Past, Current and Future of Fish Diversity in the Alakol Lakes (Central Asia: Kazakhstan)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Descriptive Statistics
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, C.B.; Waylen, K.A.; Schmidt-Kloiber, A.; Thackeray, S.J.; Kalinkat, G.; Martens, K.; Domisch, S.; Lillebø, A.I.; Hermoso, V.; Grossart, H.-P.; et al. Safeguarding freshwater life beyond 2020: Recommendations for the new global biodiversity framework from the European experience. Conserv. Lett. 2021, 14, e12771. [Google Scholar] [CrossRef]
- Revenga, C.; Campbell, I.; Abell, R.; de Villiers, P.; Bryer, M. Prospects for monitoring freshwater ecosystems towards the 2010 targets. Philos. Trans. R. Soc. B-Biol. Sci. 2005, 360, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D.; Arthington, A.; Gessner, M.; Kawabata, Z.-I.; Knowler, D.; Leveque, C.; Naiman, R.; Prieur-Richard, A.-H.; Soto, D.; Stianssy, M.; et al. Freshwater biodiversity: Importance, threats, status and conservation challengesю Biological Reviews. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern humaninduced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.S.; Tedesco, P.A.; Hugueny, B.; Jézéquel, C.; Beauchard, O.; Brosse, S.; Oberdorff, T. Anthropogenic stressors and riverine fish extinctions. Ecol. Indic. 2017, 79, 37–46. [Google Scholar] [CrossRef]
- Darwall, W.R.T.; Freyhof, J. Lost fishes, who is counting? The extent of the threat to freshwater fish biodiversity. In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 3–36. [Google Scholar]
- Harrison, I.; Abell, R.; Darwall, W.; Thieme, M.L.; Tickner, D.; Timboe, I. The freshwater biodiversity crisis. Science 2018, 362, 1369. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Kreft, H.; Guralnick, R.; Jetz, W. Global priorities for an effective information basis of biodiversity distributions. Nat. Commun. 2015, 6, 8221. [Google Scholar] [CrossRef] [Green Version]
- Mitrofanov, V.P. Formation of modern ichthyofauna in Kazakhstan and ichthyogeographic zoning. In Fish of Kazakhstan; Nauka: Alma-Ata, Kazakhstan, 1986; pp. 20–40. [Google Scholar]
- Reshetnikov, Y.S.; Shakirova, F.M. Zoogeographic analysis of the ichthyofauna of Central Asia according to the lists of freshwater fish. J. Ichthyol. 1993, 33, 37–45. [Google Scholar]
- Burlibaev, M.J.; Kurochkina, L.Y.; Kascheev, V.A.; Erokhov, S.N.; Ivaschenko, A.A. (Eds.) Globally Significant Wetlands of Kazakhstan. Vol.3; Alakol-Sassykkol Lake System: Astana, Kazakhstan, 2007; pp. 1–295. [Google Scholar]
- Filonets, P.P. Essays on the Geography of Inland Waters of Central, Southern and Eastern Kazakhstan; Nauka: Alma-Ata, Kazakhstan, 1981; pp. 1–292. [Google Scholar]
- Amirgaliev, N.A.; Timirkhanov, S.R.; Alpeisov, S.A. Ichthyofauna and Ecology of the Alakol Lake System; Bastau: Almaty, Kazakzhstan, 2006; pp. 1–368. [Google Scholar]
- Yelshibekova, A.M.; Dan’ko, E.K.; Doukravets, G.M.; Zharkenov, D.K. To history of the formation and development of fish fauna of Alakol Lakes basin. Selevinia. Zool. Yearb. Kazakhstan Cent. Asia 2015, 23, 235–240. [Google Scholar]
- Dukravets, G.M.; Mitrofanov, V.P. History of fish acclimatization in Kazakhstan. In Fish of Kazakhstan; Nauka: Alma-Ata, Kazakhstan, 1992; Volume 5, pp. 6–44. [Google Scholar]
- Mitrofanov, V.P.; Dukravets, G.M. Some theoretical and practical aspects of fish acclimatization in Kazakhstan. In Fish of Kazakhstan; Nauka: Alma-Ata, Kazakhstan, 1992; Volume 5, pp. 329–371. [Google Scholar]
- Tereshchenko, V.G.; Strelnikov, A.S. Analysis of rearrangements in the fish part of the Lake Balkhash community as a result of the introduction of new fish species. J. Ichthyol. 1995, 35, 71–77. [Google Scholar]
- Petr, T.; Mitrofanov, V.P. The impact on fish stocks of river regulation in Central Asia and Kazakhstan. Lakes Reserv. Res. Manag. 1998, 3, 143–164. [Google Scholar] [CrossRef]
- Mamilov, N.S.; Balabieva, G.K.; Koishybaeva, G.S. Distribution of alien fish species in small waterbodies of the Balkhash basin. Russ. J. Biol. Invasions 2010, 1, 181–186. [Google Scholar] [CrossRef]
- Sokolovsky, V.R.; Timirkhanov, S.R. Fishes of the Alakol-Sasykkol system of lakes. In Proceedings of the Alakol State Nature Reserve; Mekte: Almaty, Kazakhstan, 2004; Volume 1, pp. 175–191. [Google Scholar]
- Mamilov, N. Schizothorax Argentatus. The IUCN Red List of Threatened Species, 2020, e.T156744412A156744418. Available online: https://www.iucnredlist.org/species/156744412/156744418 (accessed on 20 January 2021).
- Mamilov, N. Phoxinus Brachyurus. The IUCN Red List of Threatened Species, 2020, e.T156742076A156742081. Available online: https://www.iucnredlist.org/species/156742076/156742081 (accessed on 20 January 2021).
- Mamilov, N.; Karimov, B. Triplophysa Labiata. The IUCN Red List of Threatened Species, 2020, e.T156722567A156722639. Available online: https://www.iucnredlist.org/species/156722567/156722639 (accessed on 20 January 2021).
- Mamilov, N.; Karimov, B. Triplophysa Sewerzowi. The IUCN Red List of Threatened Species, 2020, e.T156721428A156721452. Available online: https://www.iucnredlist.org/species/156721428/156721452 (accessed on 20 January 2021).
- Greenville, A.C.; Newsome, T.M.; Wardle, G.M.; Dickman, C.R.; Ripple, W.J.; Murray, B.R. Simultaneously operating threats cannot predict extinction risk. Conserv. Lett. 2021, 14, e12758. [Google Scholar] [CrossRef]
- Kenzhebekov, B.K.; Dan’ko, Y.K.; Sansyzbayev, Y.T. Current state of the lakes in the Alakol system. Hydrometeorol. Ecol. 2018, 3, 145–151. [Google Scholar]
- French, B.; Wilson, S.; Holmes, T.; Kendrick, A.; Rule, M.; Ryan, N. Comparing five methods for quantifying abundance and diversity of fish assemblages in seagrass habitat. Ecol. Indic. 2021, 124. [Google Scholar] [CrossRef]
- Deacon, A.E.; Mahabir, R.; Inderlall, D.; Ramnarine, I.W.; Magurran, A.E. Evaluating detectability of freshwater fish assemblages in tropical streams: Is hand-seining sufficient? Environ. Biol. Fish 2017, 100, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Bonar, S.A.; Hubert, W.A. Standard Sampling of Inland Fish: Benefits, Challenges, and a Call for Action. Fisheries 2020, 27, 10–16. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org (accessed on 15 November 2021).
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; 853p. (In English) [Google Scholar]
- Braak, C.J.E.; Verdonschot, P.E.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Nekrashevich, N.G. Materials on the ichthyology of the Alakol lakes. In Alakol Depression and Its Lakes; Nauka: Alma-Ata, Kazakhstan, 1965; pp. 236–268. [Google Scholar]
- Strelnikov, A.S. Fish and Biological Foundations of Fisheries in the Alakol Lakes; Tomsk State University: Tomsk, Russia, 1974; 19p. [Google Scholar]
- Ward, J.V.; Stanford, J.A. The serial discontinuity concept: Extending the model of floodplain rivers. Regul. Rivers: Res. Manag. 1995, 10, 159–168. [Google Scholar] [CrossRef]
- Martynova, A.L.; Vasil’yeva, E.D. Problems of taxonomy and diagnostics of gudgeons of the genus Gobio (Cyprinidae) of the Urals, Siberia, Kazakhstan and the Amur river basin. J. Ichthyol. 2021, 61, 529–544. [Google Scholar] [CrossRef]
- Villéger, S.; Blanchet, S.; Beauchard, O.; Oberdorffe, T.; Brossea, S. Homogenization patterns of the world’s freshwater fish faunas. Proc. Natl. Acad. Sci. USA 2011, 108, 18003–18008. [Google Scholar] [CrossRef] [Green Version]
- Takács, P.; Abonyi, A.; Bánó, B.; Erős, T. Effect of non native species on taxonomic and functional diversity of fish communities in different river types. Biodivers. Conserv. 2021, 30, 2511–2528. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Lepori, F.; Keck, F. Effects of atmospheric nitrogen deposition on remote freshwater ecosystems. AMBIO 2012, 41, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, W.H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, S.E.; Williams, Y.M.; VanDerWal, J.; Isaac, J.L.; Shoo, L.P.; Johnson, C.N. Ecological specialization and population size in a biodiversity hotspot: How rare species avoid extinction. Proc. Natl. Acad. Sci. USA 2009, 106, 19737–19741. [Google Scholar] [CrossRef] [Green Version]
- Graham, N.A.; Pueppke, S.G.; Uderbayev, T. The current status and future of Central Asia’s Fish and Fisheries: Confronting a wicked problem. Water 2017, 9, 701. [Google Scholar] [CrossRef] [Green Version]
- Mischke, S.; Zhang, C.; Plessen, B. Lake Balkhash (Kazakhstan): Recent human impact and natural variability in the last 2900 years. J. Great Lakes Res. 2020, 46, 267–276. [Google Scholar] [CrossRef]
- Ussenaliyeva, A. Save Kazakhstan’s shrinking Lake Balkhash. Science 2020, 370, 303. [Google Scholar] [CrossRef] [PubMed]

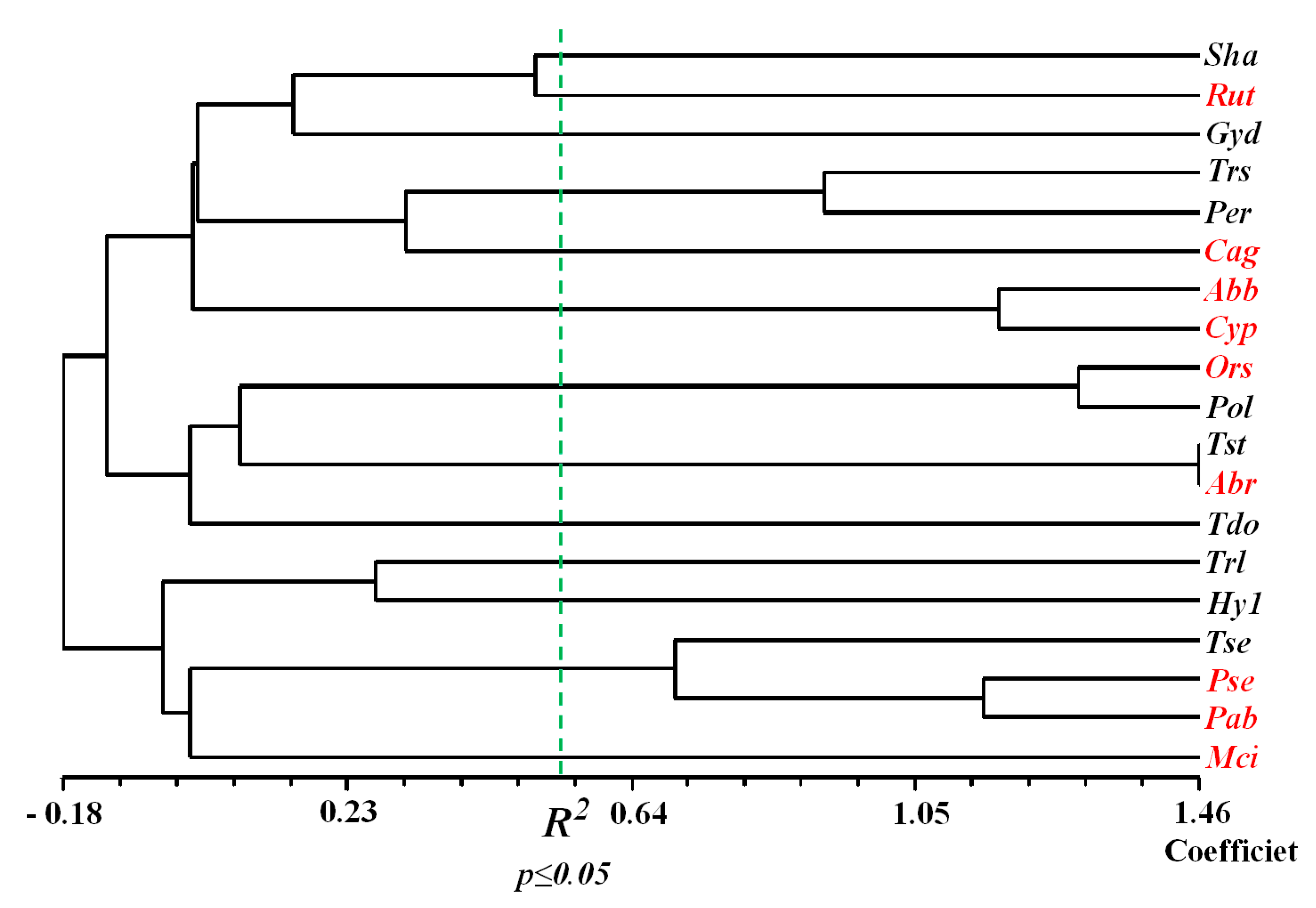


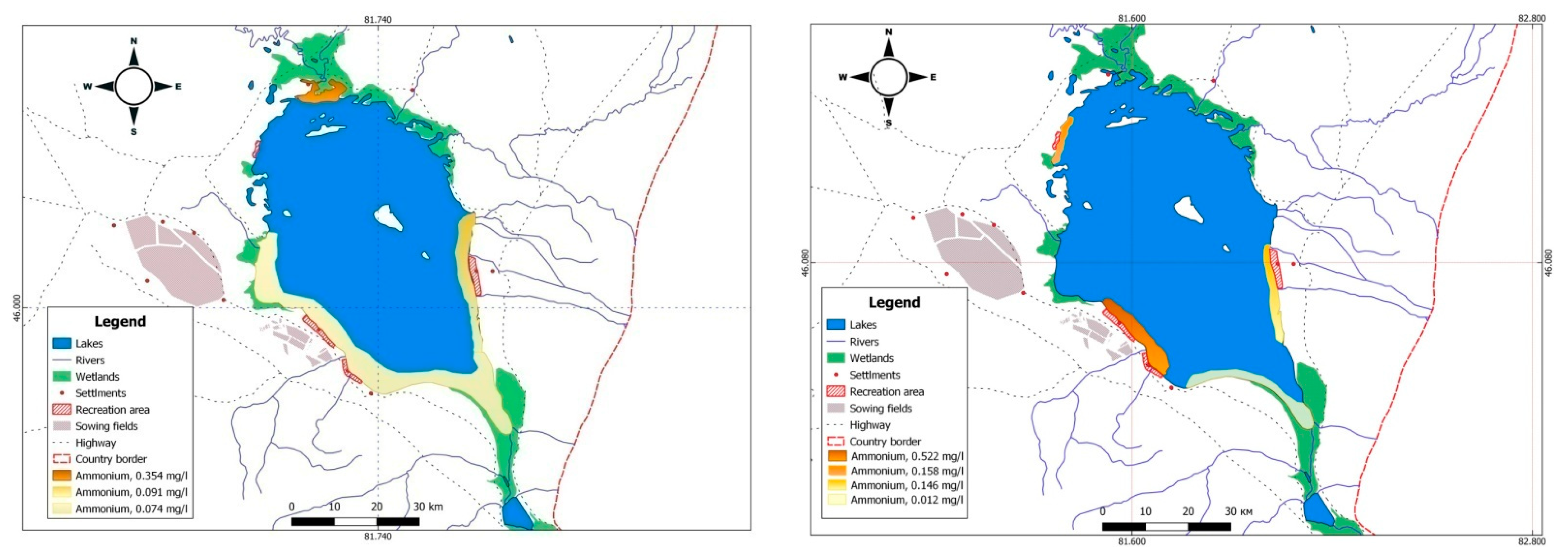

| Lake | Surface Area, km2 | Maximum Depth, m | Mineralization, g L−1 | Inflows (Average % of Total Coming Water) |
|---|---|---|---|---|
| Sassykkol | 736 | 4.7 | 0.27–2.16 | Tentek (95–100%), Karakol, Aiy |
| Koshkarkol | 120 | 5.8 | 0.85–1.28 | Zheniskesu (wet years only) |
| Alakol | 2650 | 54.0 | 1.20–11.60 | Urzhar (50%), Emel (27%) Katynsu (9%), Yrgayity and Zhamanty (9%), Zhamanotkel (5%) and 10 other |
| Zhalanashkol | 38 | 3.3 | 1.20–5.00 | Has not any |
| Site | Coordinates | Years | Biotopes | Abbreviation | |
|---|---|---|---|---|---|
| N | EO | ||||
| Alakol Lake, near Akshi settlement (recreation area) | 45°54′53″ | 81°37′00″ | 2021 | Lake | Al21a |
| 2021 | Estuary | Al21z | |||
| 2020 | Lake | Al20a | |||
| 2020 | Estuary | Al20z | |||
| 2017 | Lake | Al17a | |||
| 2016 | Lake | Al16a | |||
| 2015 | Lake | Al15a | |||
| Alakol Lake, eastern side | 45°54′53″ | 82°03′37″ | 2020 | Wetland | Al20e |
| 2020 | Lake | Al20l | |||
| Alakol Lake, near Kabanbay settlement (recreation area) | 46°05′41″ | 82°01′29″ | 2021 | Lake | Al21b |
| 2021 | Wetland | Al21lim | |||
| Alakol Lake, near Koktuma settlement (recreation area) | 45°50′13″ | 81°55′20″ | 2021 | Wetland | Al21k |
| Alakol Lake, near estuary of Zhamanty River | 45°54′47″ | 81°37′30″ | 2021 | Estuary | Zm21 |
| 2020 | Estuary | Zm20 | |||
| 2017 | Estuary | Zm17 | |||
| 2016 | Estuary | Zm16 | |||
| Sassykkol Lake | 46°40′48″ | 80°35′11″ | 2021 | Wetland | SK21 |
| 2020 | Wetland | SK20 | |||
| Zhamanty River, headwater zone | 45°51′34″ | 81°24′58″ | 2015 | Straight channel, stream | Zm15t |
| 2016 | (no fish) | ||||
| 2017, 2020, 2021 | Dry channel | (no water) | |||
| Tentek River, depositional zone | 46°12′25″ | 80°53′09″ | 2021 | Meandering channel | Tk21 |
| 2020 | Tk20 | ||||
| 2017 | Tk17 | ||||
| 2016 | Tk16 | ||||
| Tentek River, headwater zone | 46°03′17″ | 81°01′37″ | 2021 | Winding channel | Tk21t |
| 46°03′17″ | 81°01′37″ | 2016 | Tk16t | ||
| 46°03′17″ | 81°01′37″ | 2015 | Tk15t | ||
| 46°03′17″ | 81°01′37″ | 2014 | Tk14t | ||
| Right side tributary of Tentek River | 46°12′24″ | 80°51′10″ | 2017 | Meandering channel | Ua17 |
| 2016 | Ua16 | ||||
| 2015 | Ua15 | ||||
| Tokty River, transfer zone | 45°26′03″ | 82°15′13″ | 2020 | Winding channel | T20 |
| Shynzhyly River, headwater zone | 45°49′28″ | 80°33′51″ | 2021 | Meandering channel | Sh21t |
| 2020 | Sh20t | ||||
| Shynzhyly River, transfer zone | 45°23′38″ | 80°27′54″ | 2021 | Winding channel | Sh21m |
| 2020 | Sh20m | ||||
| 2017 | Sh17m | ||||
| 2016 | Sh16m | ||||
| 2015 | Sh15m | ||||
| P.Shynzhyly, depositional zone | 46°12′20″ | 80°52′15″ | 2021 | Meandering channel | Sh21l |
| 46°12′20″ | 80°52′15″ | 2020 | Sh20l | ||
| Urzhar River, transfer zone | 47°3′10″ | 81°32′19″ | 2021 | Meandering channel | U21 |
| 47°3′10″ | 81°32′19″ | 2020 | U20 | ||
| Karakol River, transfer zone | 46°57′53″ | 80°44′32″ | 2021 | Meandering channel | Kr21 |
| Yrgayity River | 45°40′10″ | 82°01′48″ | 2015,2016,2020 | Straight channel, stream | Y20 |
| Emel River, transfer zone | 46°22′46″ | 82°15′16″ | 2021 | Meandering channel | E21 |
| 46°22′46″ | 82°15′16″ | 2020 | E20 | ||
| Shagyntogay River, transfer zone | 46°16′43″ | 82°13′14″ | 2020 | Meandering channel | (no fish) |
| 2021 | Dry | (no water) | |||
| Zhalanashkol Lake | 45°36′17″ | 82°09′33″ | 2021 | Lake | ZK21 |
| 45°36′17″ | 82°09′33″ | 2020 | ZK20 | ||
| Katynsu River, transfer zone | 46°46′39″ | 82°03′17″ | 2021 | Meandering channel | K21 |
| 46°46′39″ | 82°03′17″ | 2020 | K20 | ||
| Latin Name | English Name | Abbreviation for Revealed Species | Until 1960 [37,38] | 1960–2014 [17,18,24] | Original Data 2015–2017, 2020, 2021 | |
|---|---|---|---|---|---|---|
| Number of Samples | Number of Fishes | |||||
| Indigenous species: | ||||||
| Phoxinusphoxinus (Linnaeus, 1758) | Eurasian minnow | Php | + | + | 1 | 118 |
| Phoxinus brachiurus Berg, 1912 | Seven River’s minnow | Phb | 0 | + | 1 | 28 |
| Rhynchocypris poljakowii (Kessler, 1879) | Balkhash minnow | Pol | 0 | + | 13 | 282 |
| Schizothorax argentatus Kessler, 1874 | Balkhsh marinka (snowtrout) | Sha | + | + | 17 | 344 |
| Gymnodiptychus dybowskii (Kessler, 1874) | Naked osman | Gyd | + | + | 24 | 806 |
| Triplophysa strauchii (Kessler, 1872) | Spotted thicklip loach | Trs | + | + | 28 | 489 |
| Triplophysa stoliczkai (Steindachner, 1866) | Tibetan stone loach | Tst | + | + | 15 | 157 |
| Triplophysa dorsalis (Kessler, 1872) | Gray loach | Tdo | + | + | 2 | 131 |
| Triplophysa labiata (Kessler, 1874) | Plain loach | Trl | + | + | 12 | 278 |
| Triplophysa sewerzowii (G.Nikolsky, 1938) | Severtsov’s loach | Tse | + | + | 18 | 90 |
| Triplophysa dorsalis × Triplophysa stolickai | (Hybrid) | Hy1 | 0 | 0 | 3 | 7 |
| Triplophysa labiata × Triplophysa stolickai | (Hybrid) | Hy2 | 0 | 0 | 1 | 3 |
| Perca schrenkii Kessler, 1874 | Balkhash perch | Per | + | + | 16 | 448 |
| Alien species: | ||||||
| Acipenser ruthenus Linnaeus, 1758 | Sterlet sturgeon | + | 0 | 0 | 0 | |
| Cyprinus carpio Linnaeus, 1758 | Carp | Cyp | + | + | 3 | 17 |
| Abramis brama (Linnaeus, 1758) | Bream | Abr | 0 | + | 4 | 48 |
| Tinca tinca (Linnaeus, 1758) | Tench | + | 0 | 0 | 0 | |
| Ctenopharyngodon idella (Valenciennes, 1844) | Grass carp | 0 | + | 0 | 0 | |
| Hypophthalmichthysmolitrix (Valenciennes, 1844) | Silver carp | 0 | + until 2000 | 0 | 0 | |
| Hypophthalmichthys (Aristichthys) nobilis (Richardson, 1845) | Bighead carp | 0 | + until 2000 | 0 | 0 | |
| Pseudorasbora parva (Temminck et Schlegel, 1846) | Pseudorasbora, or topmouth gudgeon | Pse | 0 | + | 16 | 176 |
| Carassius gibelio (Bloch, 1872) | Prussian carp | Cag | 0 | + | 10 | 46 |
| Rhodeus ocellatus (Kner, 1866) | Rosy bitterling | Rho | 0 | 0 | 1 | 1 |
| Rutilus rutilus (Linnaeus, 1758) | Roach | Rut | 0 | + | 3 | 21 |
| Parabramis pekinensis (Basilewsky, 1855) | White amur bream | Pab | 0 | 0 | 2 | 3 |
| Hemiculter leusiculus (Basilewsky, 1855) | Sharpbelly | 0 | + | 0 | 0 | |
| Abbottina rivularis (Basilewsky, 1855) | Abbottina or false gudgeon | Abb | 0 | + | 2 | 64 |
| Gobio cynocephalus Dybowski, 1869 | Siberian gudgeon | Gcy | 0 | 0 | 1 | 31 |
| Lefua costata (Kessler, 1876) | Eightbarbel loach | 0 | + | 0 | 0 | |
| Oncorhnchus mykiss (Walbaum, 1792) | Rainbow trout | 0 | + | 0 | 0 | |
| Orizias latipes (Temminck et Schlegel, 1846)/Orizias sinensis Chen, Uwa et Chu, 1989 | Japanese rice fish | Ors | 0 | + | 5 | 21 |
| * Sander lucioperca (Linnaeus, 1758) | Pike-perch | 0 | + | + | + | |
| Sander volgensis (Gmelin, 1789) | Volga pikeperch | 0 | + | 0 | 0 | |
| Micropercops sinctus (Dabry de Thiersant, 1872) | Mci | 0 | + | 3 | 9 | |
| Rhinogobius cheni (Nichols, 1931) | Rhs | 0 | + | 2 | 2 | |
| Fish Species | Abbreviation | Principal Components | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Indigenous: | ||||
| Schizothorax argentatus | Sha | 0.0071 | 0.0843 | 0.2987 |
| Gymnodiptychus dybowskii | Gyd | −0.0401 | −0.0272 | 0.2695 |
| Rhynchocypris poljakowii | Pol | −0.0556 | −0.0436 | −0.4802 |
| Triplophysa labiata | Trl | 0.1469 | 0.1648 | 0.1446 |
| Triplophysa dorsalis | Tdo | 0.0019 | −0.0068 | −0.1023 |
| Triplophysa sewerzowii | Tse | 0.3078 | −0.2884 | 0.0728 |
| Triplophysa stoliczkai | Tst | −0.4990 | −0.3940 | 0.1230 |
| Triplophysa strauchii | Trs | −0.0317 | 0.0741 | 0.3327 |
| Triplophysa dorsalis × Triplophysa stoliczkai | Hy1 | 0.4522 | 0.3757 | −0.1560 |
| Perca schrenkii | Per | −0.0880 | 0.1030 | 0.1109 |
| Phoxinus phoxinus | Php | invariant | ||
| Phoxinus brachiurus | Phb | invariant | ||
| Triplophysa labiata × Triplophysa stoliczkai | Hy2 | invariant | ||
| Alien: | ||||
| Carassius gibelio | Cag | −0.0522 | 0.0577 | 0.0628 |
| Cyprinus carpio | Cyp | −0.2241 | 0.2905 | −0.0796 |
| Pseudorasbora parva | Pse | 0.2222 | −0.3077 | −0.0742 |
| Abbottina rivularis | Abb | −0.3097 | 0.3986 | −0.0575 |
| Abramis brama | Abr | −0.3495 | −0.2516 | −0.1354 |
| Rutilus rutilus | Rut | 0.0086 | 0.0208 | 0.2356 |
| Parabramis pekinensis | Pab | 0.3097 | −0.3986 | 0.0575 |
| Orizias sinensis | Ors | −0.0144 | −0.0569 | −0.5579 |
| Micropercops sinctus | Mci | 0.0120 | −0.0152 | −0.0309 |
| Gobio cynocephalus | Gcy | invariant | ||
| Rhodeus ocellatus | Rho | invariant | ||
| Rhinogobius cheni | Rhs | invariant | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamilov, N.; Sharakhmetov, S.; Amirbekova, F.; Bekkozhayeva, D.; Sapargaliyeva, N.; Kegenova, G.; Tanybayeva, A.; Abilkasimov, K. Past, Current and Future of Fish Diversity in the Alakol Lakes (Central Asia: Kazakhstan). Diversity 2022, 14, 11. https://doi.org/10.3390/d14010011
Mamilov N, Sharakhmetov S, Amirbekova F, Bekkozhayeva D, Sapargaliyeva N, Kegenova G, Tanybayeva A, Abilkasimov K. Past, Current and Future of Fish Diversity in the Alakol Lakes (Central Asia: Kazakhstan). Diversity. 2022; 14(1):11. https://doi.org/10.3390/d14010011
Chicago/Turabian StyleMamilov, Nadir, Sayat Sharakhmetov, Fariza Amirbekova, Dinara Bekkozhayeva, Nazym Sapargaliyeva, Gulnar Kegenova, Ainur Tanybayeva, and Kanatbek Abilkasimov. 2022. "Past, Current and Future of Fish Diversity in the Alakol Lakes (Central Asia: Kazakhstan)" Diversity 14, no. 1: 11. https://doi.org/10.3390/d14010011
APA StyleMamilov, N., Sharakhmetov, S., Amirbekova, F., Bekkozhayeva, D., Sapargaliyeva, N., Kegenova, G., Tanybayeva, A., & Abilkasimov, K. (2022). Past, Current and Future of Fish Diversity in the Alakol Lakes (Central Asia: Kazakhstan). Diversity, 14(1), 11. https://doi.org/10.3390/d14010011








