‘Rose Arey’; (b) N. ‘Perry’s Fire Opal’; (c) N. ‘Peter Slocum’.

Table 1.
Materials used for the crossbreeding experiment.
2.2. Observation of the Microstructures of Hardy Water Lily Pollen Grains
At the full bloom stage, the flowers predicted to open the following day (i.e., when the buds were slightly loose) were selected for marking. Pollen grains from three hardy water lilies were sampled at approximately 10:00 a.m. on the 2nd day of floral anthesis. The pollen grains were double fixed with 2.5% glutaraldehyde and 1% osmic acid; washed with 0.1 mol/L phosphoric acid buffer (pH 7.2); dehydrated with ethanol gradients of 30%, 50%, 70%, 90%, and 100%; replaced with isoamyl acetate; and dried at the critical point. The dried sections were mounted on stubs, coated with gold using an ion-sputtering apparatus, and then observed under a Quanta 200 scanning electron microscope (FEI Company, Netherlands) [29]. A representative field of vision was selected to observe and photograph the morphology of the pollen population, and the far-polar view, near-polar view, equatorial view, and exine ornamentation of the pollen specimens. The pictures were imported into AutoCAD v17.0 (Autodesk, Inc., San Rafael, CA, USA); 20 pollen grains of each variety were randomly selected for the measurement of the length of the polar axis (P) and equatorial axis (E), and the length and width of the pollen grooves. The shape of the pollen grains was expressed by the ratio of P/E [30], and pollen size was expressed by the formula P × E [31].
2.3. Cross Breeding Experiment
2.3.1. Collection, Storage, and Viability of Hardy Water Lily Pollen Grains
At the full bloom stage in July 2019, the pollen of three hardy water lilies was collected, dried with silica gel, and then sealed and stored in a refrigerator at −20 °C for later use. Pollen viability was measured by an in vitro germination method, referring to previous studies [32], and the corresponding changes were made through preliminary experiments. The medium used was 5% sucrose, 15 mg/L boric acid, 20 mg/L calcium chloride, and 1% agar, and the pollen grains were cultured in an incubator at 30 °C for 12 h; the germination conditions of the pollen grains were observed under an Olympus BX51 optical microscope (Tokyo, Japan). Each treatment was repeated three times, and each observation was repeated in five fields; the number of pollen grains in each field was not less than 100.
2.3.2. Artificial Pollination Experiment
Referring to the study on the artificial pollination technology of tropical water lilies by Huang et al. [33], 1 day before the flowering of N. hybrid flower (i.e., when the buds were slightly loose), the emasculation was carried out, and the flower bud was separated using a sulfuric acid paper bag. On the 1st day of floral anthesis, the pollen of hardy water lilies was collected for pollination, bagged, and listed. The seed-setting rate of each hybrid combination was counted 4–5 weeks after pollination; the following formula was used: Seed-setting rate/% = (total number of seed-setting flowers/total number of pollinated flowers) × 100%.
2.4. Data Analysis
The values of various morphological parameters of the pollen grains and the percentage viability data were analysed by calculating the means and standard deviations and one-way analysis of variance. The means were separated using Duncan’s multiple range test at the 5% probability level using the SPSS statistical package (version 22.0; IBM Corp., Armonk, NY, USA).
3. Results
3.1. Pollen Characteristics of the Three Hardy Water Lily Varieties
The pollen of three hardy water lilies was oblate, single-grained, triangular in the polar view, and oblong in the equatorial view (Figure 2). The pollen morphology, including the exine ultrastructure of the three different hardy water lilies, showed no evident difference, with a certain degree of conservation.
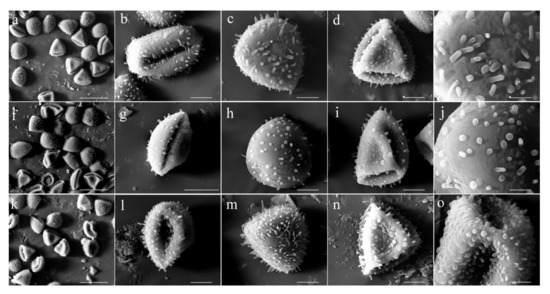
Figure 2.
Microcosmic morphology of the pollen of three hardy water lilies. (a–e) N. ‘Rose Arey’. (a) Pollen population. Scanning electron microscope (SEM), bar = 50 µm. (b) Equatorial view. SEM, bar = 10 μm. (c) Distal polar view. SEM, bar = 10 μm. (d) Proximal polar view. SEM, bar = 10 μm. (e) Surface ornamentation. SEM, bar = 5 μm. (f–j) N. ‘Perry’s Fire Opal’. (f) Pollen population. SEM, bar = 50 μm. (g) Equatorial view. SEM, bar = 20 μm. (h) Distal polar view. SEM, bar = 10 μm. (i) Proximal polar view. SEM, bar = 10 μm. (j) Surface ornamentation. SEM, bar = 5 μm. (k–o) N. ‘Peter Slocum’. (k) Pollen population. SEM, bar = 50 μm. (l) Equatorial view. SEM, bar = 10 μm. (m) Distal polar view. SEM, bar = 10 μm. (n) Proximal polar view. SEM, bar = 10 μm. (o) Surface ornamentation. SEM, bar = 5 μm.
The comparison of the pollen characteristic values (Table 2) showed that the length of the pollen equatorial axis was 32.51–37.64 μm. The length of the equatorial axis of N. ‘Perry’s Fire Opal’ pollen was the largest (37.64 ± 2.80 μm), followed by that of N. ‘Peter Slocum’ pollen (34.84 ± 3.0 μm); the lowest value was observed for N. ‘Rose Arey’ pollen (32.51 ± 2.28 μm), which was significantly smaller than that of N. ‘Perry’s Fire Opal’ pollen (p < 0.05). The length of the pollen polar axis was 18.31–20.47 μm, and there was no significant difference among the pollen from the three water lilies. N. ‘Peter Slocum’ pollen had the largest P/E value (0.59 ± 0.06 μm), and N. ‘Perry’s Fire Opal’ pollen had the smallest (0.50 ± 0.06 μm; significantly smaller than the P/E value of N. ‘Peter Slocum’). Additionally, N. ‘Peter Slocum’ pollen had the largest P × E value of 20.47 × 34.84 μm2, and N. ‘Rose Arey’ pollen had the smallest P × E value of 18.31 × 32.51 μm2. According to Wang’s classification standard for pollen shape [34], it can be determined that the pollen from all the three hardy water lilies examined herein were oblate-shaped. According to Erdtman’s pollen classification standard for pollen size [31], the pollen grains from the three hardy water lilies were determined to be medium-sized.

Table 2.
Pollen morphological characteristic values of the three hardy water lily varieties.
3.2. Characteristics of Pollen Grooves and Exine Ornamentations of the Three Hardy Water Lily Varieties
Through the observation of the microstructure of pollen from the three different hardy water lilies, it was found that the length–width ratios of the three hardy water lilies’ pollen grooves were all >2; thus, the pollen from all three hardy water lilies was deemed to possess long apertures. Among them, N. ‘Perry’s Fire Opal’ had the longest pollen aperture, which was significantly longer than that of the other two water lily varieties, and N. ‘Peter Slocum’ had the largest width of pollen aperture, which did not differ significantly from the pollen aperture width of the other two water lilies (Table 3). The apertures of pollen from all of the three hardy water lilies were of the ring-groove type (Figure 2). At the top corners of the triangular pollen, the pollen grooves had no distinct depression; the pollen grooves of the remaining parts were wide, and the edges were deeply depressed inward.

Table 3.
Pollen groove characteristic values of the three hardy water lily varieties.
The pollen exine ornamentation patterns of the three hardy water lilies were relatively similar (Figure 2). The pollen cover ornamentation on the far polar surface showed rod-shaped carvings of different lengths, and the paraxial surface of pollen was distributed with tumour patterns of different sizes. Among them, the rod-shaped carvings on the surface of the N. ‘Peter Slocum’ pollen cover were relatively long and densely distributed, and the tumour-shaped ornamentations on the paraxial surface of the pollen were also more distinct. The rod-shaped carvings on the surface of the N. ‘Rose Arey’ pollen cover were shorter than those on the surface of N. ‘Peter Slocum’, and the distribution was also sparser. Although the rod-shaped carvings on the surface of the N. ‘Perry’s Fire Opal’ pollen cover were shorter and their distribution was the sparsest, there was almost no rod-shaped carving ornamentation on it; furthermore, the tumour-shaped ornamentation on the paraxial surface of the N. ‘Perry’s Fire Opal’ pollen was not evident.
3.3. Viability of Pollen from the Three Hardy Water Lilies
According to the pollen viability results of the three hardy water lilies (Figure 3, Table 4), the pollen germination rates of the three hardy water lilies were significantly different. The pollen germination rate of N. ‘Rose Arey’ was the highest, at 74.20 ± 1.79%. The pollen germination rate of N. ‘Perry’s Fire Opal’ was 56.48 ± 12.18%, and that of N. ‘Peter Slocum’ was the lowest, at 14 ± 4.45%. There was no significant difference in the pollen germination rate between N. ‘Perry’s Fire Opal’ and N. ‘Rose Arey’, but they were significantly different from that of N. ‘Peter Slocum’.
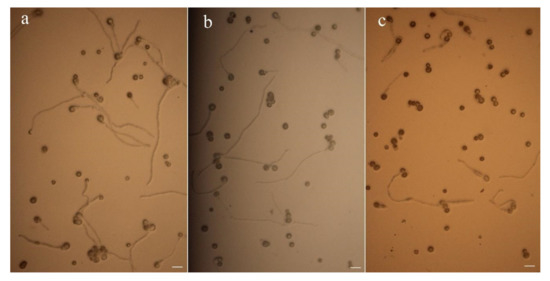
Figure 3.
Pollen germination of three hardy water lily varieties on solidified medium. (a) N. ‘Rose Arey’; (b) N. ‘Perry’s Fire Opal’; (c) N. ‘Peter Slocum’. Optical microscope, bar = 50 μm.

Table 4.
Pollen germination rate of three hardy water lilies.
3.4. Seed-Setting Rates of Different Hybrid Combinations
The results of the cross-pollination experiment (Table 5) showed that all hybrid combinations, except NH-1 × N. ‘Peter Slocum’, NH-2 × N. ‘Peter Slocum’, and NH-3 × N. ‘Peter Slocum’ can bear seeds; there were significant differences between the seed-setting rates of different hybrid combinations. Among them, the hybrid combination of NH-2 × N. ‘Rose Arey’ had the highest seed-setting rate (33.33%), followed by NH-3 × N. ‘Rose Arey’ (25.53%) and NH-2 × N. ‘Perry’s Fire Opal’ (22.03%); the seed-setting rates of the other hybrid combinations were lower.

Table 5.
Comparison of the seed-setting rates of different hybrid combinations.
Upon comparing the seed-setting rate of each strain for which N. hybrid was used as the female parent and hybridised with the three hardy water lilies, the hybrid combinations with NH-2 as the female parent had the highest seed-setting rate (average seed-setting rate, 18.45%). The average seed-setting rate of hybrid combinations with NH-3 as the female parent was 12.02%, and that of hybrid combinations with NH-1 as the female parent was the lowest (8.94%).
Upon comparing the seed-setting rate of each strain for which the three hardy water lilies were used as the male parents and hybridised with three strains of N. hybrid, the hybrid combinations with N. ‘Rose Arey’ as the male parent had the highest seed-setting rate (average seed-setting rate, 24.56%). Hybrid combinations with N. ‘Perry’s Fire Opal’ as the male parent showed an average seed-setting rate of 14.85%, and those with N. ‘Peter Slocum’ as the male parent showed a seed-setting rate of 0%.
4. Discussion
Pollen is a unique reproductive structure of plants and is mainly affected by genetic factors. The pollen of different plant species can form unique morphological characteristics that are both stable and conserved. Therefore, pollen can provide strong evidence for studies on plant phylogeny, classification among different species and varieties, and genetic relationships [35,36]. The observation and analysis of the morphology, surface characteristics, and exine ornamentation of pollen from three hardy water lilies revealed that pollen from these species showed a similar micromorphology. All pollen was oblate and single-grained, and the apertures of pollen from all three hardy water lilies were of the ring-groove type, which was consistent with the common characteristics of water lilies [21]. The results of this study showed that the pollen grains of the three hardy water lilies were oblate, triangular in the polar view, and oblong in the equatorial view; the pollen exine ornamentation was found to be rod-shaped and tumour-shaped, which was similar to the results of the study by Volkova et al. on pollen from hardy water lilies [24] but was different from the results of the studies by Bodhipadma et al. on N. nouchali var. versicolor [25], Coiro et al. on N. caerulea and N. gigantea [26], Taylor et al. on N. ondinea [21], and Zhang et al. on N. hybrid [28] and pollen from other tropical water lilies. These studies showed that the pollen grains of these tropical water lilies were oval to nearly spherical, nearly circular in the polar view, and oval or navicular in the equatorial view, and the pollen exine ornamentation was psilate or verrucate. In the current study, we also found that the pollen grains of three hardy water lilies showed certain differences in size, the obviousness of tumour ornamentation, and the size and density of rod-like ornamentation. These differences among the varieties were evident. This showed that the morphology of pollen grains from different subgenera, populations, and varieties of plants in the genus Nymphaea exhibits a certain degree of difference. Differences between these characteristics may have important taxonomic values; the application of such characteristics in the classification and identification of water lilies requires further studies.
Pollen viability plays an important role in the success and fruiting rates of plant pollination and fertilisation. Therefore, it is particularly important to measure the pollen viability of male parents before plant breeding is performed [37]. This study found that the viability of pollen from the three hardy water lilies was different. Among them, the viability of N. ‘Peter Slocum’ pollen was the lowest, at only 14 ± 4.45%. The reasons for the low pollen viability of N. ‘Peter Slocum’ may be attributed to inherent factors such as genetic characteristics and plant nutrition level, as well as external environmental factors such as temperature, light, and weather conditions [38,39]. The specific factors influencing pollen viability need to be studied further. The pollen germination rate of ‘Rose Arey’ and N. ‘Perry’s Fire Opal’ reached more than 50%, which meets the pollen vitality requirements for cross pollination; thus, pollen from these two varieties can be used as materials for cross breeding.
Interspecific hybridisation is an important means of cultivating new plant varieties. Through this method, the excellent traits of two or more species can be combined to create new and superior plant varieties [40]. However, interspecific hybridisation often limits the success rate of hybridisation owing to reproductive isolation, resulting in a phenomenon termed hybridisation incompatibility [41]. The results of this study showed that the seed-setting rates of different strains of N. hybrid, which was used as the female parent, hybridised with the three hardy water lilies were significantly different. This may be owing to varying hybridisation incompatibility between the female parent and the pollen from different male parents. To a certain extent, this shows that the seed-setting rates of the hybrid combinations of N. hybrid and hardy water lilies are closely related to the selection of N. hybrid strains as the female parents, which is similar to the conclusions drawn from research on Rhododendron [42]. Upon comparing the seed-setting rates of the strains derived from the hybridisation between the three different hardy water lilies used as male parents and the three strains of N. hybrid, it was found that the seed-setting rate of strains with N. ‘Rose Arey’ as the male parent was the highest (24.56%), followed by the strains with N. ‘Perry’s Fire Opal’ (14.85%) as the male parent; the seed-setting rate for strains with N. ‘Peter Slocum’ as the male parent was always 0%. These findings are consistent with the results of the pollen viability determinations of the three hardy water lilies. This shows that the pollen viability of male parents is an important factor affecting the success of cross-pollination experiments [43,44]. Additionally, we found that the seed-setting rates of all hybrid combinations were low, and the seed-setting rate of the combination NH-2 × N. ‘Rose Arey’ was the highest, at 33.13%. Considering that the temperature during the hybridisation experiment was high in this study and that the bagging treatment was performed during the hybridisation, the temperature inside the N. hybrid flower may have been higher, which was not conducive to the germination of pollen from hardy water lilies. Therefore, the reason for the low seed-setting rates of N. hybrid strains hybridised with hardy water lilies may be attributed to the weather conditions during hybridisation. In addition, this may also be owing to the hybridisation incompatibility between N. hybrid and the hardy water lilies [45,46].
5. Conclusions
In this study, the pollen microstructure and pollen viability of three hardy water lilies: N. ‘Rose Arey’, N. ‘Perry’s Fire Opal’, and N. ‘Peter Slocum’ were studied for the first time. The cross-compatibility of N. hybrid as female parents and three hardy water lilies as male parents was preliminarily investigated. The results showed that the pollen of the three hardy water lilies was single-grained and oblate, and the pollen exine ornamentation was rod-shaped and tumour-shaped. The pollen grains from different species differed with regard to size, the obviousness of tumour ornamentation, and the size and density of rod-like ornamentation. The viability of N. ‘Rose Arey’ pollen was the highest, and that of N. ‘Peter Slocum’ pollen was the lowest. The results of the crossbreeding experiment showed that all hybrid combinations except for NH-1 × N. ‘Peter Slocum’, NH-2 × N. ‘Peter Slocum’, and NH-3 × N. ‘Peter Slocum’ could bear seeds. The hybrid combinations with NH-2 as the female parent had the highest seed-setting rate, and the hybrid combinations with N. ‘Rose Arey’ as the male parent had the highest seed-setting rate. Therefore, for future hybridisations between N. hybrid and hardy water lilies, the NH-2 and NH-3 varieties with high seed-setting rate and N. ‘Rose Arey’ and N. ‘Perry’s Fire Opal’ varieties with high pollen viability can be used to improve the seed-setting rate. Our findings provide valuable palynological evidence for understanding the systematic taxonomy of water lilies and useful information for breeding high-quality hardy water lilies by enabling the selection of varieties with high pollen viability.
Author Contributions
Conceptualisation, Z.Z.; methodology, Z.Z.; investigation, H.Z. and H.W.; data curation, H.Z.; writing—original draft preparation, H.Z. and Q.Z.; writing—review and editing, Q.Z., Q.S. and Z.Z.; supervision, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31770752; the 333 Project of Jiangsu Province, grant number BRA2018065; the Industry-University-Research Cooperation Project of Jiangsu Province, grant number FZ20200041; the Science and Technology Project of Nanjing Greening and Gardens Bureau, grant number YLKJ202011ZD; the National Natural Science Foundation for Young Scientists of China, grant number 32101582; the Natural Science Foundation for Young Scientists of Jiangsu Province, grant number BK20210613; and the Natural Science Foundation for Universities of Jiangsu Province, grant number 20KJB220006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Yuesheng Ding, from Nanjing Yilianyuan Flower Co., Ltd., Nanjing, Jiangsu Province, China, for providing plants of three hardy water lilies. We also thank Huan Hu, from Hangzhou Longmen ancient town scenic area, Hangzhou, Zhejiang Province, China, for his help in the conduct of cross breeding experiment.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Li, S.J.; Wei, Q.; Chen, C.; Zhan, Y.; Wu, Y.P.; Yu, G. Breeding progress of waterlilies in China. J. Plant Genet. Res. 2019, 20, 829–835. [Google Scholar] [CrossRef]
- Yin, D.D.; Yuan, R.Y.; Wu, Q.; Li, S.S.; Shao, S.; Xu, Y.J.; Hao, X.H.; Wang, L.S. Assessment of flavonoids and volatile compounds in tea infusions of water lily flowers and their antioxidant activities. Food Chem. 2015, 187, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.B.; Karakas, F.P.; Turker, A.U. In vitro antibacterial and antitumor activities of some medicinal plant extracts, growing in Turkey. Asian Pac. J. Trop. Med. 2013, 6, 616–624. [Google Scholar] [CrossRef]
- Thippeswamy, B.S.; Mishra, B.; Veerapur, V.P.; Gupta, G. Anxiolytic activity of Nymphaea alba Linn. in mice as experimental models of anxiety. Indian J. Pharmacol. 2011, 43, 50–55. [Google Scholar] [CrossRef]
- Bhandarkar, M.R.; Khan, A. Antihepatotoxic effect of Nymphaea stellata willd.; against carbon tetrachloride induced hepatic damage in albino rats. J. Ethnopharmacol. 2004, 91, 61–64. [Google Scholar] [CrossRef]
- Conard, H.S. The Waterlilies: A Monograph of the Genus Nymphaea; The Carnegie Institution of Washington: Washington, DC, USA, 1905. [Google Scholar]
- Heslop-Harrisson, J. Nymphaea L. J. Ecol. 1955, 43, 719–734. [Google Scholar] [CrossRef]
- Kupriyanova, L.A. Pollen morphology of Nymphaea species in the European part of the USSR. Bot. Zhurn 1976, 61, 1558–1563. [Google Scholar]
- Xu, F.; de Craene, L.P.R. Pollen morphology and ultrastructure of selected species from Annonaceae. Plant Syst. Evol. 2013, 299, 11–24. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, H.; Wang, H. Pollen morphology of the genus Lasianthus (Rubiaceae) and related taxa from Asia. J. Syst. Evol. 2008, 46, 60–70. [Google Scholar] [CrossRef]
- Wang, X.R.; Tang, H.R.; Huang, L.; He, Z.Z.; Dong, X.L.; Fu, H.Q.; Deng, Q.X. Comparative studies on pollen submicroscopic morphology of some wild species and cultivars of Bramble (Rubus L.). Acta Hortic. Sin. 2007, 34, 1395. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, H.; Zhang, L.; Zang, D.K. Pollen morphology and cultivar classification of the genus Chaenomeles. Sci. Sil. Sin. 2008, 44, 53. [Google Scholar] [CrossRef]
- Walker, J.W. Aperture evolution in the pollen of primitive angiosperms. Am. J. Bot. 1974, 61, 1112–1137. [Google Scholar] [CrossRef]
- Walker, J.W. Evolutionary significance of the exine in the pollen of primitive angiosperms. In The Evolutionary Significance of the Exine; Ferguson, I.K., Muller, J., Eds.; Academic Press: London, UK, 1976. [Google Scholar]
- Walker, J.W.; Doyle, J.A. The bases of angiosperm phylogeny: Palynology. Ann. Mo. Bot. Gard. 1975, 62, 664–723. [Google Scholar] [CrossRef]
- Donoghue, M.J.; Doyle, J.A. Phylogenetic analysis of angiosperms and the relationships of Hamamelidae. In Evolution, Systematics, and Fossil History of the Hamamelidae; Crane, P.R., Blackmore, S., Eds.; Clarendon: Oxford, UK, 1989; pp. 17–45. [Google Scholar]
- Doyle, J.A.; Hotton, C.L. Diversification of early angiosperm pollen in a cladistic context. In Pollen and Spores: Patterns of Diversification; Blackmore, S., Barnes, S.H., Eds.; Clarendon: Oxford, UK, 1991; pp. 169–195. [Google Scholar]
- Doyle, J.A.; Donoghue, M.J. Phylogenies and angiosperm diversification. Paleobiology 1993, 19, 141–167. [Google Scholar] [CrossRef]
- Doyle, J.A.; Endress, P.K. Morphological phylogenetic analysis of basal angiosperms: Comparison and combination with molecular data. Int. J. Plant Sci. 2000, 161, S121–S153. [Google Scholar] [CrossRef]
- Wiersema, J.H. Reproductive biology of Nymphaea (Nymphaeaceae). Ann. Mo. Bot. Gard. 1988, 75, 795–804. [Google Scholar] [CrossRef]
- Taylor, M.L.; Cooper, R.L.; Schenider, E.L.; Jeffrey, M.O. Pollen structure and development in Nymphaeales: Insights into character evolution in an ancient angiosperm lineage. Am. J. Bot. 2015, 102, 1685–1702. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, M.S.; Lu, X.M.; Zhang, Y. Correlation between diurnal variation of purification effects and physiological characteristics of Nymphaea tetragona. J. Jiangsu Univ. Nat. Sci. Ed. 2011, 32, 482–486. [Google Scholar] [CrossRef]
- Shi, N.; Liu, X.J.; Du, F.F.; Chang, Y.J.; Li, N.W.; Yao, D.R. GC-MS analysis on components of essential oil from fresh flowers of tropical water lily. J. Plant Resour. Environ. 2017, 26, 104–106. [Google Scholar] [CrossRef]
- Volkova, P.A.; Shipunov, A.B. Morphological variation of Nymphaea (Nymphaeaceae) in European Russia. Nord. J. Bot. 2007, 25, 329–338. [Google Scholar] [CrossRef]
- Bodhipadma, K.; Noichinda, S.; Thaiyanto, P.; Leung, D. Morphology, viability, and germinability of pollen from two forms of Nymphaea nouchali var. Versicolor, a day-blooming waterlily. Sci. Asia 2013, 39, 214–218. [Google Scholar] [CrossRef][Green Version]
- Coiro, M.; Barone Lumaga, M.R. Aperture evolution in Nymphaeaceae: Insights from a micromorphological and ultrastructural investigation. Grana 2013, 52, 192–201. [Google Scholar] [CrossRef]
- Jiang, H.F.; Zhou, H.Y.; Shi, B.J.; Shan, C.Y.; Xu, H.; Zhang, J.; Zhang, W.M. Extraction, isolation and structural determination of the polysaccharides from Nymphaea hybrid. Chin. Wild Plant Res. 2017, 36, 19–22. [Google Scholar] [CrossRef]
- Zhang, H.H.; Wu, H.Y.; Zhou, Q.; Zhao, R.N.; Zhu, Z.L. Flowering characteristics and reproductive biology of Nymphaea hybrid, a precious water lily. Sci. Hortic. 2021, 287, 110268. [Google Scholar] [CrossRef]
- Xu, L.P.; Yu, F.Y. Microstructure of pistils and stamens in Styrax tonkinensis. J. Nanjing For. Univ. 2017, 41, 34–40. [Google Scholar] [CrossRef]
- Niu, L.X.; Zhang, Y.L. The study on pollen morphology of the wild Vitis varieties in China. Acta Hortic. Sin. 2000, 27, 361–363. [Google Scholar]
- Erdtman, G. Handbook of Palynology; Munksgaard: Copenhagen, Denmark, 1969. [Google Scholar]
- Zhang, Y.D. Research on pollen viability of hardy water lily in Qingdao. Adv. Ornam. Hortic. China 2015, 57–61. [Google Scholar]
- Huang, G.Z.; Deng, H.Q.; Li, Z.X.; Li, G. Water Lilies; China for Press: Beijing, China, 2008. [Google Scholar]
- Wang, F.X. Pollen Flora of China; Science Press: Beijing, China, 1995. [Google Scholar]
- Song, Y.Y.; Zhao, C.H.; Zhao, Y.Y.; Liu, J.X. Pollen morphology of Aletris L. (Nartheciaceae) and its systematic significance. Microsc. Res. Tech. 2019, 82, 2061–2071. [Google Scholar] [CrossRef]
- Li, H.C.; Wu, T.Y.; Gong, L.; Luo, J. Floral phenotypes and pollen morphological characteristics of 13 Species from Calanthe in Tibet. Bull. Bot. Res. 2021, 41, 547–556. [Google Scholar] [CrossRef]
- Hu, K.X.; Zhang, X.M.; Zheng, Y.F. Characteristics of pollen germination of Primula obconica. J. Northwest For. Univ. 2017, 32, 170–173. [Google Scholar] [CrossRef]
- Wang, Q.L.; Lu, L.D.; Wu, X.Q.; Li, Y.Q.; Lin, J.X. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Lv, F.D.; Wang, S.; Guo, Y.F.; Jiang, H.B.; Huang, M.Z.; Chang, S.S. Comparison of pollen characteristics and fertility of six cultivars of blueberry. Nonwood For. Res. 2016, 34, 101–108. [Google Scholar] [CrossRef]
- Dai, H.J.; Zhu, Z.B.; Shen, X.L.; Zhou, J.M.; He, J.H. Essences and approaches of distant hybridization in crops breeding. Genomics Appl. Biol. 2010, 29, 144–149. [Google Scholar] [CrossRef]
- Deng, Y.M.; Teng, N.J.; Chen, S.M.; Chen, F.D.; Guan, Z.Y.; Song, A.P.; Chang, Q.S. Reproductive barriers in the intergeneric hybridization between Chrysanthemum grandiflorum (Ramat.) Kitam. and Ajania przewalskii Poljak. (Asteraceae). Euphytica 2010, 174, 41–50. [Google Scholar] [CrossRef]
- Geng, X.M.; Zhang, C.Y.; Luo, F.X.; Wang, L.G. Study on seed setting of wild Rhododendron in China. Jiangsu Agric. Sci. 2013, 41, 159–161. [Google Scholar] [CrossRef]
- Preston, R.E. The intrafloral phenology of Streptanthus tortuosus (Brassicaceae). Am. J. Bot. 1991, 78, 1044–1053. [Google Scholar] [CrossRef]
- Soares, T.L.; Jesus, O.N.; Souza, E.H.; Oliveira, E.J. Floral development stage and its implications for the reproductive success of Passiflora L. Sci. Hortic. 2018, 238, 333–342. [Google Scholar] [CrossRef]
- Peng, X.L.; Liao, K.; Jia, Y.; Liu, H.; Ma, W.; Xu, L. Study on the cross compatibility among 9 apricot cultivars in Xinjiang. J. Fruit Sci. 2015, 32, 192–199. [Google Scholar] [CrossRef]
- Sun, C.Q.; Ma, Z.H.; Zhang, Z.C.; Sun, G.S.; Dai, Z.L. Factors influencing cross barriers in interspecific hybridizations of water lily. J. Am. Soc. Hortic. Sci. 2018, 143, 130–135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).