Abstract
The conservation of species should be based on knowledge of habitat requirements, population structure and conservation status. This knowledge is quite important to design conservation areas for species and to promote long-term persistence. In this study, we investigated habitat suitability, population size structure and conservation status of Pinanga arinasae in Bali. Plots with palms and adjacent areas with no palms were sampled to characterize key habitat variables. Habitat suitability was modeled using Artificial Neural Network (ANN) and Random Forest (RF) methods. The population size structure was characterized by counting and measuring the height and reproductive status of the individuals found in plots. Furthermore, we assessed the extinction risk of the species using the IUCN Red List Criteria. The ANN variables that best explained occurrence were litter depth, elevation, canopy openness and slope. The RF variables that best explained the data were elevation, litter depth, slope, and aspect. Both ANN and RF are robust models that can be used to predict the occurrence of P. arinasae. The population size structure included many seedlings, but juvenile and mature individuals were found in relatively small numbers. Based on the findings, we proposed Endangered B1+B2ab(i,ii,iii,v); D as the conservation status of P. arinasae.
1. Introduction
Indonesia is a center of palm diversity, with approximately 570 palm species (out of about 2600 known species) [1,2]. Endemism and habitat specificity are factors often used in evaluating species rarity [3]. Endemism is highly correlated with the small species ranges and restricted distributions [4]. Endemic palms tend to have relatively small population sizes, small habitat ranges, and prolonged evolutionary isolation, as seen in Borassodendron borneense J. Dransf. [5], Calamus glaucescens (Blume) D. Dietr. [6], Pinanga javana Blume, and P. arinasae Witono [7]. Therefore, they are likely to be more vulnerable to human or natural threats [8].
Pinanga arinasae Witono is an endemic palm species in Bali, Indonesia. The species is reported only in Bukit Tapak, part of the Batukahu nature reserve. The species is probably more widely distributed, but its existence is now highly degraded, since the species was collected as an ornamental palm, its young fruits are a substitute for betel nut (Areca catechu), its young leaves (cabbage or umbut) can be eaten, its stems are used for traditional cremation ceremonies (“ngaben”), and its leaf-sheaths are used for making “cukup” (Balinese umbrella). This palm is generally uncommon in its natural habitat, with palm seedlings seldom growing to be reproductively mature [7]. To develop a comprehensive conservation action for an endemic palm species, basic information on population size, range and the habitat requirement of the species is needed [9,10].
Statistical and machine learning techniques are nowadays used to understand and model the habitat requirements of plant species. We selected Artificial Neural Networks (ANN) and Random Forests (FR) to elaborate the species distribution model of this endemic palm. Artificial Neural Networks [11,12] were originally developed as a model of human brains. Artificial neural network techniques are well suited for modeling complex phenomena with non-linear relationships [13]. These techniques have been widely used for applications such as remote sensing image classification [14] and related modeling [15]. Artificial neural networks have been effective tools for habitat suitability modelling [16], population prediction, community development [17,18,19], classification and association [12], and patterning complex relationships [20,21]. Random Forests (RF) or random decision forests are a machine learning method for classification and regression. The Random Forest technique was created to provide accurate predictions while reducing the problem of overfitting the data [22,23]. Random Forest modeling in species distribution models has been shown to have higher prediction accuracy than decision trees and other models [24,25].
Habitat suitability, population size structure and conservation status of P. arinasae are very important to study in view of its status as endemic palm species in Bali. This study aims to document the ecology of P. arinasae for conservation planning and to maintain its sustainability in its natural habitat.
2. Materials and Methods
2.1. Study Sites
We assessed the habitat suitability and population structure of P. arinasae by direct field observations of known locations for this species. The distribution of P. arinasae was assessed in three locations in Bali, i.e., Bukit Tapak (Batukahu nature reserve), Jatiluwih village (Tabanan), and Pilan Customary Forest (Gianyar) (Figure 1). Bukit Tapak covers 810.40 ha area [26]. This location has various topographies from flat to steep, with a slope range of approximately 8% to 45%. The dominant species in this location are Dacrycarpus imbricatus (Blume) de Laub., Casuarina junghuhniana, Magnolia sp., Crypteronia paniculata Blume, and Pometia sp. [27].
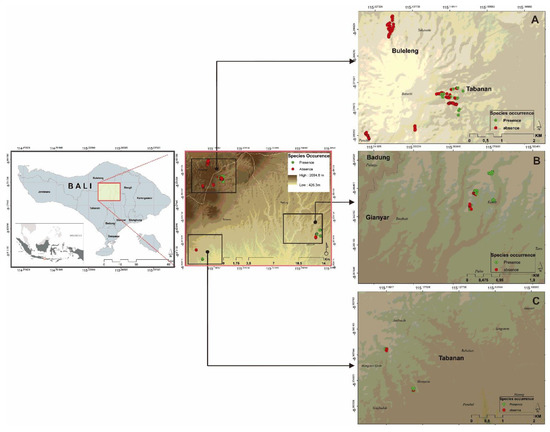
Figure 1.
Research locations: (A). Bukit Tapak nature reserve, (B). Pilan customary forest, and (C). Jatiluwih. Green dot represent the presence of this species and red dot represent the absence of this species in those locations.
Jatiluwih village is located in the Penebel subdistrict, District of Tabanan, Bali. Jatiluwih is in the highland below Batukaru mountain. The elevation of this site ranges from 500–1500 m above sea level and the annual precipitation rate is 2500 mm/year. The average temperature in this area is approximately 19 °C [28]. The topography is mountainous [29].
Pilan Customary Forest is located in Kerta village, Payangan sub-district, district of Gianyar. Kerta village has 4 customary forests that are located in four villages, including Pakraman Panyabangan, Pilan, Seming and Marga Tengah. The species target of this study was only found in Pilan Village in five small patches of customary forest, namely Pure Puseh, Pucak Tegeh, Pucak Sari, Pure Dalem, and Tegal Suci.
Those locations were chosen because unverified information was obtained from interviews with local people and forest authorities who know of the presence of this species in those locations. The locations where the species was found to be present and absent were used to provide data to model habitat suitability using Artificial Neural Network (ANN) and Random Forest (RF) models.
2.2. Sampling Data
Transect sampling was used to assess the habitat suitability of P. arinasae. A straight line transect going upslope from the first point was made where P. arinasae was found. Then, we established a square plot along the transect line to assess the presence and absence records. A 10 × 10 m square plot was used to assess juvenile and mature individuals, while a 2 × 2 m square plot anchored inside the corner of a 10 × 10 m plot was used to assess P. arinasae seedlings. We also expanded our sampling to create other plots surrounding the presence plots until we did not find P. arinasae individuals. Each of the plots was located using GPS. We recorded those data for 191 plots (Figure 1), consisting of presence and absence plots in three different locations.
2.3. Habitat Suitability
The environmental attributes recorded at each quadrat plot include elevation, slope, aspect, litter depth, canopy openness, soil moisture, soil pH, humidity, temperature, and light intensity. The measurement of those environmental attributes was conducted in all observation plots. Soil moisture and pH were measured using a standard soil tester. Relative humidity was measured with a hygrometer hanging upon a branch within each plot, after waiting some minutes to obtain a stable measurement. A Lux meter was used to measure light intensity within the plot. A vertical photograph was taken from the center of plot, then the photo was analyzed using the Gap Light Analyzer (GLA), Version 2.0 software [30]. Two different model approaches were applied in this study to predict habitat suitability. Environmental data coupled with data on the locations of palm occurrence were used to train an ANN and a RF with ten predictor environmental variables (Figure 2). These variables included elevation, slope, aspect, litter depth, canopy openness, soil moisture, soil pH, humidity, temperature, and light intensity. All observation plots were assigned, based on the presence or absence of the species. Using all of the data for model fitting allows no testing of model generality. The data were partitioned into training and testing sets. The proportion of testing data should be , where p is the number of predictors. If there are only two predictors, the train test ratio should be 50:50, whereas if there five predictors, it should be 67:33. If there are many (>10) predictors, it should be 75:25 [31]. The observation data were therefore divided into training data and test data with a ratio of 75:25, because the input data contained ten environmental variables. The data partitioning in this study consisted of 143 training data and 48 test data. This data partitioning was made without using the presence/absence status of the plots to assign groups.
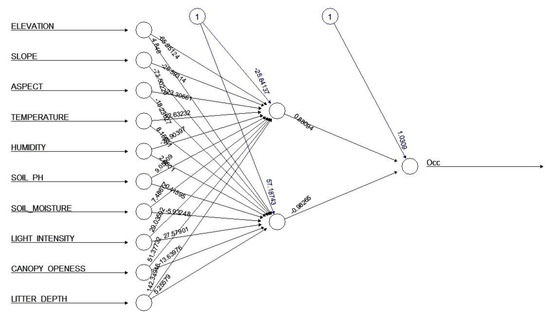
Figure 2.
Artificial Neural Network (ANN) model with ten input variables, two neurons in one hidden layer, and one output.
The first layer of the ANN consisted of an input layer with the ten environmental variables. One output layer represents the predicted presence or absence (occurrence) of the species based on the explanatory variables. A single hidden layer ANN architecture was employed in this study because fitting this type of model using backpropagation is commonly used in ecology research [32]. The number of nodes (neuron units) within the hidden layer that yielded the best model performance and smallest model error predictions was tested. These tests were conducted in a single hidden layer with numbers of nodes from 1 to 9. This model was implemented using the “neuralnet” version 1.33 package [33]. Model performance or fit was measured by using AUC (area under ROC curve). The model was run 1000 times. The kappa statistic was used to evaluate the performance of model. Four repeated random partitions of the data set were created. The relative importance method was used in order to determine the environmental variables that most influenced the occurrence of the species in the sampling area [34]. The relative importance of the input variables in the neural networks was calculated as the sum of the product of raw, input-hidden, hidden-output connection weights [34]. A confusion matrix was used to describe the performance of a classification model on a set of test data. The confusion matrix was displayed as a table that indicated presence, false presence, absence and false absence. The prediction error of ANN was also calculated.
In the RF model, an iterative process was used to hierarchically split the decision tree using the variables available for splitting at each tree node (“mtry”) Parameter adjustments were applied to obtain the best model performance or fitting model of the random forest. We used “mtry” from 1 to 9 to determine the best model performance. We used the MASS [35] and randomForest [36] packages to run this model. Model performance was represented by AUC. The fitting process was set to use 1000 trees for model development. The prediction error was calculated using the “curay metric” that is specific for random forest models. Four folds of cross-validation were used to test this model. The kappa statistic was the measure of model fit for cross-validation. The “VarImp” function was used to estimate the relative importance of variables that influenced the occurrence predictions of the species.
The statistical software R was used to perform the analysis [37]. We used the “neuralnet” R package [33] for the neural network and ‘‘randomForest’’ for the RF [38].
2.4. Population Structure
The measurements recorded for this study included the numbers of individual plants in each plot to determine palm tree density classified as mature, juvenile or seedling, and the height of the individual palm specimens from the visible stem up to the upper part of the leaf sheath. The population structure was assessed by grouping individuals into the mature, juvenile, and seedling classifications, based on height. Mature individuals were those that had entered the generative reproduction phase. Juvenile individuals were classified as having more than five leaves. We also classified individuals into ten height classes, including 0–2, 2–4, 4–6, 6–8, 8–10, 10–12, 12–14, 14–16, 16–18, and 18–20 m.
2.5. Conservation Status Assessment
The conservation status of P. arinasae was assessed by criteria B and D, as defined by the IUCN Red List Category and Criteria Version 3.1 [39]. The extent of occurrence (EOO) and area of occupancy (AOO) used in criterion B were calculated using the Geospatial Conservation Assessment Tool (GeoCAT) [40]. For criterion D, population size was calculated from the total number of mature individuals of the species.
3. Results
3.1. Model Performance or Fitting Model
The Artificial Neural Network (ANN) and Random Forest (RF) models were used to predict the occurrence of P. arinasae. Single hidden layer ANNs with 2 nodes, 4 nodes, 6 nodes, 7 nodes, and 9 nodes had an AUC > 0.90 (Table 1). The highest kappa score was found in the model with 2 nodes, with the kappa score = 0.63. For the prediction error of the model, a single hidden layer ANN with 2 nodes showed the lowest prediction error (error model = 0.04). In the RF model, several “mtry” (the number of variables available for splitting at each tree node) parameters were manipulated to obtain the best performance of RF model prediction. The RF model prediction with “mtry” values from 2 to 9 had a similar AUC score (AUC = 0.94) (Figure 3). The highest kappa score (kappa = 0.74) was found in RF model with “mtry” = 6. Th RF models with “mtry” from 2 to 9 had similar model prediction error (error model = 0.12) (Table 2).

Table 1.
AUC, kappa, and error model of ANNs.
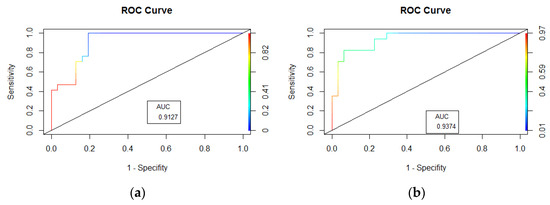
Figure 3.
Area under ROC curve: (a). Artificial Neural Network (ANN) and (b) Random Forest (RF) of P. arinasae.

Table 2.
AUC, kappa and error model of RFs.
The confusion matrix represented true presence, false presence, true absence, and false absence. The confusion matrix was calculated using the 48 test data sets that were selected from total number of observations. The ANN model with 2 nodes yielded a confusion matrix with 29 true absences, 0 false absences, 17 true presences, and 2 false presences (Figure 4). The confusion matrix of RF model with “mtry” = 6 showed that there were 30 true absences, 1 false absence, 17 true presences, and 5 false presences (Figure 5).
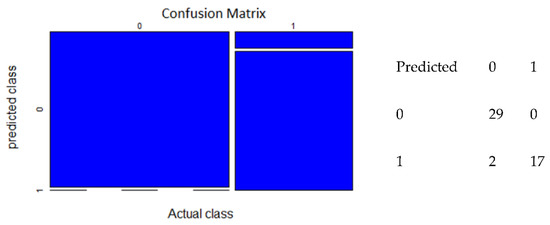
Figure 4.
Confusion matrix of ANN model (predicted class vs. actual class).
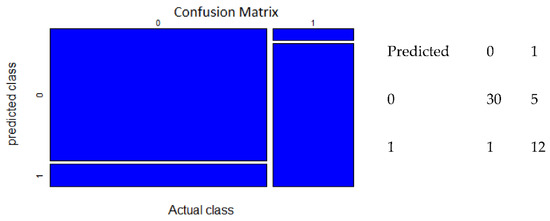
Figure 5.
Confusion matrix of RF model (predicted class vs. actual class).
3.2. Habitat Suitability
The Artificial Neural Network (ANN) and Random Forest (RF) models were used to assess the environmental variables that influenced the predicted occurrence of P. arinasae. Based on a qualitative assessment of ten environmental variables, it appeared that elevation, slope, aspect, litter depth and humidity were different between the locations where the species was present and absent (Figure 6).
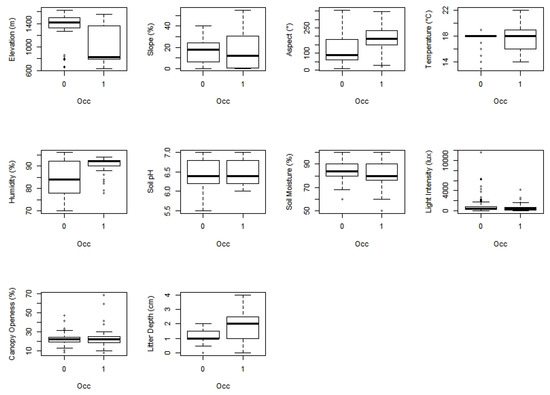
Figure 6.
Ten environmental variables in predicting of P. arinasae occurrence. 0 represents an absence location and 1 represents a presence location.
The ANN suggested that there were several environmental variables that influenced the species’ occurrence. These environmental variables were litter depth, elevation, canopy openness and slope. Those variables had higher scores than the others. The elevation indicated that there was a negative association with species occurrence. Conversely, litter depth and canopy openness had a positive association with species occurrence (Figure 7).
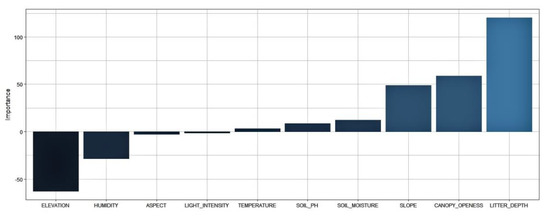
Figure 7.
The importance of the ten explanatory variables for the response variable in determining species presence using ANN.
The RF predictive model implied that there were four environmental variables that most influenced the species occurrence. Those variables were elevation, litter depth, slope, and aspect (Figure 8). The score of each environmental variable is presented in Table 3.
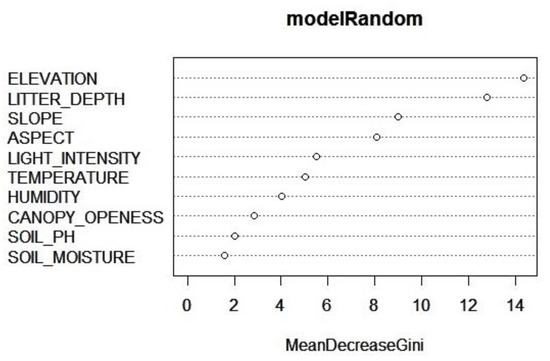
Figure 8.
The importance of the ten explanatory variables for the response variable in determining species presence using RF.

Table 3.
The values of ten explanatory variables for the response variable in determining species presence using Random Forest (RF).
3.3. Population Structure
In the sampled plots, mature individuals represented the bulk of the population across size classes. Mature individuals were found with heights from 4 to 20 m. The highest number of mature individuals was seen in the height class of 10–12 m (Figure 9). Juvenile individuals were found in the class grouping with heights from 2 to 10 m. The highest number of juvenile individuals were seen in height class of 4 to 6 m (Figure 10). Seedling individuals were only found in the height class from 0 to 2 m. A total of 3327 individuals were located at all the study sites. Most of the individuals (91.6%) were seedlings, with only 134 of them (4%) in the mature stage.
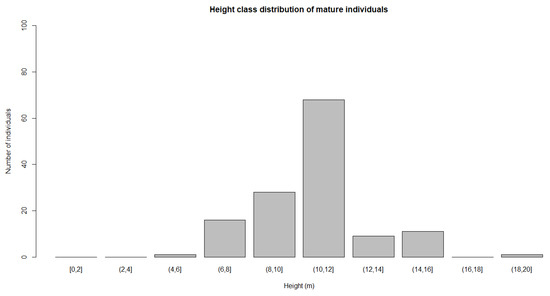
Figure 9.
Height class distribution of mature individuals.
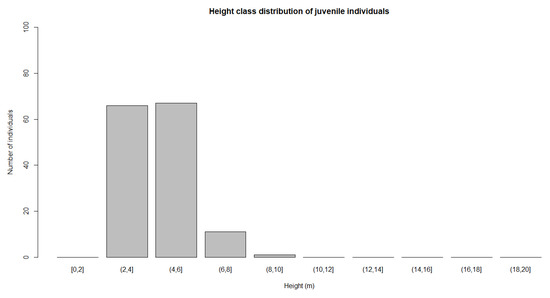
Figure 10.
Height class distribution of juvenile individuals.
3.4. Conservation Status
According to our GeoCAT calculation, P. arinasae had an EOO and AOO of 128.8 km2 and 48 km2, respectively. The species is distributed in three locations and is threatened by unsustainable harvesting [7]. Therefore, the species was qualified for the Endangered category under the criteria B1+B2(i,ii,iii,v). i.e., EOO < 5000 km2, AOO < 500 km2, number of locations <5 and continuing decline in EOO, AOO, quality of habitat and number of mature individuals. Furthermore, based on the total number of mature individuals found during the survey, the population size of P. arinasae was 134 individuals. Under criterion D, the species could also be assessed as Endangered. Based on this assessment, we proposed Endangered B1+B2ab(i,ii,iii,v); D as the conservation status of P. arinasae.
4. Discussion
4.1. Model Performance
The RF and ANN models resulted in good model performance for the prediction of P. arinasae occurrence in the study locations. This ANN model had an AUC score of 0.91, indicating an excellent model with low prediction error. In this model, the number of iterations used for model training had little effect on the model performance. The back-propagation method of fitting model weights can be subject to getting stuck in a local optimum or overfitting the training data. The decay parameter reduces some weights during fitting in order to avoid overfitting. Changes of the decay parameter had little effect on the model performance over 1000 runs. In order to evaluate the ANN, the highest score of the kappa statistic from the repeated runs was used. The kappa statistic had a score of 0.63, indicating substantial agreement. The confusion matrix of the ANN model showed that there were 29 true predicted absences, 0 predicted false absences, 17 predicted true presences, and predicted 2 false presences. This model had 2 commission errors and 0 omission errors, which means that this model had a high classification accuracy.
The RF model with the number of variables available for splitting at each tree node (“mtry”) = 6 provided the best model performance to predict the occurrence of P. arinasae. The RF with “mtry” = 6 had the AUC score (AUC) = 0.94. This model was identified as an excellent predictor of presence and absence. The highest kappa statistic was found in the random forest model with “mtry” = 6. The kappa statistic score of 0.74 indicated substantial agreement. Data on 1000 growing trees was used in fitting this model. The number of growing trees in this model did not seem to affect the model’s performance and prediction error. The confusion matrix for the RF model showed that there were 30 predicted true absences, 1 predicted false absence, 17 predicted true presences, and 5 predicted false presences. This model had 5 commission errors and 1 omission error, which means that this model also has high classification accuracy.
4.2. Habitat Suitability
The ANN model suggested that litter depth, elevation, canopy openness, and slope were the most important environmental variables for prediction of P. arinasae occurrence. Those environmental variables had high scores in the variable importance assessment (Figure 7). Litter depth is probably associated with suitable environmental conditions in the germination and establishment of seedlings. A deep litter may increase seed germination and survival by reducing variation in moisture and soil temperature. Deep litter could reduce the chance of predators finding the seeds [41]. The establishment of a thick leaf litter layer affects microsite factors, such as light, temperature, moisture, and nutrient availability. These factors have significant consequences for germination and seedling survivorship [42]. The leaf litter was considered an important factor in maintaining higher seedling diversity and establishment success of plants growing in a tropical forest. Different species should be expected to respond differently to the same litter environment. Litter can promote species diversity at the seedling stage in several ways. Environmental heterogeneity can be created by litter. The presence of litter in the environment can alter the relative proportions of present species [43]. Some studies reported that Gustavia had more seedlings emerging under litter than on bare ground exposed to the sun. The positive effect on Gustavia seedlings was attributed to the higher moisture and humidity under the litter. The presence of litter creates a more suitable habitat for Gustavia in gaps and allows Gustavia seedlings to use areas of the gap that may be inaccessible to other species, since Gustavia germinates later than these other species [44]. The number of Dipteryx seeds that successfully germinated under litter was about four times higher than above litter. The litter depth was relatively thick where the species is present in all of observation plots. The litter layer included a lot of plant material debris, such as fallen leaves and rotting branches that have not been completely decomposed. The soil usually has a thick layer of humus [45].
The ANN model implicated elevation as another environmental variable that influences the occurrence of P. arinasae. Based on the model, elevation has a negative association with species distribution. Elevation likely affects the temperature, precipitation, and radiation regimes. The limitations of our sampling regime (transects along the slope from known locations with present individuals) makes this environmental variable more likely to be a reflection of absence plots that differ consistently, but by small amounts in elevation. It is possible that variation of the underlying geology in the plots could reflect variation in mineral nutrient availability. Many factors such as soil pH, available nutrient content, and availability of soil water are important for plants and influenced by topography [46,47]. The occurrence of P. arinasae in Bukit Tapak was seen at elevations from 1378 to 1533 m above sea level. In the area with an elevation of 1378 m, where the first point of occurrence was recorded in Bukit Tapak, the palm were growing in a relatively flat area. They typically appear to be somewhat spatially clumped, and they are typically shorter than surrounding vegetation. The height of many individuals was about 11.5 m. Only a few mature and juvenile individuals were seen in this location. We found that some seedlings were growing under mature individuals, but the number was relatively small. In the area with the elevation up to 1400 m, some individuals were growing well on steep slopes. They were mostly classified as in the reproductive phase and reached heights up to 18 m. Some individuals at this location were quite difficult to assess because the slope was quite steep. Those individuals tended to establish on different hills far away from each other, but we were still able to see the larger palms from adjacent sites. The palm was seen in several locations at the Jatiluwih site, at an elevation of around 670 m above sea level. These were locations where the palm was seen surrounding a rice farm (paddy farm) and a vegetable plantation. The palm mostly grows on the slope, with a small river on the lower slope. The palm is not believed to be intentionally planted by local people but was naturally growing on that location, based on reports from the local people. In Pilan customary forest, the occurrence of the palm was seen in all customary forest patches. Pilan customary forest has an elevation of around 820 m above sea level.
Canopy openness was the third relatively important factor that affected the occurrence predictions. The ANN model showed a positive association between canopy openness and species occurrence. Canopy openness is correlated with the light intensity that can reach the vegetation. If the canopy cover is dense, there is reduced light availability for the forest floor vegetation. The occurrence of mature individuals in the three research locations (Bukit Tapak, Jatiluwih, and Pilan Customary Forest), tended to be associated with relatively high canopy openness. Some seedlings, juvenile and mature individuals were also seen beneath the surrounding canopy vegetation.
The ANN showed there was a positive correlation between slope and species occurrence. Slope is potentially related to the flow of water, therefore it affects potential soil moisture and soil characteristics (texture, moisture regime, and development) [48]. The occurrence of the palm was seen on steep slopes in both Bukit Tapak and Jatiluwih. In contrast, the Pilan customary forest palms were found in places with little slope. The RF model analysis found several environmental variables that influenced the occurrence of P. arinasae. Those variables were similar to those from the ANN model analysis, namely elevation, litter depth, and slope. The direction of the slope (aspect) is facing affects the amount of solar radiation received on the slope and the seasonal and annual patterns of solar insolation (radiation). Slope direction may affect soil moisture availability [48]. The RF model does not represent the correlation of each environmental variable (predictors) with species occurrence (response variable).
4.3. Population Structure
Height was chosen as a variable to represent the population structure in this study. Height has been found to be a good predictor of palm fecundity than age in a threatened Madagascar palm species, Neodypsis decaryi Jumelle [49]. We found that mature individuals with a height of 4 m, have already entered the reproduction phase (Figure 9). Those individuals seemed to mature earlier, assuming an age/size relationship. Plant growth and development is likely influenced by climatic factors, such as rainfall, temperature, relative humidity, and wind. For instance, temperature might be associated with the plant’s metabolic rate. Rising temperature could accelerate photosynthesis and respiration rates, perhaps producing plants that tend to mature earlier. However, excessively high temperature will inhibit the growth and development of a plant [50]. Some mature individuals can reach 20 m tall. Their heights are taller than surrounding vegetation. They produce a huge number of fruits. In Pilan customary forest, mature individuals had a height up to 15 m, taller than the surrounding vegetation. Here mature individuals were producing many mature fruits. Some of the mature individuals were growing close to each other. In Bukit Tapak, mature individuals were growing well on the steep hill, and the palm reached up to 18 m tall. The mature individuals were flowering and fruiting. Some of the mature individuals that were recorded had a height up to 20 m. There were 134 totally mature individuals found during the field survey.
Juvenile individuals were classified as those with heights of 2–10 m (Figure 10). Even though they had a height around 10 m, they had not yet entered the reproducing phase. In Pilan customary forest, juvenile individuals were growing well, surrounded by mature trees. It was suggested that those individuals were the offspring of the mature trees. In Bukit Tapak, juvenile individuals were established far away from the mature trees. We saw those individuals clustered in areas without mature individuals. A small number of seedlings were recorded in Jatiluwih.
Seedling individuals were only found in one class height (0 to 2 m). A total of 3048 seedlings were observed in this study. Seedling individuals were found in large numbers in the Pilan customary forest. They were growing well under mature individuals. Even fallen inflorescences from mature individuals were able to germinate a local high density of seedlings. In contrast, we did not observe a large number of seedlings growing at the higher-elevation Bukit Tapak site. The dense forest floor vegetation covered a large fraction of the slope. Only few seedlings were seen in the location where mature individuals were present, and no seedlings were found in the other locations beneath the mature palms. There were several possibilities as to why seedlings were not growing in locations where mature palms were found, including: (1) the seeds might have moved down to the lower part of the hills by water and germinated in that area, (2) the seeds might be dispersed by civet cat, since the animal has been reported feed on and defecate viable seeds of P. javana, a species closely related to P. arinasae [7], (3) the seeds germinate but were outcompeted by the surrounding vegetation. The higher mortality rate in the early stage of growth could be caused by dense cover from palm crowns and Pandan leaves that reduce sunlight exposure and by competition for space [51]. Finally, (4) the upper slope could have a higher soil temperature because of light directly hitting this surface [52]. The soil conditions might inhibit the seedling growth. Seedlings were recorded in the Jatiluwih site, but the abundance of the seedlings was relatively small. Canopy openness was one factor that influenced habitat suitability. Based on the ANN model, it has positive correlation with occurrence, which means this palm preferred locations with bigger canopy openness.
Pilan customary forest consisted of several forest patches and belongs to an indigenous customary forest. Local people have protected those forests from encroachment for a long time. Nobody can enter the location without a permit from the leader of the customary community. Those forests were dense compared to the other study sites. All growth stages of palm were seen in this location, the abundance there was the highest among the study sites. The high palm abundance occurs here, although some plantations (vegetables and citrus) have been established surrounding Pilan customary forest. The status of the Bukit Tapak is a protected area, with limited access and legal permits required for any kind of activities inside of the reserve. Human threats to the palms on the reserve are currently minimal. Furthermore, these palms were growing well on the steep hills (slope).
The population demography of this palm is an important research question to be addressed because only few juvenile and mature individuals were found in the field survey. Seedlings were rarely seen under the mature palm. In the Jatiluwih study site, the abundance and occurrence was lower and they were typically found to grow in slope valleys. The status of the palm in the Jatiluwih was is the most vulnerable because the locations where the palm was present were owned by local people. They could convert their lands into paddy farms or plantations in the future. These study areas, including Bukit Tapak, Pilan customary forest and Jatiluwih were located around Batukahu mountain. A long time ago, this palm was alleged to grow on Batukahu mountain, then it was dispersed to the lower locations in surrounding.
4.4. Conservation Status and Implications
The IUCN Red List is the most widely used tool to assess the extinction risk of a species. It has also become a powerful instrument for monitoring, management, decision making and conservation planning [53]. Pinanga arinasae currently has not been assessed for its conservation status based on the IUCN Red List Category and Criteria. Based on the findings of the present study, we proposed Endangered B1+B2ab(i,ii,iii,v); D as the conservation status of the species. Under this category, P. arinasae is considered to be facing a very high risk of extinction in the wild. Therefore, further studies and comprehensive conservation action need to be implemented immediately in order to conserve the species and its habitat.
Studies on the genetic diversity and structure of the species would be essential to support both in situ and ex situ conservation actions. A prediction of the distribution of the species across Bali Island could be conducted to identify unsurveyed potential habitats of the species. It is also important to identifying suitable habitats for species reintroduction and restoration. In addition, seeds and living collections of the species from the study sites should be collected at botanic gardens to serve as ex situ collections. The collections can be used for public education and awareness as well as for the source of propagation material to support reintroduction programs in the future.
5. Conclusions
Both Artificial Neural Network (ANN) and Random Forest (RF) are robust models that can be used to predict the occurrence of P. arinasae. Based on a habitat suitability assessment, those models implicate many of the same environmental variables that affect the occurrence of P. arinasae. Population demography (survivorship and fecundity) over time (stochasticity) of P. arinasae needs to be addressed in order to properly evaluate the sustainability of this endemic palm species in Bali, Indonesia. According to the IUCN Red List criteria, the conservation status of this species is Endangered B1+B2ab(i,ii,iii,v); D. Although there were several limitations of this study, the results can be used as a basic starting point for management and conservation actions of endemic palm P. arinasae.
Author Contributions
Conceptualization, A.Y., W.P.C.J.; methodology, A.Y., P.D.R.; software, A.Y. and P.D.R.; validation, A.Y., J.R.W., Y. and W.P.C.J.; formal analysis, A.Y., W.P.C.J. and P.D.R.; investigation, E.M. and I.P.A.; resources, E.M., I.P.A., I.A.F., I.R. and R.N.Z.; data curation, R.N.Z., I.R., I.A.F., Y. and A.Y.; writing—original draft preparation, All authors.; writing—review and editing, All authors; visualization, A.Y., P.D.R. and W.P.C.J.; supervision, A.Y., J.R.W. and W.P.C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Center for International Forestry Research (CIFOR) Indonesia and USAID Forestry Fellowship Program.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data are not yet publicly available. Individual requests can be addressed to the corresponding author.
Acknowledgments
We thank the Director of CIFOR Indonesia, who provided a fund to accomplish this study and the Director of Research Center for Plant Conservation and Botanic Gardens, who allowing us to conduct this study. Our great appreciation is delivered to our research coordinator that support all of our research activities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uhl, N.W.; Dransfield, J.; He Moore, J.R. Genera Palmarum; The LH Bailey Hortorium and The International Palm Society: Lawrence, KS, USA, 1987. [Google Scholar]
- Dransfield, J. Conservation of the Diversity of Indonesian Palms. In Strategic for Flora Conservation in Asia; Suhirman, Butler, G., Fuaddini Pfeiffer, J., Richardson, M.S., Eds.; Kebun Raya Bogor: Bogor, Indonesia, 1994; pp. 77–84. [Google Scholar]
- Goerck, J.M. Patterns of Rarity in the Birds of the Atlantic Forest of Brazil: Patrones de Rareza En Las Aves Del Bosque Atlántico de Brasil. Conserv. Biol. 1997, 11, 112–118. [Google Scholar] [CrossRef]
- Savage, J.M. The Dispersal Centres of Terrestrial Vertebrates in the Neotropical Realm. A Study in the Evolution of the Neotropical Biota and Its Native Landscapes; Springer: Cham, Switzerland, 1974. [Google Scholar]
- Dransfield, J. The Genus Borassodendron (Palmae) in Malesia. Reinwardtia 2014, 8, 351–363. [Google Scholar]
- Dransfield, J. A Monograph of Ceratolobus (Palmae). Kew Bull. 1979, 34, 1–33+ii. [Google Scholar] [CrossRef]
- Witono, J.R.; Mogea, J.P.; Somadikarta, S. Pinanga in Java and Bali. Palms 2002, 46, 193–202. [Google Scholar]
- Frankham, R. Inbreeding and Extinction: Island Populations. Conserv. Biol. 1998, 12, 665–675. [Google Scholar] [CrossRef]
- Kozlowski, G.; Matthies, D. Habitat Differentiation in the Threatened Aquatic Plant Genus Baldellia (L.) Parl.(Alismataceae): Implications for Conservation. Aquat. Bot. 2009, 90, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Zhang, Q.; Wang, Z.; Guo, Q.; Wang, J.; Liu, N.; Liang, K. Conservation and Possible Reintroduction of an Endangered Plant Based on an Analysis of Community Ecology: A Case Study of Primulina Tabacum Hance in China. Plant. Species Biol. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Ripley, B.D. Pattern Recognition and Neural Networks; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lek, S.; Giraudel, J.L.; Guégan, J.-F. Neuronal Networks: Algorithms and Architectures for Ecologists and Evolutionary Ecologists. In Artificial Neuronal Networks; Springer: Cham, Switzerland, 2000; pp. 3–27. [Google Scholar]
- Özesmi, S.L.; Özesmi, U. An Artificial Neural Network Approach to Spatial Habitat Modelling with Interspecific Interaction. Ecol. Modell. 1999, 116, 15–31. [Google Scholar] [CrossRef]
- Benediktsson, J.A.; Swain, P.H.; Ersoy, O.K. Conjugate-Gradient Neural Networks in Classification of Multisource and Very-High-Dimensional Remote Sensing Data. Int. J. Remote Sens. 1993, 14, 2883–2903. [Google Scholar] [CrossRef]
- Shoemaker, D.A.; Cropper, W.P. Application of Remote Sensing, an Artificial Neural Network Leaf Area Model, and a Process-Based Simulation Model to Estimate Carbon Storage in Florida Slash Pine Plantations. J. For. Res. 2010, 21, 171–176. [Google Scholar] [CrossRef]
- Paruelo, J.; Tomasel, F. Prediction of Functional Characteristics of Ecosystems: A Comparison of Artificial Neural Networks and Regression Models. Ecol. Modell. 1997, 98, 173–186. [Google Scholar] [CrossRef]
- Tan, S.S.; Smeins, F.E. Predicting Grassland Community Changes with an Artificial Neural Network Model. Ecol. Modell. 1996, 84, 91–97. [Google Scholar] [CrossRef]
- Recknagel, F.; French, M.; Harkonen, P.; Yabunaka, K.-I. Artificial Neural Network Approach for Modelling and Prediction of Algal Blooms. Ecol. Modell. 1997, 96, 11–28. [Google Scholar] [CrossRef]
- Chon, T.-S.; Park, Y.-S.; Park, J.H. Determining Temporal Pattern of Community Dynamics by Using Unsupervised Learning Algorithms. Ecol. Modell. 2000, 132, 151–166. [Google Scholar] [CrossRef]
- Lek, S.; Delacoste, M.; Baran, P.; Dimopoulos, I.; Lauga, J.; Aulagnier, S. Application of Neural Networks to Modelling Nonlinear Relationships in Ecology. Ecol. Modell. 1996, 90, 39–52. [Google Scholar] [CrossRef]
- Tuma, A.; Haasis, H.-D.; Rentz, O. A Comparison of Fuzzy Expert Systems, Neural Networks and Neuro-Fuzzy Approaches. Controlling Energy and Material Flows. Ecol. Modell. 1996, 85, 93–98. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Using Models to Infer Mechanisms. IMS Wald Lect. 2002, 2, 59–71. [Google Scholar]
- Prasad, A.M.; Iverson, L.R.; Liaw, A. Newer Classification and Regression Tree Techniques: Bagging and Random Forests for Ecological Prediction. Ecosystems 2006, 9, 181–199. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards Jr, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Balai Konservasi Sumber Daya Alam. Available online: http://www.ksda-bali.go.id/kawasan-hutan/kawasan-konservasi/cagar-alam-batukahu/ (accessed on 9 February 2018).
- Satyanti, A.; Siregar, H.-M. Microclimate Preference and Habitat of Begonia in Bedugul, Bali. Biotropia 2012, 19, 80–91. [Google Scholar]
- Putra, P.A.M.; Pamungkas, I.N.A. Membangun Brand Awareness Objek Wisata Jatiluwih Tabanan Bali. eProc. Manag. 2019, 6, 1561–1567. [Google Scholar]
- Dewi, M.H.U. Pengembangan Desa Wisata Berbasis Partisipasi Masyarakat Lokal Di Desa Wisata Jatiluwih Tabanan, Bali. J. Kawistara 2013, 3, 129–139. [Google Scholar]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs; Users Manual and Program Documentation; Simon Fraser University: Burnaby, BC, Canada, 1999; Volume 36. [Google Scholar]
- Huberty, C.J. Why Multivariable Analyses? Educ. Psychol. Meas. 1994, 54, 620–627. [Google Scholar] [CrossRef]
- Olden, J.D.; Lawler, J.J.; Poff, N.L. Machine Learning Methods without Tears: A Primer for Ecologists. Q. Rev. Biol. 2008, 83, 171–193. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, S.; Guenther, F.; Suling, M.; Mueller, S.M. Neuralnet: Training of Neural Networks (R Package Version 1.33). 2016. Available online: http://cran.nexr.com/web/packages/neuralnet/neuralnet.pdf (accessed on 24 December 2021).
- Olden, J.D.; Joy, M.K.; Death, R.G. An Accurate Comparison of Methods for Quantifying Variable Importance in Artificial Neural Networks Using Simulated Data. Ecol. Modell. 2004, 178, 389–397. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Exploratory Multivariate Analysis. In Modern Applied Statistics with S; Springer: Cham, Switzerland, 2002; pp. 301–330. [Google Scholar]
- Liaw, A.; Wiener, M. RandomForest: Breiman and Cutler’s Random Forests for Classification and Regression. R Package. Version 2015, 4, 6–10. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: http://r.meteo.uni.wroc.pl/web/packages/dplR/vignettes/intro-dplR.pdf (accessed on 24 December 2021).
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1. IUCN Species Survival Commission; IUCN: Gland, Switzerland; Cambridge, UK, 2001. [Google Scholar]
- Bachman, S.; Moat, J.; Hill, A.W.; De La Torre, J.; Scott, B. Supporting Red List Threat Assessments with GeoCAT: Geospatial Conservation Assessment Tool. Zookeys 2011, 150, 117. [Google Scholar] [CrossRef]
- Sork, V.L. Distribution of Pignut Hickory (Carya Glabra) along a Forest to Edge Transect, and Factors Affecting Seedling Recruitment. Bull. Torrey Bot. Club 1983, 110, 494–506. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett, S.T.A. Plant Litter: Its Dynamics and Effects on Plant Community Structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Molofsky, J.; Augspurger, C.K. The Effect of Leaf Litter on Early Seedling Establishment in a Tropical Forest. Ecology 1992, 73, 68–77. [Google Scholar] [CrossRef]
- Garwood, N.C. Seed Germination in a Seasonal Tropical Forest in Panama: A Community Study. Ecol. Monogr. 1983, 53, 159–181. [Google Scholar] [CrossRef]
- Cintra, R.; Horna, V. Seed and Seedling Survival of the Palm Astrocaryum Murumuru and the Legume Tree Dipteryx Micrantha in Gaps in Amazonian Forest. J. Trop. Ecol. 1997, 13, 257–277. [Google Scholar] [CrossRef]
- Sabatier, D.; Grimaldi, M.; Prévost, M.-F.; Guillaume, J.; Godron, M.; Dosso, M.; Curmi, P. The Influence of Soil Cover Organization on the Floristic and Structural Heterogeneity of a Guianan Rain Forest. Plant. Ecol. 1997, 131, 81–108. [Google Scholar] [CrossRef]
- Sollins, P. Factors Influencing Species Composition in Tropical Lowland Rain Forest: Does Soil Matter? Ecology 1998, 79, 23–30. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Ratsirarson, J.; Silander, J.A., Jr.; Richard, A.F. Conservation and Management of a Threatened Madagascar Palm Species, Neodypsis Decaryi, Jumelle. Conserv. Biol. 1996, 10, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Poincelot, R.P. Horticulture: Principles and Practical Applications.; Prentice-Hall Inc.: Hoboken, NJ, USA, 1980. [Google Scholar]
- Widyatmoko, D.; Burgman, M.A.; Guhardja, E.; Mogea, J.P.; Walujo, E.B.; Setiadi, D. Population Status, Demography and Habitat Preferences of the Threatened Lipstick Palm Cyrtostachys Renda Blume in Kerumutan Reserve, Sumatra. Acta oecologica 2005, 28, 107–118. [Google Scholar] [CrossRef]
- Binkley, D.; Stottlemyer, R.; Suarez, F.; Cortina, J. Soil Nitrogen Availability in Some Arctic Ecosystems in Northwest Alaska: Responses to Temperature and Moisture. Ecoscience 1994, 1, 64–70. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The Value of the IUCN Red List for Conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).