Abstract
Land and freshwater molluscs are the most abundant non-arthropod invertebrates from inland habitats worldwide, playing important ecological roles and some being important pests in agriculture. However, despite their ecological, and even economic and sanitary importance, their local diversity in many European regions is not perfectly understood, with a particularly notableknowledge gap in the northern Iberian malacofauna. This work aims at providing a revised checklist of continental gastropods and bivalves from the Asturias (northern Spain), based on the examination of newly collected and deposited material and on the critical analysis of published and gray literature. A total of 165 molluscan species are recognized. Ten species constitute new records from Asturias and seven from northern Iberian Peninsula. Seventeen species are introduced or invasive, evidencing the current increase of the bioinvasion rate in continental molluscs. Furthermore, all these exotic species are parasite transmitters or trematode intermediate hosts, and thus represent a potential bio-sanitary risk for human and other animal health. The provided data strongly suggest that the increase of invasive freshwater snail species can lead to an increase in parasitic infections, and this is a crucial point that transcends the merely scientific to the political-social sphere.
1. Introduction
Land and freshwater snails and slugs are mostly heterobranch gastropods (except for some operculate snails that are caenogastropods) inhabiting many different inland habitats worldwide. Overall continental gastropods and bivalves play important ecological roles as prey for many species of birds and small mammals, predators of other invertebrates or as scavengers or filter-feeding organisms. Furthermore, they have attracted attention in the last decade not only for its surprising and unknown diversity [,,,,] but also for other aspects, from being indicators of climate change and evolutionary responses (e.g., Cepaea nemoralis) to a sentinel species of wetlands, ponds, and streams environmental quality [,]. Some species of land snails and slugs eat plants, including vegetables, fruits and garden flowers and thus they can destroy young plants, ruin crops and constitute an important pest in many countries [,]. Other species feed on another invertebrate groups such as earthworms and flatworms or even on other snails or slugs [,].
Several snail and slug species are also important from a medical or veterinary point of view, since they can act as first hosts of several trematode worms or being transmitters of nematodes or other parasites [,,]. Parasitic diseases cause serious public health problems worldwide and according to the World Health Organization (WHO) and the Institute for Health Metrics and Evaluation (IHME), the number of worldwide deaths from infectious parasitic diseases exceeded one and a half million only in 2017 [].
Many species of parasitic worms (called ‘helminths’ in medical-veterinary terminology) are responsible for several of the most worldwide prevalent parasitosis (e.g., hookworms or schistosomiasis). ‘Helminth’ is a term which groups animals distributed among three phyla: Nematoda, Platyhelminthes and Acanthocephala []. However, helminth- caused diseases have suffered a lack of attention on the part of medicine and human sociology []. The trematodes are divided into two subclasses: Digenea Carus 1863, the one with the greatest medical and veterinarian importance [] and Aspidogastrea Faust & Tang, 1936. Digenea flatworms (commonly known as ‘digestive flukes’) include about 9000 species that can parasitize both humans and domestic animals and can be found thoughout the entire body, from the digestive tract and bile and pancreatic ducts to the lung circulatory and genitourinary system [,].
Trematodes have a complex life cycle that involves at least four larval stages that occur inside the first intermediate host, which is usually a mollusc [,]. The mollusc species that act as trematode intermediate hosts generally belong to the Class Gastropoda, which includes both slugs and land, marine and freshwater snails [,] that are widespread in most geographic regions []. Most trematodes are highly specific and need a particular species for completing their life cycle, while a smaller part of them are more generalist and can complete their cycle using different species as intermediate hosts [,].
Land and freshwater snails, slugs and bivalves have been introduced unintentionally and intentionally to many regions worldwide [,]. These introduced species, when become invasive, have greatly impacts on native biodiversity, i.e., preying upon or outcompeting native species, transferring pathogens and parasites, causing community and ecosystem level imbalances or even facilitating the introduction of other species [,,]. In the same way, they may generate significant economic losses, mainly to agriculture, and even they can affect human health [,,,]. At this critical juncture, these introduced molluscs, together with their trematodes and other parasites, can spread to new areas, generating new bio-sanitary risks in the receiving ecosystems that can affect the human population nearby. Therefore, it is imperative to exactly know the real diversity and the specific identity of the snails that can act as intermediate hosts in one region for evaluate the potential bio-sanitary risk, prevalence and dissemination of trematode infections.
The Iberian Peninsula is one of the largest European peninsulas and constitutes the south-westernmost part of Europe. It is bordered on the southeast and east by the Mediterranean Sea and on the north, west, and southwest by the Atlantic Ocean. Due to these geographical features, the Iberian Peninsula harbors different climate types, with the Oceanic climate from the North to northwestern part and the Mediterranean one in the Centre and southern regions standing out. It also presents Alpine and the Subarctic climate zones in the higher mountains of northern Spain (mainly the Cantabrian Mountains and the Pyrenees) []. From the biogeographical perspective, the Iberian Peninsula is considered one of the most important Pleistocene glacial refugia of the European subcontinent, harboring a remarkable biological diversity including endemic species [].
The Principality of Asturias is one of the largest territories of the north of the Iberian Peninsula. There are many favorable habitats for being colonized by multiple species of terrestrial and freshwater snails, slugs and bivalves. However, the knowledge of Asturian malacofauna is scarce, fragmentary and insufficient []. In the same way, even less is known about the effects of mollusc invasions, their potential risks or the prevalence and number of species that act as intermediate hosts of medical-veterinary importance trematodes. So, the main goals of this work are: (a) to carry out an updated review of freshwater and terrestrial molluscs of Asturias, (b) to assess the invasive potential of the new arrived species to the studied territory and (c) to evaluate the bio-sanitary risk associated with the reported molluscs through the elaboration of a list confronting the recorded species with the potential trematodes that can host and the subsequent trematodiasis in humans.
2. Materials and Methods
Several seasonal samplings were carried out throughout the Principality of Asturias (2010–2020, Figure 1) looking for molluscs (details of collection date and locality are specified in the respective species sections in ‘Results’ for the new records). In addition, the material from the Collection of the Department of Organisms and Systems (BOS) of the University of Oviedo, in which several mollusc species from previous studies are stored [,,], was revised. For the elaboration of the mollusc checklist, an exhaustive review of the Asturian available bibliography was carried out. The species status was assigned following the guidelines of the International Union for the Conservation of Nature (IUCN). A ‘native species’, ‘autochthonous’ or ‘indigenous’ species is one original from a specific geographical site (here, the Principality of Asturias) without human intervention of any kind. Terms of ‘introduced species’, ‘exotic’, ‘alien’ or ‘non-native’ refer to that species introduced by humans (intentionally or not) outside its present or past range of distribution. Furthermore, an ‘invasive alien species’ is considered that species established outside its range of natural distribution, both present and past, whose introduction and/or expansion supposed a threat to indigenous biological diversity. In addition to these terms, the term ‘cryptogenic species’ refers to a species without certainty about its native or introduced origin, due to a lack of study or other impediments []. The European distribution of molluscs was obtained from Welter-Schultes [] and the Iberian ones from Cadevall and Orozco [] and our own observations. The ecological data of the Malacofauna was obtained from Cadevall and Orozco [] and our own observations. The list of trematodes was obtained through a literature review of trematodes associated with all the potential intermediate host species in the Principality of Asturias of molluscs. Only those trematode species with confirmed cases of infection in humans (i.e., trematodiasis) were considered to cause disease. Distribution maps were generated with ArcMap 10.8.1 (ArcGis, Redlands, CA, USA).
Figure 1.
Geographical situation of the Principality of Asturias (red) in Spain (dark green) and within Europe.
3. Results
3.1. Annotated Checklist
A total of 165 molluscs species has been recorded in the Principality of Asturias. The malacological fauna is composed by 138 snails (83.6%), 25 slugs (15.2%) and two mussels (1.2%) (Table A1 and Table A2). Among snails, there are 37 aquatic species (26.8%) (Table A1) and 101 (73.2%) with terrestrial habits (Table A2).
According to their status, 141 native species (85.45%) have been recorded (117 snails, 22 slugs, two mussels). Furthermore, there are 15 (9%) introduced species (14 snails and one slug), seven (4.2%) with no clear origin catalogued as cryptogenic (five snails and two slugs) and two (1.2%) invasive aquatic snail species. Of them, ten species constitute new records from the Asturian malacofauna (Figure 2).
Phylum Mollusca
Class Gastropoda Cuvier, 1795
Order Cycloneritida Haller, 1890
Superfamily Neritoidea Rafinesque, 1815
Family Neritidae Rafinesque, 1815
Genus Theodoxus Montfort, 1810

Figure 2.
New species records for the Asturian malacofauna. (a) Otala lactea (La Corredoria-Oviedo), (b) Cepaea hortensis (Grandiella, Riosa), (c) Rumina decollata (dune system of San Juan de Nieva-Avilés), (d) Lymnaea stagnalis (Villanueva de Oscos pond), (e) Melanoides tuberculata (Nora River-Oviedo), (f) Omphiscola glabra (temporary pond in Avilés), (g) Planorbella duryi (Nalón River-El Entrego, San Martín del Rey Aurelio), (h) Stagnicola cf. palustris (spring in Lugones-Siero), (i) Helix pomatia (Isabel la Católica park-Gijón), (j) Pomacea cf. maculata (San Andrés de los Tacones reservoir-Gijón). All scale bars 1 cm.
3.1.1. Theodoxus fluviatilis fluviatilis (Linnaeus, 1758)
Aquatic species widely distributed in Europe and the Iberian Peninsula. Frequent species in Asturias. It generally lives in the medium-high stretches of streams and rivers with clean waters. It lives on stones where it feeds on diatoms and detritus The Asturian record under the name Theodoxia bourguignati (Recluz, 1852) [,] corresponds to T. fluviatilis.
Order Architaenioglossa Haller, 1890
Superfamily Ampullarioidea Gray, 1825
Familiy Ampullariidae Gray, 1824
Genus Marisa Gray, 1824
3.1.2. Marisa cornuarietis (Linnaeus, 1758)
This aquatic species inhabits the rivers and lagoons of Central America and the North of South America []. Recently a population of this species was found in the Nora River, near the town of Colloto (Oviedo) []. They argued that this species could have arrived due to the pet and aquarium trade, followed by its subsequent escape or release of specimens in the area.
Genus Pomacea Perry, 1810
3.1.3. Pomacea cf. maculata Perry, 1810
This is an aquatic species native to South America. In recent years, it has invaded numerous countries, producing several damages, especially colonizing rice plantations []. It has been introduced in tropical and subtropical countries, as well as certain temperate regions of the northern hemisphere, such as the United States [,]. In the Iberian Peninsula, its presence has been reported in Ebro River, causing serious problems []. This species lives preferably in low-flow fresh waters (lagoons, estuaries and some rivers) where it feeds on aquatic plants. Here, we present the first record of this species for Asturias. This snail was found alive in the San Andrés de los Tacones (Gijón) reservoir in 2018. The potential introduction pathway of this species is probably associated with ornamental plant species or through pet trade and subsequent escape or release.
Material examined: Spain–Asturias: San Andrés de los Tacones reservoir (Gijón): five alive specimens. 2018. (30TTP77382018).
Superfamily Cyclophoroidea Gray, 1847
Family Megalomastomatidae Blanford, 1864
Genus Obscurella Clessin, 1889
3.1.4. Obscurella asturica Raven, 1990
Endemic species to the Iberian Peninsula only known from the Desfiladero de los Beyos and the Lagos de Covadonga where its type locality is found []. A rare and sparsely distributed species in Asturias. It lives in rocks and limestone walls in sympatry with O. hidalgoi.
3.1.5. Obscurella bicostulata (Gofas, 1989)
Endemic species to the Iberian Peninsula limited to the center and east of the Cantabrian coast. A frequent species in Asturias. It lives in rocks and limestone walls, occasionally in sympatry with O. hidalgoi. Anadón and Anadón [] recorded Cochlostoma (Anotus) berilloni (Bassoon, 1880) in Roces and Gato Mountains. However, after reviewing the material, it has turned out to correspond to an identification error with O. bicostulata, a non-described species at the time.
3.1.6. Obscurella hidalgoi (Crosse, 1864)
Species of Iberian-French distribution located in the eastern half of the Cantabrian coast. Frequent species in Asturias, it lives in rocky areas and limestone walls, occasionally in sympatry with O. bicostulata and more specifically in its limited area of distribution, with O. asturica [].
Family Aciculidae Gray, 1850
Genus Acicula W. Hartmann, 1821
3.1.7. Acicula fusca (Montagu, 1803)
Species distributed in Western Europe. It is mainly located in the north of the Iberian Peninsula. Frequent species in Asturias. It lives in very humid environments normally buried in the ground, although it can also live among moss or in the soil mulch.
Genus Menkia Boeters, E. Gittenberger & Subai, 1985
3.1.8. Menkia horsti Boeters, E. Gittenberger & Subai, 1985
Endemic species to the Iberian Peninsula. Frequent species in Asturias, although it is only known from a few localities, including its type locality, the Cueva de Tito Bustillo in Ribadesella []. It has also been found in Cantabria []. It lives in moist environments, generally buried in the ground in areas with loose soil, although it can also live among moss and other humid microenvironments.
3.1.9. Menkia rolani E. Gittenberger, 1991
Endemic species to the Iberian Peninsula. Rare species in Asturias known from few localities, including its type locality in Grado []. The habitat in which this species has been found is similar to M. horsti.
Genus Platyla Moquin-Tandon, 1856
3.1.10. Platyla callostoma (Clessin, 1911)
Species distributed throughout western Europe. Limited to the north Iberian Peninsula, specifically to the provinces of Asturias, Cantabria and certain areas of Catalonia. Rare species in Asturias, known only from the east extreme []. It lives in humid microenvironments like mosses and leaf litter.
Superfamily Viviparoidea Gray, 1847
Family Viviparidae Gray, 1847
Genus Sinotaia Gray, 1847
3.1.11. Sinotaia cf. quadrata (Benson, 1842)
This aquatic species inhabits the rivers and lagoons of China, Korea, and Taiwan and was later introduced in Japan, Thailand and the Philippines []. Sinotaia quadrata has also been introduced in South America, specifically in Argentina [] and in the Arno River of Italy []. Recently, a population of this species in the Nora River near the town of Colloto (Oviedo) was found [] and argued that this species could have been associated with an ornamental plant species (Eichhornia crassipes (Mart.) Solms) located in the nearby Nora River.
Order Littorinimorpha Golikov & Starobogatov, 1975
Superfamily Littorinoidea Children, 1834
Family Pomatiidae Newton, 1891 (1828)
Genus Pomatias S. Studer, 1789
3.1.12. Pomatias elegans (O. F. Müller, 1774)
Species widely distributed in Europe and North Africa. In the Iberian Peninsula it extends through central Portugal and the Cantabrian and the Mediterranean coast. Frequent species in Asturias that can become locally abundant once located. Well adapted to anthropized environments. It also frequents rocky walls where it hides in crevices and forests, selecting soils with a lot of humus or inhabiting very humid microhabitats.
Genus Leonia Gray, 1850
3.1.13. Leonia mammillaris (Lamarck, 1822)
Species distributed throughout North Africa and the southeast part of the Iberian Peninsula (Alicante, Murcia and Almería). In Asturias, only one single record is known: an empty shell found in August of 1988 on a small beach located in the south of Misiego, in the Villaviciosa estuary []. Its origin is uncertain, and it seems more likely that it was introduced due to passive transport in vehicles since it is a very crowded place [].
Superfamily Cerithioidea J. Fleming, 1822
Family Thiaridae Gill, 1871 (1823)
Genus Melanoides Olivier, 1804
3.1.14. Melanoides tuberculata (O. F. Müller, 1774)
This aquatic species inhabits the rivers and lagoons of tropical and subtropical regions of Africa, Asia and Australia []. This species has been introduced many times outside its native range including North, South and Central America []. It has also been introduced to many countries of Europe []. In the Iberian Peninsula it was recorded from Aragón (Zaragoza), Valencia (Castellón) and the Canary Islands [,]. In 2010, it was detected in the Ebro River basin, concretely in the town of l’Aldea []. Here, we present the first record of this species for Asturias. During a series of monitoring surveys carried out in 2017, some adults of this snail were found in the rocks of the riversides of Nora River, near to the locality of Colloto. In addition, some juveniles were found among river sand at the same locality. As an aquarium species it could come associated with ornamental plant (common in the area) or through pet trade and subsequent release.
Material examined: Spain–Asturias: Nora River (Oviedo): eight adults and ten juveniles. 2017. (30TTP74080669).
Superfamily Truncatelloidea Gray, 1840
Family Truncatellidae Gray, 1840
Genus Truncatella Risso, 1826
3.1.15. Truncatella subcylindrica (Linnaeus, 1767)
Species widely distributed along the Mediterranean and Atlantic coasts, distributed along the entire coast of the Iberian Peninsula. It is a rare species in Asturias that inhabits rocky walls and muddy areas influenced by tidal cycles.
Family Bithyniidae Gray, 1857
Genus Bithynia Leach, 1818
3.1.16. Bithynia tentaculata (Linnaeus, 1758)
Aquatic species widely distributed in Europe and throughout much of the Iberian Peninsula. Frequent species in Asturias. It usually lives in the lower-middle course of streams and rivers. It is also found in stagnant water areas and in temporary streams, either on the substrate or among the marshy vegetation, on which it feeds.
Family Hydrobiidae Stimpson, 1865
Genus Alzoniella Giusti & Bodon, 1984
3.1.17. Alzoniella asturica (Boeters & Rolán, 1988)
Endemic species to the Iberian Peninsula. Together with its type locality, the La Fontona fountain [], it is only found near Grado []. A rare species in Asturias believed to be living associated to springs.
3.1.18. Alzoniella cantabrica (Boeters, 1983)
Endemic species to the Iberian Peninsula that is distributed throughout Cantabria, the Basque Country, the north of León, Burgos, Palencia, and the eastern part of Asturias []. Frequent species in the region. Although Boeters [] does not refer to the habitat of A. cantabrica, it seems to live associated to springs and water currents. In certain localities it lives in sympatry with A. ovetensis or A. montana [].
3.1.19. Alzoniella camocaensis Rolán & Boeters, 2015
Endemic species to the Iberian Peninsula. It is only known from the vicinity of Camoca in Villaviciosa, being its type locality Fuente Tebia []. A rare species in Asturias that lives associated to the spring and is found under stones and among the characteristic reddish sediment of its fountain.
3.1.20. Alzoniella lucensis (Rolán, 1993)
Endemic species to the Iberian Peninsula that is distributed throughout Galicia, the western part of Asturias and some localities of León []. A rare species in Asturias, which tends to live associated to running and clean waters. It is generally found attached to leaf litter, wood and branches [].
3.1.21. Alzoniella marianae Arconada, Rolán & Boeters, 2007
Endemic species to the Iberian Peninsula, only known from its type locality Fuente Caliente and nearby areas in Villazón in Salas []. Due to its scarce geographical distribution, it can be considered rare in Asturias. It lives associated to the spring [].
3.1.22. Alzoniella montana (Rolán, 1993)
Peninsular endemism distributed throughout Asturias, Cantabria, the Basque Country and some localities in León []. Rare species in Asturias. It can be found in the eastern part of the region. This species lives associated to running and clean waters. Rolán [] commented that the absence of juvenile individuals in the sampled areas may indicate that their juvenile stages live in areas deeper than the interstitial levels. In certain localities it lives in sympatry with other Alzoniella species [].
3.1.23. Alzoniella ovetensis (Rolán, 1993)
Peninsular endemism located in Asturias and some localities of León and Cantabria [,]. Its type locality is Baselgas in Grado []. Frequent species in Asturias. Brodely distributed in the central-eastern zone. This species lives in springs, fountains, and streams of running and clean water. In certain localities it lives in sympatry with A. montana and A. cantabrica []. Ojea and Anadón [] recorded Bythinella brevis (Draparnaud, 1805) in Monte Naranco. However, this species does not seem to be part of the fauna of the Iberian Peninsula []. Thus, it is believed the record from Ojea and Anadón [] corresponds to A. ovetensis, a very abundant species in this area that had not been described at that time.
3.1.24. Alzoniella somiedoensis Rolán, Arconada & Boeters, 2009
Endemic species to the Iberian Peninsula. It is located near Pola de Somiedo from where its type locality La Malva is found []. This species is rare and lives under fallen leaves on flooded surfaces. It can also be found on rocks and mosses.
Genus Deganta Arconada & Ramos, 2019
3.1.25. Deganta azarum (Boeters & Rolán, 1988)
Endemic aquatic species to the Iberian Peninsula. It is only known from Asturias and Cantabria [,] being its type locality the Fuente La Broquera in Trubia (Oviedo). This fountain was destroyed for road construction [,]. A frequent species in the central area of Asturias that lives in springs, streams, washing places and peri-urban channels with clean water. It prefers shady places, so it is easily found living under stones, leaves or submerged tree branches []. Curiously, localities where D. azarum is abundant, Alzoniella species are scarce or absent and vice versa [].
Genus Islamia Radoman, 1973
3.1.26. Islamia ayalga Alonso, Quiñonero-Salgado & Rolán, 2018
Endemic Iberian species. Only known from Asturias, specifically from its type locality the cave of the Caldueñín spring in Llanes []. Nothing is known about the ecology and biology of this rare species. It seems to be a stigobiotic species which completes its entire life cycle in underground watercourses.
Genus Mercuria Boeters, 1971
3.1.27. Mercuria tachoensis (Frauenfeld, 1865)
Rolán and Boeters [] described Alzoniella camocaensis in Fuente Tebia from La Camoca in Villaviciosa. In addition, they commented that there is a species of the genus Mercuria pending description. Although the work remains unpublished, the species that lives in Asturias is Mercuria tachoensis (Jonathan Miller, pers. comm).
Family Moitessieriidae Bourguignat, 1863
Genus Spiralix Boeters, 1972
3.1.28. Spiralix asturica Quiñonero-Salgado, Ruiz-Cobo & Rolán, 2017
Endemic species to the Iberian Peninsula. Only known from Asturias, specifically from its type locality, a small spring near the train tracks on the road to Las Caldas and Fuso from Caces to Trubia (Oviedo) and from the Fuente de los Tres Caños in Priorio, Las Caldas []. It is a rare species and nothing is known about its ecology and biology, appearing to be a stigobiotic species. Rolán and Ramos [] described Paladilhiopsis septentrionalis based on material collected in the provinces of Álava, Vizcaya, Burgos and Cantabria. Rolán and Arconada [] recorded this species in Asturias. Years later P. septentrionalis was included in the genus Spiralix and the subgenus Burgosia and its distribution was restricted to Álava, Vizcaya and Burgos due to the description of two new species previously confused with S. septentrionalis: S. burgensis for Cantabria and S. asturica for Asturias [].
3.1.29. Spiralix vetusta Quiñonero-Salgado, Alonso & Rolán, 2018
Endemic species to the Iberian Peninsula. It is only known from Asturias, specifically from its locality type Fuente Vieya (Palaciós) in Lena []. Nothing about the ecology and biology of this extremely rare species is known, apart for being a stigobiotic species [].
Family Tateidae Thiele, 1925
Genus Potamopyrgus Stimpson, 1865
3.1.30. Potamopyrgus antipodarum (Gray, 1843)
This aquatic species lives in the rivers and lagoons of New Zealand []. Potamopyrgus antipodarum has been introduced in several countries of Europe, Asia and North America []. It is widely distributed throughout the Iberian Peninsula and is a very common species in Asturias. It lives in different aquatic environments, from fountains, troughs, and springs, to running waters and river mouths with brackish water, where it buries itself in the substrate. This species is listed in the Spanish Catalogue of Invasive Exotic Species (Real Decreto 630/2013, from 2 of August).
Order UNASIGNED
Superfamily Lymnaeoidea Rafinesque, 1815
Family Lymnaeidae Rafinesque, 1815
Genus Galba Schrank, 1803
3.1.31. Galba truncatula (O.F. Müller, 1774)
Aquatic species widely distributed throughout Europe. It can be found in most of the Iberian Peninsula. A frequent species in Asturias that lives in temporary ponds, humid plains and walls, swampy forests, fountains and springs.
Genus Lymnaea Lamarck, 1799
3.1.32. Lymnaea stagnalis (Linnaeus, 1758)
Aquatic species widely distributed throughout Europe, Asia and North Africa. In the Iberian Peninsula it is only known from Catalonia, Castilla y León and Andalucía []. This species lives in stagnant waters, rivers and pools. Here, we present the first record of this species for Asturias. This snail was found alive in a peatbog at Villanueva de Oscos in 2018.
Material examined: Spain–Asturias: Villanueva de Oscos pond: three specimens. 20-VIII-2018. (29TPH61559569).
Genus Omphiscola Rafinesque, 1819
3.1.33. Omphiscola glabra (O. F. Müller, 1774)
A poorly known aquatic species widely distributed across Europe (from Scandinavia to northern Spain). In the Iberian Peninsula is a quite rare species, only known from some collections from Euskadi and Galicia []. Here, we present the first published record of this species for Asturias. This snail was found in a temporary pond of a recreational area at the base of Sierra del Aramo and only one live specimen could be examined in the field. The pond was surrounded by abundant aquatic vegetation such Juncus spp. and mint. The specimen was found adhered to a submerged log in a quite muddy substrate. Although much sampling in the mud was made, no more snails were found. All the ecological remarks fit with recent works [] with the exception of the height (range 400–500 m. vs. our own observation at 888 m. high).
Material examined: Spain–Asturias: Temporal pound in Aviles: four specimens. BOS Collection. V-1979 (Coordinates not indicated). Recreational area of La Peral, Pola de Lena (Lena): one live specimen. 17-IX-2020, 888 m. (30TTN64998266).
Family Physidae Fitzinger, 1833
Genus Physella Haldeman, 1842
3.1.34. Physella acuta (Draparnaud, 1805)
This aquatic species inhabits the rivers and lagoons of North America where it coexists with other species of the same family []. Currently this species is found in Europe, Africa, South Asia, Australia, and Japan []. In the Iberian Peninsula it is distributed throughout all the territory. Physella acuta is frequent in Asturias, where it inhabits all kinds of waters such as lakes, temporary ponds, drinking troughs and rivers, ditches and fountains. It is believed that its introduction in Europe occurred through the cotton trade in the 18th century [], and it was later dispersed throughout the territory due to aquatic plants linked to aquarium and pets trade []. This highly invasive species does not appear in the Spanish Catalogue of Invasive Alien Species (Real Decreto 630/2013, from 2 of August).
Genus Radix Montfort, 1810
3.1.35. Radix auricularia (Linnaeus, 1758)
Species distributed throughout central Europe. In the Iberian Peninsula is distributed along the North. Only one Asturian record is known [], making it a rare species in Asturias. It usually lives associated with rivers where it is common to find among stones on the riversides.
3.1.36. Radix labiata (Rossmässler, 1835)
Species distributed throughout Europe. Only known from León and Huesca in the Iberian Peninsula []. In the past, there was some confusion due to the use of Radix peregra (O.F. Müller, 1774) to refer to R. labiata, when the former is a junior synonym of R. balthica. Therefore, Iberian records must be reviewed []. A rare species that lives in different types of water: rivers, fountains and springs. Thus, although it is quite probable this species occurs in Asturias, its presence in the territory needs confirmation.
Genus Ampullaceana Servain, 1882
3.1.37. Ampullaceana balthica (Linnaeus, 1758)
Species distributed throughout Europe and the whole the Iberian Peninsula. This species was recorded as Lymnaea (Radix) limosa (Linnaeus, 1758) in Piedras Blancas and Santa María del Mar (Castrillón) []. Common species that lives in different types of water courses.
Genus Stagnicola Jeffreys, 1830
3.1.38. Stagnicola cf. palustris (O. F. Müller, 1774)
Species distributed throughout much of Europe and the Iberian Peninsula. It usually habits in stagnant waters with abundant vegetation, from small swamps to lagoons and lakes, even tolerates brackish water and temporary desiccations. Here, we present the first record of this species for Asturias. The status of this species in Asturias is not clear, it could be part of the native fauna since the species is distributed throughout much of the Iberian Peninsula. However, the material here recorded belongs to the collection of molluscs of the University of Oviedo (BOS) and all the material was found in anthropized areas where it has been able to be introduced.
Material examined: Spain–Asturias: Silvota industrial estate (Siero): one specimen. BOS Collection. 29-VII-1980 (coordinates not indicated). Spring in Monte Naranco (Oviedo): one specimen. BOS Collection. 07-III-1981 (coordinates not indicated). Spring in Lugones (Siero): one specimen. BOS Collection. 28-IV-1983 (coordinates not indicated).
Family Planorbidae Rafinesque, 1815
Genus Ancylus O. F. Müller, 1773
3.1.39. Ancylus fluviatilis O.F. Müller, 1774
Aquatic species widely distributed throughout Europe except for its northern fringe. It is broadly distributed throughout the Iberian Peninsula. It is a common species in Asturias that lives in rivers, streams, lakes, and sources of clean and oxygenated water. It is generally easy to locate on stones scraping the algae on which it feeds.
Genus Gyraulus Charpentier, 1837
3.1.40. Gyraulus laevis (Alder, 1838)
Aquatic species widely distributed throughout Europe. It is widely distributed in the Iberian Peninsula and is found in most communities. It is a rare species in Asturias. Since the record from El Llano in Llordón (Cangas de Onís) [], this species has not been found again. Its typical habitat is the edges of lakes and swamps with stagnant, clean and shallow waters.
Genus Planorbella Haldeman, 1843
3.1.41. Planorbella duryi (Wetherby, 1879)
Aquatic species that inhabits waterways in Florida (North America). It has been established on numerous occasions outside its native range including much America, Jamaica and Cuba, the Middle East, Russia, Australia, Hawaii and Europe [,,]. In the Iberian Peninsula, it has been recorded in the city of Barcelona, Ebro River Basin, Tarragona [], Elche, Alicante [], Castellón, Valencia, Mallorca [] and the Canary Islands []. Here, we present the first record of this species for Asturias. These snails were found alive in highly anthropized areas, so the probable pathway of introduction of this species is linked to ornament aquatic plants and pet trade.
Material examined: Spain–Asturias: Nora River, near Colloto (Oviedo): four adults and nine juvenile specimens. VII-2013. (30TTP74080669). Nalón River (El Entrego, San Martín del Rey Aurelio): five specimens. 2017 (30TTN85699627). San Andrés de los Tacones reservoir (Gijón): five specimens. 2018. (30TTP77382018). Spring in Monte Naranco at the beginning of Pista Finlandesa (Oviedo): one juvenile specimen. 11-XI-2019. (30TTP68060658).
Order Ellobidae L. Pfeiffer, 1854 (1822)
Superfamily Ellobioidea L. Pfeiffer, 1854 (1822)
Family Ellobiidae L. Pfeiffer, 1854 (1822)
Genus Ovatella Bivona-Bernardi, 1832
3.1.42. Ovatella aequalis (Lowe, 1832)
Species distributed along the European Atlantic coasts. Regarding its distribution in the Iberian Peninsula, Bank et al. [] considered this species as a Macaronesian endemism commenting that the previous records of O. aequalis could correspond to a misidentification with O. firminii (Payraudeau, 1827). However, Alonso et al. [] provide the first records of O. aequalis outside the Macaronesian region, specifically for the coasts of Cantabria and Asturias. This rare species lives in Asturias in rocks linked to estuaries.
Genus Pseudomelampus Pallary, 1900
3.1.43. Pseudomelampus exiguus (Lowe, 1832)
Species distributed along the European Atlantic coast. It is widely distributed in the Cantabrian and Atlantic coasts of the Iberian Peninsula. A rare species in Asturias []. It can be found in coastal rocky areas, hidden under stones subjected to a strong saline influence.
Genus Myosotella Monterosato, 1906
3.1.44. Myosotella myosotis (Draparnaud, 1801)
Species of Euro-Mediterranean distribution that is distributed throughout good part of the coasts of the Iberian Peninsula. A frequent species in Asturias []. It is found in rocks linked to estuaries. It can also be found among halophilic plants and under wood and stones in humid dune areas, always subject to a certain saline influence.
3.1.45. Myosotella denticulata (Montagu, 1803)
Species distributed along the European Atlantic coast. It is located on the Cantabrian and Mediterranean coast of the Iberian Peninsula. Certain authors consider this species as a synonymous of M. myosotis and may constitute a complex of species []. Frequent in the study area living in the crevices of the coastal rocky areas [].
Genus Leucophytia Winckworth, 1949
3.1.46. Leucophytia bidentata (Montagu, 1808)
Species distributed along the European Atlantic coast and the western Mediterranean basin. In the Iberian Peninsula it is present on the Cantabrian and Atlantic coast. It can also be found scattered along the Mediterranean coasts and the Balearic Islands. Frequent species in Asturias []. Its habitat is very similar to that of other members of the Ellobiidae family.
Genus Carychium O. F. Müller, 1773
3.1.47. Carychium minimum O.F. Müller, 1774
Eurosiberian species distributed throughout a good part of the Iberian Peninsula, mainly linked to the Mediterranean, Atlantic and Cantabrian fringes. A rare species in Asturias. There is only one record from the Integral Reserve of Muniellos []. This species prefers humid microenvironments under rocks, logs, and leaf litter.
3.1.48. Carychium tridentatum (Risso, 1826)
Eurosiberian species found throughout the north of the Iberian Peninsula, with other isolated Iberian records. Frequent species in Asturias, whose microhabitat is identical to its sister species C. minimum.
Genus Zospeum Bourguignat, 1856
3.1.49. Zospeum gittenbergeri Jochum, Prieto & De Winter, 2019
Endemic species to the Iberian Peninsula. It is only known from its type locality, the cave Puente de Inguanzo in Cabrales (Asturias) []. This species lives inside caves. However, the authors do not refer to the type of habitat where Z. gittenbergeri can be found, so more studies are necessary to clarify the data on its biology, ecology and distribution.
3.1.50. Zospeum percostulatum Alonso, Prieto, Quiñonero-Salgado & Rolán, 2018
Endemic species to the Iberian Peninsula. In Asturias is known from two caves, located geographically very close to each other, in the lower part of the Sierra of Cuera []. Its type locality is the cave La Herrería (La Pereda) in Llanes []. This species lives inside caves, on damp walls covered with a clay film. It has been found in sympatry with Z. cf. praetermissum [].
3.1.51. Zospeum praetermissum Jochum, Prieto & De Winter, 2019
Endemic species to the Iberian Peninsula. Only known from Asturias, from its type locality the Puente de Inguanzo cave in Cabrales [], previouly recorded as Zospeum schaufussi Frauenfeld, 1982. This species lives inside caves, however, the authors do not refer to the type of habitat where this species can be found, so more studies are necessary to clarify its biology, ecology and distribution.
Order Stylommatophora A. Schmidt, 1855
Superfamily Achatinoidea Swainson, 1840
Family Achatinidae Swainson, 1840
Genus Rumina Risso, 1826
3.1.52. Rumina decollata (Linnaeus, 1758)
Species distributed throughout the European Mediterranean coast and north Africa. It is a common Mediterranean species in the Iberian Peninsula being absent in the northwest. Here, we present the first record of this species for Asturias. Some live adults and juveniles of this snail were found in the dune system of San Juan de Nieva in Avilés. The area where this species was found is near a car park, so the introduction of this species in Asturias is quite probably linked to tourism. Since it has been found in a highly crowded area, it has been able toculd have been introduced passively attached to vehicles.
Material examined: Spain–Asturias: dune system of San Juan de Nieva (Avilés) near the parking area: seven specimens. 8-VIII-2012. (30TTP62713027).
Family Ferussaciidae Bourguignat, 1883
Genus Cecilioides A. Férussac, 1814
3.1.53. Cecilioides acicula (O. F. Müller, 1774)
Species with a circum-Mediterranean distribution, broadly distributed in much of the Iberian Peninsula. Frequent species in Asturias. It lives buried, sometimes at great depth. However, it also lives in the humid microhabitat located under stones and in rock crevices with abundant organic matter.
Superfamily Papillodermatoidea Wiktor, R. Martin & Castillejo, 1990
Family Papillodermatidae Wiktor, R. Martin & Castillejo, 1990
Genus Papilloderma Wiktor, R. Martin & Castillejo, 1990
3.1.54. Papilloderma altonagai Wiktor, Martín & Castillejo, 1990
It is an endemic Iberian species. The only records are those used in its description (Sanctuary of Covadonga in Asturias and Puerto de las Alisas in Cantabria) and one from Alejandro Pérez-Ferrer through a citizen science platform []. Its habitat includes high mountain meadows, anthropized areas and ruderal environments []. Slug shells discovered at the entrance to the caves (without living specimens) [] remarks the importance to study the real distribution of this unknown species or other that remains undefined. It could be underground and predatory of worms [,].
Superfamily Punctoidea Morse, 1864
Family Punctidae Morse, 1864
Genus Paralaoma Iredale, 1913
3.1.55. Paralaoma servilis (Shuttleworth, 1852)
Species distributed throughout the Mediterranean part of Europe, including throughout a good part of the Iberian Peninsula. Frequent species in Asturias. It is well adapted to anthropized areas, where it lives between cracks in stone walls with abundant vegetation. It also frequents forests and riverbanks, where it is found on the vegetation, under stones or leaf litter.
Genus Punctum Morse, 1864
3.1.56. Punctum pygmaeum (Draparnaud, 1801)
Holarctic species distributed throughout a large part of the Iberian Peninsula with lack of records in the central zone. Punctum pygmaeum is common in Asturias and lives associated with banks and grasslands near watercourses, occupying high humidity microhabitats.
Family Discidae Thiele, 1931 (1866)
Genus Discus Fitzinger, 1833
3.1.57. Discus rotundatus (O. F. Müller, 1774)
Species widely distributed throughout Europe. Common in the northern half of the Iberian Peninsula. Amply distributed species in Asturias that lives in all kinds of environments such as forests, stone walls, gardens and meadows. Its most common microhabitats are those located under stones, logs and mosses.
Family Helicodiscidae Pilsbry, 1927
Genus Lucilla R. T. Lowe, 1852
3.1.58. Lucilla scintilla (Lowe, 1852)
Species distributed throughout North America and several countries in Europe. There is an open debate about its status as an introduced species in Europe []. In the Iberian Peninsula is only known from Asturias and Cantabria []. This species is considered rare in Asturias since only a few records from the eastern part of the territory are known []. Not much is known about the biology of this species. Although it is known that generally exhibits underground habits it could frequent flooded areas thanks to its high resistance to immersion in water [].
3.1.59. Lucilla singleyana (Pilsbry, 1890)
Species distributed throughout North America and a large part of Europe and Africa. Like in the case of L. scintilla, there is an open debate about its status as an introduced species in Europe []. Patchily distributed in the Iberian Peninsula. In Asturias it is rare, with only a few records in the western coast of Asturias []. It has habits that are practically identical to its sister species L. scintilla []. The recent appearance of L. scintilla in Asturias [], has made the presence of L. singleyana in the region remain in doubt, and it is possible that the records provided by Hermida [] correspond to misidentifications.
Superfamily Testacelloidea Gray, 1840
Family Testacellidae Gray, 1840
Genus Testacella Lamarck, 1819
3.1.60. Testacella haliotidea Draparnaud, 1801
Broadly distributed species in the Atlantic coast and the European Mediterranean. In the Iberian Peninsula it is distributed from Asturias to Catalonia, as well as the provinces neighboring the Mediterranean coast. Asturias records are scarce and fragmented, probably due to the underground habitat of this species that makes it difficult to detect. It is a predatory species of worms. A review of Testacella species in Asturias is provided in Robla and Alonso [].
3.1.61. Testacella maugei Férussac, 1819
Broadly distributed species in the European Atlantic. In the Iberian Peninsula it is distributed from Navarra to Galicia, continues along the Atlantic coast to Gibraltar and reaches Valencia in the Mediterranean. It is the most easily identifiable Testacella due to its large shell compared to the rest Iberian species. Same biology to other members of Testacellidae. It seems a widely distributed species in Asturian territory based on the location of slug shells [].
3.1.62. Testacella scutulum G. B. Sowerby I, 1821
This species is distributed through the western Mediterranean, easily found in the entire Iberian Mediterranean coastal area. In Asturias, no living specimens were located, but shells were found []. The lack of more exhaustive studies on this family of slugs and its apparent Mediterranean distribution, allow us to think whether the found slug shells [] could have been the result of several introductions. However, more studies are needed to clarify its status in Asturias as native or introduced.
Superfamily Succineoidea Beck, 1837
Family Succineidae Beck, 1837
Genus Oxyloma Westerlund, 1885
3.1.63. Oxyloma elegans (Risso, 1826)
Cosmopolitan Holarctic species, broadly distributed in the Iberian Peninsula. Oxyloma elegans is an amphibian aquatic species very common in Asturias. It lives on the banks of rivers, streams, water courses, canals, lagoons, wetlands and fountains, generally attached to the riverside vegetation.
Genus Quickella C.R. Boettger, 1939
3.1.64. Quickella arenaria (Potiez & Michaud, 1838)
Species distributed throughout Western Europe. In the Iberian Peninsula there are only a few records from the Arán Valley (Lérida), Castellón, Teruel and Asturias. In Asturias it is a very rare species, only known from Sta. María del Puerto (Santoña) and Tielve (Cabrales) []. It lives in humid areas near watercourses and even in high mountains.
Superfamily Pupilloidea W. Turton, 1831
Family Pupillidae W. Turton, 1831
Genus Pupilla J. Fleming, 1828
3.1.65. Pupilla muscorum (Linnaeus, 1758)
Holarctic species distributed throughout the northern half of the Iberian Peninsula and in some isolated areas in the south. Pupilla muscorum is rare in Asturias and it is highly chalky and hygrophilous. It lives mainly associated with banks and meadows near watercourses among other habitats.
Family Cochlicopidae Pilsbry, 1900 (1879)
Genus Cochlicopa A. Férussac, 1821
3.1.66. Cochlicopa lubrica (O. F. Müller, 1774)
Holarctic species widely distributed in the Iberian Peninsula. This species is common in Asturias and lives in all types of environments: banks, forests, crops, anthropized areas, usually in humid microhabitats under stones, logs, and leaf litter. It is usually found in sympatry with C. lubricella, from which it is difficult to separate due to its great morphological similarity []. Hermida [] assigned the record of Zua subcilindrica (Linnaeus, 1767) registered by Bofill and Haas [] in Covadonga, Las Caldas (Oviedo) and Sta. María del Mar (Castrillón) to Cochlicopa lubrica.
3.1.67. Cochlicopa lubricella (Porro, 1838)
Holarctic species, somewhat less distributed by the Iberian Peninsula than C. lubrica but distributed throughout the same regions. Frequent species in Asturias with identical habitat to C. lubrica. Both species have a great morphological similarity [].
Family Enidae B. B. Woodward, 1903 (1880)
Genus Merdigera Held, 1838
3.1.68. Merdigera obscura (O. F. Müller, 1774)
Widely distributed species in North Africa and southern Europe, occurring in a good part of the Iberian Peninsula. Common species in Asturias well adapted to anthropized areas, easily found in walls and leaf litter.
Genus Jaminia Risso, 1826
3.1.69. Jaminia quadridens (O. F. Müller, 1774)
Broadly distributed in southern Europe and the Iberian Peninsula. This is a thermophilic species. Not yet recorded from Asturias, it has been found in a few localities, in dry, south oriented rocky slopes with shrubs (Alonso and Raven, in press).
Family Lauriidae Steenberg, 1925
Genus Lauria Gray, 1840
3.1.70. Lauria cylindracea (Da Costa, 1778)
Species distributed throughout Africa, Asia and Europe. It is distributed throughout practically the entire Iberian Peninsula, being quite frequent in Asturias. It lives in all kinds of environments, particularly in anthropized areas and stone walls. It usually forms enormous colonies under the rocks.
3.1.71. Lauria sempronii (Charpentier, 1837)
Species distributed throughout the European Mediterranean and Atlantic fringe. It is a rare species in the Iberian Peninsula limited to a small fraction of the Cantabrian region. Very rare species in Asturias, since only one record is known in Campo de Caso []. It seems to live in limestone soils and wall cavities.
Genus Leiostyla R. T. Lowe, 1852
3.1.72. Leiostyla anglica (Férussac, 1821)
Species widely distributed across the European Atlantic coast. In the Iberian Peninsula it is distributed from the northwest to the south of Portugal with some records towards the Mediterranean area. It is a very rare species in Asturias, only known from an isolated record in the vicinity of Trubia and Proaza []. It is typical from humid and shady areas such as forests and swamps.
Family Pyramidulidae Kennard & B. B. Woodward, 1914
Genus Pyramidula Fitzinger, 1833
3.1.73. Pyramidula rupestris (Draparnaud, 1801)
Species distributed throughout Asia, Africa and central and southern Europe. In the Iberian Peninsula is found in the northwest part. It is rare in Asturias and is limited to rocks and limestone walls. This species prefers crevices where humid microhabitats are easily formed. It feeds mainly on lichens.
3.1.74. Pyramidula umbilicata (Montagu, 1803)
Species distributed throughout Western Europe. It is found in the northern part of the Iberian Peninsula. Its typical habitat is very similar to P. rupestris although it can also be found frequently on stone walls. They are very active in humid walls.
Family Valloniidae Morse, 1864
Genus Vallonia Risso, 1826
3.1.75. Vallonia costata (O. F. Müller, 1774)
Holarctic species broadly distributed in the Iberian Peninsula. Vallonia costata is frequent in Asturias and lives in a great diversity of environments that include humid places (forests and grasslands), where it is found linked to leaf litter and wood. It is also not uncommon on drier calcareous substrates. Easily observed in sympatry with V. pulchella.
3.1.76. Vallonia excentrica Sterki, 1893
Holarctic species with patchy distribution in the Iberian Peninsula. Rare species in Asturias, only recorded in several isolated localities []. It inhabits calcareous soils, coastal areas and meadows. The lack of new records of this species in Asturias, together with its similar appearance to its sister species V. pulchella, makes us think that the material recorded by Altonaga et al. [] corresponds to a misidentification. However, more studies are needed to clarify the presence of this species in Asturias.
3.1.77. Vallonia pulchella (O. F. Müller, 1774)
Holartic species with same distribution as V. costata in the Iberian Peninsula. Frequent species in Asturias that shares the same type of habitats as V. costata.
Genus Acanthinula H. Beck, 1847
3.1.78. Acanthinula aculeata (O. F. Müller, 1774)
Species of western Palearctic distribution that occupies a good part of the Iberian Peninsula. Quite a rare species in Asturias. It is distributed in very humid areas such as forests, bushes and riverbanks, generally living among leaf litter.
Family Vertiginidae Fitzinger, 1833
Genus Vertigo O. F. Müller, 1773
3.1.79. Vertigo antivertigo (Draparnaud, 1801)
Species amply distributed throughout Europe. In the Iberian Peninsula it is limited to the north-northeast region. Rare species in Asturias, where only one record from near the Lago Enol (Covadonga) is known []. It is a typical species of humid and swampy areas where it can be found among the mosses and leaf litter on which it feeds.
3.1.80. Vertigo pusilla O. F. Müller, 1774
Species distributed throughout the Central Europe and Asia, with isolated records in the Iberian Peninsula (Catalonia, Castellón and Asturias). It can be considered very rare in Asturias, since only one record from Cangas de Narcea is known []. Its typical habitat is associated with fountains and springs living among stones, wood and vegetation.
3.1.81. Vertigo pygmaea (Draparnaud, 1801)
Holarctic species broadly distributed throughout the north of the Iberian Peninsula, the Portuguese coast and some areas of the Mediterranean. Frequent species in Asturias. Vertigo pygmaea is typical in moist environments. However, it can also be found in calcareous walls.
3.1.82. Vertigo substriata (Jeffreys, 1833)
Species distributed throughout central and northern Europe, with isolated records in the Iberian Peninsula limited to Andorra, the Pyrenees, Sierra Nevada (Granada) and Asturias. Very rare species in Asturias with a single record near the town of Noriega [].
Superfamily Clausilioidea J. E. Gray, 1855
Family Clausiliidae J. E. Gray, 1855
Genus Clausilia Draparnaud, 1805
3.1.83. Clausilia bidentata abietina Dupuy, 1849
Species distributed throughout France and the northern part of the Iberian Peninsula. It is a very common species in Asturias. Typical of humid microenvironments. It is normally found attached to stones and calcareous walls, although it is also found under wood and stones. It is found from the coast to mountain areas. As a highly variable species, it has been historically confused with several subspecies of Clausilia rugosa []. Therefore, the old records of Clausilia rugosa Draparnaud, 1801 registered by Hidalgo [] and Clausilia (Kuzmicia) rugosa Draparnaud, 1801 from Bofill and Haas [] are surely misidentifications and probably correspond C. bidentata abietina.
Genus Balea Gray, 1824
3.1.84. Balea heydeni Maltzan, 1881
Species distributed throughout the Atlantic European coastal areas. Limited to the northwest part of the Iberian Peninsula. It is an uncommon species in Asturias associated with humid environments such as riverbanks and fountains. Historically this species has been confused with its sister species B. perversa by several authors. However, after recent reviews it is clarified the distribution of these two sister species in the Peninsula, B. heydeni was relegated to the northwest of the Peninsula and B. perversa to the northeast [,]. A recent study on this species in Cantabria seems to confirm because B. perversa was not found in any of the sampled localities [,]. After the mentioned works, B. perversa is recorded in the Integral Reserve of Muniellos in Asturias []. However, after reviewing the material, it has turned out to correspond to a misidentification with adults of B. heydeni and juveniles of Clausilia bidentata abietina.
Superfamily Arionoidea Gray, 1841
Family Arionidae Gray, 1840
Genus Arion A. Férussac, 1819
3.1.85. Arion ater (Linnaeus, 1758)
It is a widely distributed European species. This slug occurs in the north of the Iberian Peninsula and is perhaps a common species in Asturias. Historically, the Arion ater complex (usually denominated Arion ater s. l.) have comprised two different morphological forms: Arion ater and Arion rufus, without consensus about their status []. Both species have been considered different subspecies [,], ecotypes [] or species/clades [,]. Due to its external resemblance, most authors confused both species, so the records of Arion ater must be revaluated in Asturias. Recently, with new genetic approaches, Peláez et al. [] stablished the members of Arion complex as separated species. Arion ater, among its great external variability, tends to be a large black species usually found in gardens, meadows, orchards and other ruderal and anthropized areas. It can be found in mountainous regions and forests too. It is an important pest species.
3.1.86. Arion rufus (Linnaeus, 1758)
Records of Arion rufus from Arion ater complex needs re-examinations and confirmations after new studies []. Tentatively, with the same European, Iberian and Asturian distribution of A. ater. Arion rufus frequents gardens, orchards and other anthropized areas as an important pest for agriculture.
3.1.87. Arion hispanicus Simroth, 1886
Endemic slug distributed across the northwest and central part of the Iberian Peninsula. Asturias is the northern limit of its distribution. It seems to be a species that prefers humid meadows []. This slug is poorly known.
3.1.88. Arion hortensis Férussac, 1819
This slug species is distributed in southwest Europe with several introductions in other parts of the continent. In the Iberian Peninsula is limited to the north. Castillejo [,] and Cadevall and Orozco [] created distribution maps including Asturias but no specimens were collected from there or studied. Various specimens of Arion that fitted with the external appearance of A. hortensis were observed under a stone in a meadow with some bushes in December of 2019 in Monte Naranco (Oviedo). However, slugs were not collected, so the presence of this species in Asturias, although quite probable, needs confirmation.
3.1.89. Arion intermedius Normand, 1852
Arion species native to Western Europe. In the Iberian Peninsula it is widely distributed, only missing in the south. It is, with the members of A. ater complex, the other most common species in Asturias, frequently found in meadows, orchards, gardens and forests.
3.1.90. Arion lusitanicus auct. non Mabille, 1868/Arion vulgaris Moquin-Tandon, 1855
Under the denomination of Arion lusitanicus/Arion vulgaris, two different slugs were included without a clear specific status. Arion lusitanicus is supposed to be a microendemic slug to Portugal [,,] quite different from A. vulgaris. The separation of both species was confirmed thanks to molecular analysis [,]. On the other hand, A. vulgaris, commonly named ‘Spanish slug’, is a wide distributed species with a high expansive and invasive character [,]. Although its origin is not clear, it could be native from Central Europe [,]. Thus, all the records of this species in Asturias must be reviewed [,,,,] because only A. lusitanicus was apparently recorded. Asturian records of A. lusitanicus probably must be reassigned to A. vulgaris, a slug ranked among the hundred must invasive species in Europe [].
Género Geomalacus Allman, 1843
3.1.91. Geomalacus maculosus Allman, 1843
Endemism is limited to the northern Iberian Peninsula and Ireland [,]. It is a nocturnal species that spends most of the day hidden in crevices or under stones and logs. It occurs in very humid microhabitats of well-preserved wooded areas (oak groves, chestnuts and beech forests). It also has a mountain morph (with different color from the common morph) and can appear in anthropized areas such as stone walls covered by moss []. It is the only Iberian slug under some type of protection. It is protected under Appendix A of the Bern Convention and is included in Annex IV (a) of the EU Habitats Directive 92/43 []. It is catalogued as Vulnerable (VU B1ab(I, ii, iii)) in the Iberian Peninsula red book of invertebrates []. It is a common species in Asturias but difficult to detect due to its nocturnal habits.
Superfamily Limacoidea Lamarck, 1801
Family Limacidae Lamarck, 1801
Genus Limacus Lehmann, 1864
3.1.92. Limacus flavus (Linnaeus, 1758)
Species patchily distributed, mainly occurring in the Mediterranean region. In the Iberian Peninsula it is a broadly distributed species lacking in some regions. In Asturias it is a very common species that appears frequently in orchards, gardens and anthropic areas. It presents ruderal habits, although it also frequents forests and other natural habitats. Limacus maculatus (Kaleniczenko, 1851) is a similar species that have not be found in Iberian territory yet. Due to its external resemblance to L. flavus and its status of introduced in other regions [], it is not rare that could be found in Asturias in the future.
Genus Ambigolimax Pollonera, 1887
3.1.93. Ambigolimax valentianus (Férussac, 1822)
Amply distributed species throughout all the Iberian Peninsula, introduced in north and central parts of Europe. It is a common species in Asturias with anthropic trends, frequent in ruderal habitats and gardens []. It can be found in cities, urban parks and walls with crevices and mosses. It also occurred in natural habitats. Due to its relative abundance, it can become an agricultural pest.
Género Lehmannia Heynemann, 1862
3.1.94. Lehmannia marginata (O.F. Müller, 1774)
Common species in central and western Europe. It is distributed in the northern Iberian Peninsula. Common species in Asturias linked to ruderal and anthropized areas. However, it can also be found in forests developing arboreal trends []. It is considered a species with a great variability []. One morphological form treated as Lehmannia marginata f. rupicola, is considered as a separated species by some authors [,]. Lehmannia rupicola Lesson & Pollonera, 1882 is not still found in Asturias, but it is a species that quite probably will be found in future samplings.
Género Limax Linnaeus, 1758
3.1.95. Limax maximus Linnaeus, 1758
Widely distributed species throughout Europe that occurs in the northern Iberian Peninsula, as well as Portugal. It has specific records in southern Spain [], probably due to introductions. Common species, linked to altered environments such as orchards, walls and other habitats under man influence. It is a nocturnal species that is easily observed on the cooler and rainy nights. However, during the day it is quite difficult to find even under stones or logs in which it hides.
Family Agriolimacidae H. Wagner, 1935
Genus Deroceras Rafinesque, 1820
3.1.96. Deroceras agreste (Linnaeus, 1758)
Broadly distributed species in the western Palearctic. In the Iberian Peninsula it is common in the northern, although it has also been found in certain areas of the Mediterranean. It is an abundant species in Asturias, common in mountainous areas, both in meadows and forests [].
3.1.97. Deroceras panormitanum (Lessona & Pollonera, 1882)/D. invadens Reise, Hutchinson, Schunack & Schlitt, 2011
This species was believed to be distributed along the European Atlantic coast and some areas of the Mediterranean region. In the Iberian Peninsula it had been recorded in the Basque Country, Galicia, León, Cantabria, Valencia and Portugal. In fact, Castillejo [] drew distribution maps of this species that included Asturias, even though no Asturian specimen had been studied at the time. The only Asturian record of D. panormitanum is one from Muniellos []. Cadevall and Orozco [] did not include this reference in their book, but they included Asturias as the region where D. panormitanum occurred. Exhaustive analyses and studies have determined that D. panormitanum is a species limited to Sicily and Malta [] and all the records of this species from the rest of the world need revision. It could actually correspond to Deroceras invandens [,]. Therefore, D. panormitanum does not occur in Asturias. The specimen of D. panormitanum from the Integral Reserve of Muniellos could not be revised due to its bad conservation state. However, this record probably belongs to D. invadens. More studies are needed to clarify its status in Asturias, but it is probably an extended anthropical species introduced from Italy [].
3.1.98. Deroceras ercinae De Winter, 1985
Iberian endemism exclusively limited to the Cantabrian region. Species distributed in a variety of environments, but usually preferring calcareous soils []. This species is quite unknown and since its description and some later works [] that contribute with new records for Galicia, it has not been re-studied.
3.1.99. Deroceras laeve (O.F. Müller, 1774)
Holarctic species that in the Iberian Peninsula is distributed in the north, lacking in southern and central regions. It is a species linked to very moist microenvironments. In Asturias it is preferably found in areas close to rivers [] among other habitats.
3.1.100. Deroceras lombricoides (Morelet, 1845)
Peninsular endemism distributed in northwest Spain and that extends to the south of Portugal. Its typical habitat is pine forests inhabiting the needles layer, but it is also found in other habitats [] Like D. ercinae, it is a little studied species that, since the works of Castillejo [] and Pesqueira et al. [] has not been restudied and its distribution and biology in the Asturian region is still an enigma.
3.1.101. Deroceras reticulatum (O.F. Müller, 1774)
The most abundant Iberian species of Deroceras widely distributed across Europe. It is a very common species in Asturias, particularly in anthropized areas, gardens and crops. It is an important pest.
3.1.102. Deroceras rodnae Grossu & Lupu, 1965
A species restricted to certain areas of central and eastern Europe. In the Iberian Peninsula is limited to the northern section. It is a typically forest species that frequents forest edging on wet nights, although it can also be found in leaf litter or dead wood []. It is a common species in Asturias.
Genus Furcopenis Castillejo & Wiktor, 1983
3.1.103. Furcopenis darioi Castillejo & Wiktor, 1983
Iberian endemism, only found in a small region of Galicia, the Sil Valley (León) and the south of Asturias []. It is typical from chestnut forests, holm oaks and broom scrub [] However, like other species such as D. ercinae and D. lombricoides it is a species quite unknown.
Family Vitrinidae Fitzinger, 1833
Genus Vitrina Draparnaud, 1801
3.1.104. Vitrina pellucida (O. F. Müller, 1774)
A Holarctic species widely distributed in the Iberian Peninsula, except the southwestern area. In Asturias, this species prefers humid environments such river meadows and forests with logs, trunks and leaf litter.
Superfamily Gastrodontoidea Tryon, 1866
Family Gastrodontidae Tryon, 1866
Genus Aegopinella Lindholm, 1927
3.1.105. Aegopinella nitidula (Draparnaud, 1805)
Species distributed throughout Western Europe that in the Iberian Peninsula is limited to the northern part. It is a frequent species in Asturias that lives in forests and edges of orchards.
3.1.106. Aegopinella pura (Alder, 1830)
Species distributed throughout central Europe. In the Iberian territory it is limited to the northeast region with some records in certain provinces of Portugal. It is not a common species in Asturias, which tends to select relatively dry and exposed environments, being easy to find under stones.
Genus Zonitoides Lehmann, 1862
3.1.107. Zonitoides nitidus (O. F. Müller, 1774)
Holarctic species distributed throughout much of the Iberian Peninsula. Rare species in Asturias, there is only evidence of an isolated record in Cangas de Onís [] and it has not been located again. In other parts of the Peninsula where it is abundant, it lives associated with watercourses such as sources and riversides, generally among the vegetation or leaf litter.
Family Oxychilidae Hesse, 1927 (1879)
Genus Oxychilus Fitzinger, 1833
3.1.108. Oxychilus alliarius (J. S. Miller, 1822)
Western European species. In the Iberian Peninsula is distributed throughout the northwest region and other several localities (Salamanca, Ávila and Tarragona). Rare species in Asturias that lives in forests among the leaf litter.
3.1.109. Oxychilus anjana Altonaga, 1986
Endemic Iberian species with a limited distribution to Cantabria and western Asturias. This species is rare in Asturias because there is only one known record in Trescares from Peñamellera Alta [], so little can be said about its habitat in Asturias. It could live in riverside forests with organic matter.
3.1.110. Oxychilus cellarius (O. F. Müller, 1774)
Species distributed throughout western and central Europe, widespread in the northern half of the Iberian Peninsula. It is one of the most frequent species of Oxychillus in Asturias. This species lives in riverside forests and gardens. It can adapt to certain degree of anthropization.
3.1.111. Oxychilus draparnaudi (H. Beck, 1837)
Species distributed throughout the western Mediterranean and Europe. Well widespread over the entire Iberian Peninsula. It is one of the most frequent species in Asturias with O. cellarius. It lives in the same habitat as O. cellarius. In front of the snails of the region it is partially carnivorous and feeds on worms and snails.
3.1.112. Oxychilus navarricus (Bourguignat, 1870)
Endemism to the Cantabrian-Pyrenean region in the northern Iberian Peninsula. It is a frequent species in Asturias that usually lives in humid beech trees forest among the leaf litter. This species has been recorded in Asturias many times under the name of O. helveticus (Blum, 1881) (= O. navarricus) [,,]. Bech [] registered Oxychilus (Oxychilus) cfr. psaturus Bourguignat, 1864 in Covadonga and later, Altonaga [] assigned this record to O. helveticus (= O. navarricus).
Genus Perpolita H. B. Baker, 1928
3.1.113. Perpolita hammonis (Strøm, 1765)
Species distributed throughout much of central and northern Europe. It lives in the northern half of the Iberian Peninsula. It is a common species in Asturias that lives in deciduous forests and gardens, among leaf litter and mosses.
Genus Retinella P. Fischer, 1877
3.1.114. Retinella incerta (Draparnaud, 1805)
Cantabrian-Pyrenean endemism distributed throughout the northeast of the Iberian Peninsula. There are only two records belonging to a establish population detected in 2015 in Baldornón (Gijón) and an empty shell found in El Monte in Pico San Martín from Siero []. Its origin is uncertain, and it seems introduced through ornamental plants [].
Genus Morlina A. J. Wagner, 1914
3.1.115. Morlina glabra (Rossmässler, 1835)
This species was recorded by Hermida [] under the name of Oxychilus (Morlina) glaber (Rossmässler, 1835) in Luarca (Valdés), on the riverside. Its distribution in the Iberian Peninsula includes the coast of Catalonia, as well as other provinces such as Galicia, Ávila, Castellón, Salamanca and Cáceres. Its presence in Galicia was ratified by Altonaga []. However, he mentions that he had not found it anywhere else in the north. It is absent in the eastern half of Galicia. This distribution gap, with the difficulty that this family presents regarding its identification, makes us question the presence of this species in Asturias. It may be a misidentification with another species of the genus Oxychilus.
Family Pristilomatidae Cockerell, 1891
Genus Vitrea Fitzinger, 1833
3.1.116. Vitrea subrimata (Reinhardt, 1871)
Species distributed throughout southern Europe and the northeast Iberian Peninsula. A rare species in the region, it lives in humid places, usually under stones, logs or leaf litter. This species has historically been recorded throughout the Iberian Peninsula under the name of Vitrea narbonensis (Clessin, 1877). However, it is currently considered an endemism from southern France and therefore absent on the Iberian Peninsula. The records of this species by Bofill and Haas [] under the name of Hyalinia (Vitrea) narbonnensis Clessin, 1877 on the road from Castrillón to Sta. Maria del Mar correspond to V. contracta as indicated by Hermida []. Thus, V. narbonensis are not part of the fauna of the region.
3.1.117. Vitrea contracta (Westerlund, 1871)
Species with a Euro-Mediterranean distribution found in practically all the Iberian Peninsula. It is frequent in Asturias and usually lives in the humid microhabitat formed under stones, logs and among the leaf litter. Álvarez-Cuesta et al. [] recorded V. crystallina (O. F. Müller, 1774) in the Integral Reserve of Muniellos. However, after reviewing the material it turned out to correspond to a misidentification with V. contracta.
Superfamily Parmacelloidea P. Fischer, 1856 (1855)
Family Milacidae Ellis, 1926
Genus Milax Gray, 1855
3.1.118. Milax nigricans (Philippi, 1836)
It is a slug broadly distributed in central Europe. In the Iberian Peninsula occurs in the Mediterranean. In the north, it is distributed until Asturias, where its status is completely unknown. Hermida [] collected some specimens, after which it has not been recorded again. It is a slug that prefers ruderal and anthropized areas.
3.1.119. Milax gagates (Draparnaud, 1801)
Slug with a patchy distribution in Europe, widely distributed in the Iberian Peninsula. The records from Asturias are scarce [,,]. Curiously, Asturias is the northern region with the least records of this species. It is an anthropical and ruderal slug that occurs in orchards, gardens, walls and forests. M. gagates and M. nigricans are similar species difficult to distinguish without anatomical analysis. More studies are needed to reach the real abundance of Milax species and for clarifying the status of these two species in the study area. However, specimens from the Integral Reserve of Muniellos could not be reviewed due to its bad conservation condition.
Superfamily Trochomorphoidea Möllendorff, 1890
Family Euconulidae H. B. Baker, 1928
Genus Euconulus Reinhardt, 1883
3.1.120. Euconulus fulvus (O. F. Müller, 1774)
Holarctic species. It is widely distributed in the Iberian Peninsula missing in some provinces, probably due to sampling deficiencies. Frequent species in Asturias. It lives in a wide variety of environments: montane meadows, deciduous forests, riverbanks and gardens. A recent review of Euconulus is provided in Horsáková et al. [].
Superfamily Helicoidea Rafinesque, 1815
Family Helicidae Rafinesque, 1815
Genus Cepaea Held, 1838
3.1.121. Cepaea hortensis (O. F. Müller, 1774)
Species distributed throughout central and western Europe. In the Iberian Peninsula it is only distributed along the Pyrenees, in the mountains systems of Guadalajara, Cuenca and Teruel and La Rioja. It usually lives in forests close to riversides. Here, we present the first record of this species for Asturias. This snail was found alive in Alto L’Angliru Mountain.
Material examined: Spain–Asturias: Grandiella, L’Angliru (Riosa). one specimen. 15—X-2016. (30TTN63429172).
3.1.122. Cepaea nemoralis (Linnaeus, 1758)
Species widely distributed throughout Europe. An abundant species in the Iberian Peninsula more frequent in the northern. It is a very common species in Asturias. Highly adapted to anthropized environments, it presents a great variability of different color morphs. It lives in a wide variety of environments being especially common in gardens, orchards and walls.
Genus Cornu Born, 1778
3.1.123. Cornu aspersum (O. F. Müller, 1774)
Species widely distributed throughout Europe and the entire Iberian Peninsula. Probably one of the most common and known species in Asturias, well adapted to anthropization. Its habitat is very similar to that of C. nemoralis which it lives in sympatry. It is a species with gastronomic interest.
Genus Eobania P. Hesse, 1913
3.1.124. Eobania vermiculata (O. F. Müller, 1774)
Species distributed throughout the European Mediterranean area that mainly occupies the eastern part of the Iberian Peninsula. There are only two known records in Asturias: one empty shell found in 1989 in a private garden located in Baldornón (Gijón) and another empty shell found in 2015 in a roadside adjacent to a farm in La Campa Torres in Gijón []. Its origin is uncertain. It seems more likely that they are punctual introductions related to the trade of garden and horticulture plants [].
Genus Helix Linnaeus, 1758
3.1.125. Helix pomatia Linnaeus, 1758
Species distributed throughout central and southern Europe. In the Iberian Peninsula the only isolated specimens have been recorded as introductions in Catalonia and Valencia. Here, we present the first record of this species for Asturias. This snail was found alive in Isabel la Católica park in 2016, one of the busiest urban parks in Gijón, with numerous native Iberian and ornamental plants. The potential pathway of introduction of this species is, probably, the gardening of ornamental plants.
Material examined: Spain–Asturias: Isabel la Católica park (Gijón): one live specimen. 2016. (30TTP86442395).
Genus Otala Schumacher, 1817
3.1.126. Otala lactea (O. F. Müller, 1774)
Species distributed throughout the southern part of the Iberian Peninsula and the north of Morocco. It lives in all kinds of environments from calcareous surfaces to dry and sunny areas, among rocks and vegetation. Here, we present the first record of this species for Asturias. Some adults of this snail were found alive in the in the facilities of a greenhouse in La Corredoria (Oviedo) during 2013–2019. The introduction of this species is probably linked to the importation of ornamental plants to the greenhouse from southern regions of Spain where this species is really common.
Material examined: Spain–Asturias: La Corredoria (Oviedo), near a greenhouse: 21 specimens. 2013–2019. (30TTP71310756).
3.1.127. Otala punctata (O. F. Müller, 1774)
Species distributed throughout the western European Mediterranean area, inhabiting the eastern part of the Iberian Peninsula. One single record is known in Asturias from an empty shell found in 1981 in a private garden in Baldornón (Gijón). Its origin is uncertain. Due to the lack of records, it could be an introduction related to the garden and horticulture trade [].
Genus Theba Risso, 1826
3.1.128. Theba pisana pisana (O. F. Müller, 1774)
Species distributed along the European Atlantic and Mediterranean coast. In the Iberian Peninsula is distributed throughout the southern half and the northern coast. Frequent species in Asturias. It has a highly thermophilic and xerophilic character. It frequents arid coastal places, although it can be found inner, essentially in ruderal and roadsides. It is locally abundant where it is located, constituting colonies of large numbers of estivating individuals. Bofill & Haas [] recorded Helix (Theba) cantiana Montagu, 1803 (= Monacha cantiana (Montagu, 1803)) in Salinas (Avilés). However, after the review by Prieto [] it is concluded that it was a misidentification with T. pisana.
Family Elonidae E. Gittenberger, 1977
Genus Elona H. Adams & A. Adams, 1855
3.1.129. Elona quimperiana (Blainville, 1821)
Species with a vicarious distribution: French Brittany and the north of the Iberian Peninsula. In Asturias it lives mainly in humid forests, on the banks of rivers and rocky crevices. It is also a common species at the entrance of caves and even in somewhat deeper parts. Both juveniles and adults can even be found in areas with a certain degree of anthropization. It usually lives among leaf litter or under wood. It is a species protected internationally included in Appendix A of the Bern Convention and in Habitats Directive 92/43. It is protected at national level (Real Decreto 139/2011, from 4 of February). However, it is a common, abundant and amply distributed species throughout the territory of Asturias.
Family Geomitridae C.R. Boettger, 1909
Genus Cochlicella A. Férussac, 1821
3.1.130. Cochlicella acuta (O. F. Müller, 1774)
Species distributed along the Atlantic and Mediterranean coasts of Europe. Preferably distributed along the coastal provinces of the Iberian Peninsula. Common species in Asturias, with a very xerothermic character. It is easily found in dune systems and other coastal territories, occasionally inner.
3.1.131. Cochlicella barbara (Linnaeus, 1758)
Species of similar European distribution to C. acuta that nevertheless is easily found in the entire Iberian Peninsula. Unlike C. acuta, C. barbara prefers more humid environments such as meadows, crops and riversides. However, it is commonly found in dune areas.
Genus Ponentina P. Hesse, 1921
3.1.132. Ponentina revelata (Michaud, 1831)
Species distributed throughout Europe widely distributed in northern Iberian Peninsula, reaching the western part of Portugal. It is a rare species in Asturias. It lives in meadows and rocky areas. The genus Ponentina has been recorded in Asturias on several occasions. First, Bofill & Haas [] registered Helix (Hygromia) occidentalis Récluz, 1845 (= P. ponentina (Morelet, 1845)) in Covadonga, Piedras Blancas and Pravia. These records were subsequently collected by Manga-González [] and Raven [] as P. ponentina. Puente [] mentioned material identified as P. ponentina in Cabo Peñas in his doctoral thesis. Finally, Rolán [] found P. ponentina in Alto de la Cobertoria (Pola de Lena). However, Holyoak and Holyoak [] exhaustively reviewed the genus Ponentina in the Iberian Peninsula and indicate that P. ponentina is endemic from Portugal. Thus, the species extended through the northwest part of the Iberian Peninsula is P. revelata, so all the aforementioned records should be reassigned to P. revelata.
Genus Cernuella Schlüter, 1838
3.1.133. Cernuella virgata (Da Costa, 1778)
Species distributed throughout North Africa and the European Mediterranean coast. It is found in the majority of the Iberian Peninsula. Quite common species in Asturias, well adapted to anthropized areas. It lives in an important variety of environments such as ruderal areas, gardens and dune systems. It is generally found attached to wooden posts and to herbaceous or shrubby vegetation of a certain size, estivating. A recent work [] points out that this species has been the subject of successive introductions to the Cantabrian coast from other places in the Iberian Peninsula, based on a historical literature review and new data provided on distribution and habitat.
Genus Helicella A. Férussac, 1821
3.1.134. Helicella itala itala (Linnaeus, 1758)
Species distributed throughout much of Western Europe. However, in the Iberian Peninsula it is limited to the north. Helicella itala itala is frequent in Asturias. It lives in a great variety of environments from dune areas to mountains. Altimira [] recorded Helicella pampelonensis minor in Monte Naranco (Oviedo) andTapia de Casariego. In her doctoral thesis, Puente [] corrected several records of Helicella itala pampelonensis (A. Schmidt, 1856) from the Iberian Peninsula, such those of Altimira [] finally attributed to H. itala itala.
Genus Plentuisa Puente & Prieto, 1992
3.1.135. Plentuisa vendia Puente & Prieto, 1992
Endemic species to the Iberian Peninsula, whose distribution is limited to Picos de Europa [,]. Rare species in Asturias that lives in exposed areas. Usually, it is found on limestone substrates or among the herbaceous vegetation of these areas.
Genus Xeroplexa Monterosato, 1892
3.1.136. Xeroplexa intersecta (Poiret, 1801)
Species distributed along the western coast of Europe. It occupies the west part of the Iberian Peninsula. Very rare species in the region. There is only one record in Tapia de Casariego []. Generally, it can adapt to a great variety of environments. Although the known distribution of this species could reach the eastern part of Asturias, it has not been reported again since 1969.
Genus Xerosecta Monterosato, 1892
3.1.137. Xerosecta cespitum (Draparnaud, 1801)
Species distributed throughout southwestern Europe. Broadly distributed in the Iberian Peninsula except for the westernmost areas. Common in Asturias. It has been recorded in numerous localities in the center of the region []. It prefers highly anthropized environments such as ruderals, roadsides and industrial estates. Its presence in the roadsides of Puerto de Pajares suggests that industrial vehicles can act as the main vector of dispersal of this species from other regions of the Peninsula []. The record provided by Anadón and Anadón [] in the Roces and Gato Mountains has turned out to correspond to a misidentification with H. itala itala after reviewing the material.
Genus Xerotricha Monterosato, 1892
3.1.138. Xerotricha apicina (Lamarck, 1822)
Species distributed throughout the Atlantic and Mediterranean European and African coast. It is more abundant in the southern part of the Iberian Peninsula. It is a rare species in Asturias. It is highly xerophilic and thermophilic, so it prefers dune and coastal environments.
3.1.139. Xerotricha corderoi (E. Gittenberger & Manga, 1977)
Endemic Iberian species limited to the provinces of León and Asturias. It is restricted to high mountain environments between rocks and vegetation, where it is relatively common [].
Family Hygromiidae Tryon, 1866
Genus Ashfordia J. W. Taylor, 1917
3.1.140. Ashfordia granulata (Alder, 1830)
Species distributed along the European Atlantic coast. In the Iberian territory it is located in the northwest part. Frequent species in Asturias. It is well adapted to anthropized areas. However, it also prefers forest vegetation and can appear in coastal environments.
Genus Cryptosaccus Prieto & Puente, 1994
3.1.141. Cryptosaccus asturiensis Prieto & Puente, 1994
Endemic Iberian species only found in Asturias and its border with León. Quite rare species that only has been reported in Pola de Somiedo and Picos Albos from Asturias and Sta. María del Puerto from León []. It lives in limestone walls and cliffs, and rocky slopes, hidden in crevices or under vegetation. Its type locality is a calcareous rock between Caunedo and Gúa, near Pola de Somiedo []. It is a poorly known species within the Asturian malacofauna, even though it is a practically Asturian endemism.
Genus Mengoana Ortiz de Zárate López, 1949
3.1.142. Mengoana jeschaui (Kobelt, 1878)
Endemic Iberian species found in the northwest part. Mengoana jeschaui is common in Asturias and lives in a great variety of environments: forests, riversides, limestone areas, roadsides and stone walls. Ojea and Anadón [] recorded Hygromia lymbata (Draparnaud, 1805) in Monte Naranco (Oviedo) and later, Prieto and Puente [] reviewed the material and concluded that it was a misidentification with M. jeschaui. Bofill and Haas [] registered Helix (Monacha) incarnata (O. F. Müller, 1774) (= Monachoides incarnatus (O. F. Müller, 1774)) in Monte Naranco (Oviedo), Avilés and Covadonga. Later, Puente [] corrected these records reassigning them to Mengoana jeschaui.
Genus Monacha Fitzinger, 1833
3.1.143. Monacha cartusiana (O. F. Müller, 1774)
Species distributed along the Mediterranean part of Europe. Widely distributed in the eastern part of the Iberian Peninsula. Common species in Asturias, mainly distributed in the western. It is a species with calcareous trends distributed from the coast to mountainous areas.
Genus Portugala E. Gittenberger, 1980
3.1.144. Portugala inchoata (Morelet, 1854)
It is an endemic species widely distributed throughout the eastern part of the Iberian Peninsula. It is abundant in Asturias, more common in the western part of the region. It selects forests, meadows and limestone areas.
Genus Pyrenaearia P. Hesse, 1921
3.1.145. Pyrenaearia cantabrica (Hidalgo, 1873)
Endemic species to the Iberian Peninsula, distributed exclusively along the northern provinces of Spain. Frequent species in Asturias whose type locality is located in Oviedo. It usually prefers living attached to limestone rocks on walls, in highly thermophilic and xerophilic environments; however, they can select more humid areas in the northern Iberian Peninsula [,,]. Various species and varieties have been described that have become synonymized with this species, including P. cantabrica var. covadongae Ortiz de Zárate López, 1956, P. poncebensis Ortiz de Zárate López, 1956, P. schaufussi (Kobelt, 1876) and P. oberthuri (Ancey, 1884) [,,]
Genus Zenobiellina Holyoak, D. T. & Holyoak, G. A., 2018
3.1.146. Zenobiellina graminicola Holyoak, D. T. & Holyoak, G. A., 2018
Endemic Iberian species. Only known from Cantabria (Desfiladero de la Hermida) and Asturias (Covadonga) []. It lives on fresh vegetation, humid places and flooded areas.
3.1.147. Zenobiellina subrufescens (J. S. Miller, 1822)
Species distributed along the western coast of Europe occupying a good part of the northern Iberian Peninsula. Its habitat is identical to that of Z. subrefescens. The recent description of Z. graminicola [] evidences the necessity of reviewing all the Asturian material.
Family Trissexodontidae H. Nordsieck, 1987
Genus Oestophora P. Hesse, 1907
3.1.148. Oestophora barbella (Servain, 1880)
Endemic Iberian species. It occupies the western part of the Iberian Peninsula and Asturias. It is found in humid places like forests and riversides, usually hidden in humid micro-environments. This species has historically been confused with its sister species O. barbula (Rossmässler, 1838) which has been recorded on several occasions in Asturias [,,,,,,,]. However, after reviewing these two species by Holyoak and Holyoak [] it was concluded that O. barbula is an endemism restricted to the eastern and central regions of Portugal, considering the aforementioned records belonging to O. barbella.
3.1.149. Oestophora lusitanica (L. Pfeiffer, 1841)
Endemic species to the Iberian Peninsula, located on the Atlantic coast. Rare species in Asturias, distributed throughout the western part of the region. Its typical habitat is similar to that of O. barbaella and the rest of the species of the family Trissexodontidae.
3.1.150. Oestophora silvae Ortiz de Zárate López, 1962
Endemic species to the Iberian Peninsula. It mainly occupies the northern part of the territory. An abundant species in Asturias that can be found in a wide variety of environments such as stone walls, rocky areas and forests.
Genus Oestophorella G. Pfeffer, 1930
3.1.151. Oestophorella buvinieri (Michaud, 1841)
Endemic Iberian species broadly distributed along the Cantabrian coast. Oestophorella buvinieri and Oestophora silvae are the two most abundant representatives of Trissexodontidae family in Asturias. It shares the same habitat as O. silvae.
Superfamily Azecoidea H. Watson, 1920
Family Azecidae H. Watson, 1920
Genus Azeca J. Fleming, 1828
3.1.152. Azeca goodalli (A. Férussac, 1821)
Species distributed throughout the northwestern part Europe. In the Iberian Peninsula it is distributed throughout the Cantabrian coast. It is common in Asturias and usually lives under mosses and leaf litter in forest environments.
Genus Cryptazeca Folin & Bérillon, 1877
3.1.153. Cryptazeca subcylindrica Folin & Bérillon, 1877
Cantabrian-Pyrenean endemism distributed throughout the southwest France and the northeast of the Iberian Peninsula. Rare species in Asturias that lives associated with caves and calcareous rocks. Historically, it has been recorded in Asturias under the name of Cryptazeca vasconica (Kobelt, 1894), a name that is later synonymized with C. subcylindrical []. Holyoak and Holyoak [] expressed their doubts about the Bech [] record of Cryptazeca monodonta (de Folin & Bérillon, 1877) in Torca de Lusil from Ruenes (Peñamellera Alta). Probably, it is a misidentification with C. subcylindrica.
Superfamily Chondrinoidea Steenberg, 1925
Family Chondrinidae Steenberg, 1925
Genus Chondrina Reichenbach, 1828
3.1.154. Chondrina avenacea (Bruguière, 1792)
Species distributed throughout Western Europe. It occupies a good part of the northeast Iberian Peninsula. Quite a rare species in Asturias, only known in the north of Pendueles []. There is another record of this species in Monte Naranco from Oviedo []. However, it is a misidentification with C. kobelti indicated by Altonaga []. The Bofill and Haas [] record of Pupa (Modicella) avenacea (Bruguière, 1792) in Covadonga should be re-examined since C. kobelti is a very common species in this particular area. Where it is frequent, it tends to choose limestone substrates such as rocky and vertical walls.
3.1.155. Chondrina cantabroccidentalis Somoza-Valdeolmillos & Vázquez-Sanz, 2021
Endemic species to the Iberian Peninsula distributed along the Cantabrian Mountain. This species was recently described, and it is little known []. However, it seems to present similar habits to the rest species of the family.
3.1.156. Chondrina cliendentata E. Gittenberger, 1973
Endemic species to the Iberian Peninsula distributed throughout the southern of the Picos de Europa []. Rare species in Asturias which lives in limestone walls and rocks in thermophilic and xerophilic environments.
3.1.157. Chondrina kobelti kobelti (Westerlund, 1887)
Endemic species to the Iberian Peninsula amply distributed along the Cantabrian coast. Frequent species in Asturias. This species lives in limestone walls subjected to a strong sunlight. Generally, it selects shaded rock ledges looking for shady and humid environments.
3.1.158. Chondrina kobelti ordunensis Pilsbry, 1918
Endemic species to the Iberian Peninsula. The recent molecular study of the genus Chondrina in the Cantabrian coast [] revealed that this species occurs in the easternmost part of the region, essentially in Picos de Europa. It seems to present similar habits to C. kobelti kobelti. More studies are needed to increase the knowledge of this species.
3.1.159. Chondrina kobeltoides E. Gittenberger, 1973
Endemic species to the Iberian Peninsula. It is distributed throughout the area of Picos de Europa and western parts of Cantabria. It is not a frequent species in Asturias. Although it has an identical habitat to C. kobelti. Its type locality is Lago de la Ercina in Covadonga [].
Genus Abida W. Turton, 1831
3.1.160. Abida vasconica (Kobelt, 1882)
Endemic species to the Iberian Peninsula that, like other members of the family Chondrinidae, is distributed throughout the Cantabrian coast. Frequent species in Asturias that, although it lives in limestone walls, it can also be found among the leaf litter and under stones of limestone substrates. It usually appears in sympatry with other species such as C. kobelti and C. kobeltoides. Anadón and Anadón [] recorded Abida secale (Draparnaud, 1801) in Roces and Gato mountains. However, after reviewing the material, it turned out to correspond to a misidentification with A. vasconica. Bofill and Haas [] registered Chondrina lusitanica (L. Pfeiffer, 1848) under the name of Pupa (Modicella) lusitanica (Rossmässler, 1839). However, it is an identification error with A. vasconica, as Hermida [] had already indicated.
Family Truncatellinidae Steenberg, 1925
Genus Truncatellina R. T. Lowe, 1852
3.1.161. Truncatellina callicratis (Scacchi, 1833)
Widely distributed species along European and some Asian countries. T. callicratis is distributed along the eastern part of the Iberian Peninsula. Frequent species in Asturias. It lives in leaf litter and under wood and stones of calcareous areas. Its status in the Iberian Peninsula is discussed in Holyak et al. [].
Genus Columella Westerlund, 1878
3.1.162. Columella aspera Waldén, 1966
Species distributed throughout Western Europe. In the Iberian Peninsula it is mainly distributed along the Atlantic coast. It is a very rare species in Asturias. Several isolated records are known in the region [,]. Its typical habitat is forests occupying the leaf litter.
3.1.163. Columella edentula (Draparnaud, 1805)
Holarctic species. It is found in Galicia, Asturias and the northeast of the Iberian Peninsula. However, it is very rare in Asturias. Like C. aspera, some isolated records are known in the region [,] More studies are needed to clarify the real distribution of Columella species in Asturias.
Class Bivalvia Linnaeus, 1758
Order Unionida Gray, 1854
Superfamily Unionoidea Rafinesque, 1820
Family Margaritiferidae Henderson, 1929 (1910)
Genus Margaritifera Schumacher, 1815
3.1.164. Margaritifera margaritifera (Linnaeus, 1758)
Species with a patchy distribution from the northwest of the Iberian Peninsula to northern Europe []. Rare species in Asturias. It inhabits rivers with clean waters and not strong currents poor in calcium buried in bottoms of boulders, gravel and sand. It is protected under Appendix B of the Bern Convention and is included in Annex II and V of the EU Habitats Directive 92/43. It is catalogued as Endangered (EN A2ac+3ac; B1ab(i,ii,iii,iv); E) in the Red Book of Endangered Invertebrates of Spain [].
Order Sphaeriida Lemer, Bieler & Giribet, 2019
Superfamily Sphaerioidea Deshayes, 1855 (1820)
Family Sphaeriidae Deshayes, 1855 (1820)
Genus Euglesa Jenyns, 1832
3.1.165. Euglesa casertana (Poli, 1791)
Palearctic species distributed along the Iberian Peninsula. Frequent species in Asturias. It inhabits all types of watercourses, such as fountains, streams, rivers or lakes. It usually buries itself in sandy or muddy substrates. Ocharan-Larrondo et al. [] recorded Pisidium amnicum (O. F. Müller, 1774) in Tablizas from Integral Reserve of Muniellos river. However, after reviewing the material it has turned out to be a misidentification with E. casertana.
3.2. Questionable Species
A total of three taxa are considered doubtful, so they are excluded of the final malacological catalogue of Asturias.
3.2.1. Abida bigerrensis (Moquin-Tandon, 1856)
This species was recorded near Lago Enol in Covadonga []. Raven [] reviewed the family Chondrinidae of the Cantabrian Mountain and indicated that he had not found this species in any of his samplings. The confirmed distribution of this species is from the extreme east of Cantabria to the north of Lleida. Their almost total absence in the western half of Cantabria makes us question altogether the presence of this species in Asturias. It could be a misidentification or translocation of the information on the origin of the samples.
3.2.2. Succinea spp.
Different species of the genus Succinea have been recorded in Asturias on numerous occasions. First of all, Bofill and Haas [] recorded Succinea longiscata Morelet, 1845 and Succinea pfeifferi Rossmässler, 1835 in Piedras Blancas and Sta. María del Mar (Castrillón). Currently the validity of the first species is in doubt (Taxum inquirendum), while the second is considered synonymous of Oxyloma elegans. Later, Ojea and Anadón [] registered Succinea putris (Linnaeus, 1758) in Monte Naranco (Oviedo). Due to the difficult identification of this group and its recurrent confusion with Oxyloma genus, we believe that the aforementioned records are misidentifications with Oxyloma elegans. More studies are thus needed to verify the presence of this genus and species in Asturias.
3.2.3. Obscurella crassilabrum (Dupuy, 1849)
This species was recorded in Santa Albas [] as Pomatias crassilabrum Dupuy, 1849. Later, Raven [] made a review of the genus Obscurella and Gofas [] one of the genus Cochlostoma of the Cantabrian Mountains and Pyrenees. Neither of them mentioned the material recorded by Hidalgo []. The confirmed distribution of this species is from the eastern half of Cantabria to the north of Huesca. Its absence in the westernmost part of Cantabria makes the presence of this species in Asturias questionable, being surely a misidentification with O. bicostula not yet described at that time. Later, Ojea and Anadón [] registered Cochlostoma obscurum in Monte Naranco (Oviedo) and later Altonaga et al. [] commented this record as doutbful. Gofas [] in their review considered that C. obscurum is not part of the Cantabrian fauna. Therefore, the record from Ojea and Anadón [] it is surely an identification error with O. hidalgoi or O. bicostulata, common species in this area.
3.3. Diversity of Molluscs
The most diverse terrestrial mollusc families are Geomitridae (10 species), Agriolimacidae (eight species), Helicidae (eight species), Hygromiidae (eight species), Oxychilidae (eight species) and terrestrial Ellobidae (five species) (Figure 3). On the other hand, the most diverse aquatic mollusc families are Hydrobiidae (11 species), Lymnaeidae (seven species) and aquatic Ellobidae (five species) (Figure 4).
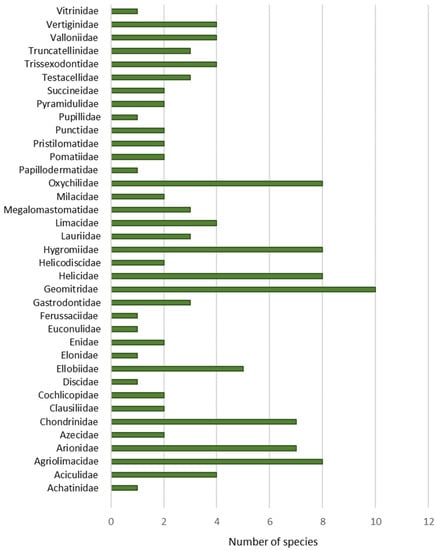
Figure 3.
Diversity of species of the different terrestrial mollusc families recorded in Asturias.
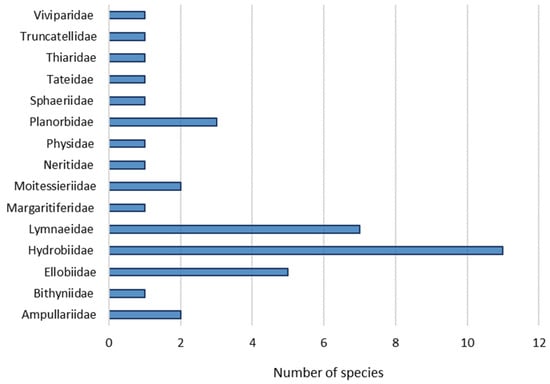
Figure 4.
Diversity of species of the different freshwater mollusc families recorded in Asturias.
3.4. Diversity of Trematodes
A total of 131 species of parasites belonging to 34 families included in the class Trematoda, subclass Digenea were found in the literature (Table A3). Of these, a total of 22 species included in eight families can parasitize humans (Table 1).

Table 1.
Diversity of digenean flukes that can parasitize humans.
The study of the diversity of intermediate hosts of the set of 22 trematode species that can parasitize humans revealed a total of 29 mollusc taxa (10 freshwater and 19 terrestrial) belonging to 14 families (four freshwater and 10 terrestrial) (Figure 5 and Figure 6; Table 2). The most representative families of mollusc intermediate hosts of Digenean flatworms are the families Lymnaeidae (seven species; 24.1%), Geomitridae (six species; 20.7%), Helicidae (four species; 13.8%) and Agriolimacidae (two taxa; 6.9%) (Figure 7).
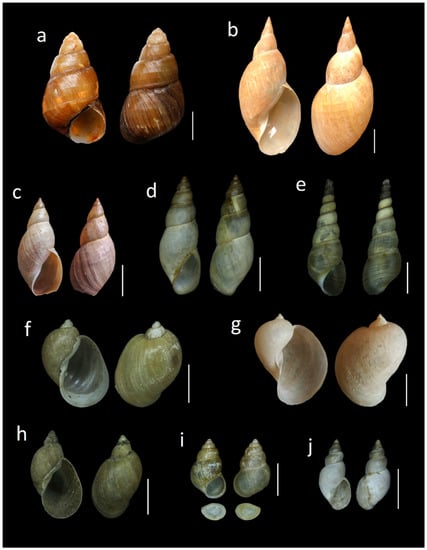
Figure 5.
Intermediate freshwater gastropod hosts of trematodes in Asturias. (a) Sinotaia cf. quadrata (Nora River-Oviedo), (b) Lymnaea stagnalis (Villanueva de Oscos pond), (c) Stagnicola cf. palustris (spring in Lugones-Siero), (d) Omphiscola glabra (temporary pond in Avilés), (e) Melanoides tuberculata (Nora River-Oviedo), (f) Ampullaceana balthica (Trubia River-Oviedo), (g) Radix auricularia (Trubia River-Oviedo), (h) Radix cf. labiata (Bascones River-Grado), (i) Bithynia tentaculata (Embalse de Valduno–Las Regueras), (j) Galba truncatula (Bascones River-Grado). All scale bars 1 cm.

Figure 6.
Terrestrial molluscs that act as intermediate hosts of trematodes in Asturias. (a) Cepaea nemoralis (Monte Naranco-Oviedo), (b) Cornu aspersum (Somines-Grao), (c) Cepaea hortensis (Grandiella, Riosa), (d) Helicella itala itala (Areñas–Cangas de Narcea), (e) Xerosecta cespitum (Pola de Lena-Lena), (f) Theba pisana pisana (Xagó beach-Oviedo), (g) Cochlicella acuta (Xagó beach-Oviedo), (h) Cochlicella barbara (Monte Naranco-Oviedo) (i) Clausilia bidentata abietina (Monte Naranco-Oviedo), (j) Rumina decollata (dune system of San Juan de Nieva-Avilés), (k) Merdigera obscura (Belmonte–Belmonte de Miranda), (l) Cochlicopa lubrica (Monte Naranco-Oviedo), (m) Xerotricha corderoi (Gamoniteiro Mountain-Quirós), (n) Monacha cartusiana (Peña Pegadín-Oviedo), (o) Vallonia costata (Monte Naranco-Oviedo), (p) Cernuella virgata (Xagó beach-Oviedo), (q) Arion ater (Forest of Navelgas–Tineo), (r) Deroceras spp. (Particular house in Navelgas-Tineo). All scale bars 5 mm.

Table 2.
Diversity of mollusc hosts of digenean trematodes that can parasitise humans. * The status of genus Deroceras depends on the specific species.
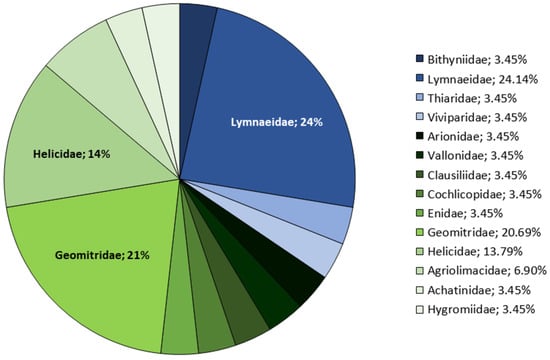
Figure 7.
Percentages of molluscan families that act as intermediate hosts of medical importance digenean trematodes. Families of freshwater molluscs in blue colour and the families of terrestrial molluscs in green colour.
Regarding the families and species of terrestrial molluscs, all transmit a single parasite and a single disease except for the species Cornu aspersum, Cochlicella barbara, Cochlicella acuta and Cernuella virgata, which transmit two parasites and two diseases. However, in freshwater molluscs, the family Lymnaeidae (10 parasites and four diseases) and the family Thiaridae (seven parasites and six diseases) are the ones that transmit the highest number of parasites and diseases (Figure 8a). The freshwater species that transmit the most parasites and diseases are Melanoides tuberculata (F. Thiaridae: seven parasites and six diseases), Radix auricularia (F. Lymnaeidae: six parasites and three diseases), Lymnaea stagnalis (F. Lymnaeidae: seven parasites and four diseases), R. labiata (F. Lymnaeidae: six parasites and four diseases), Ampullaceana balthica (F. Lymnaeidae: six parasites and four diseases) and Stagnicola cf. palustris (F. Lymnaeidae: four parasites and four diseases) (Figure 8b).
Figure 8.
Diversity of parasites (blue) and diseases (red) transmitted by (a) Freshwater mollusc families and (b) Freshwater mollusc species.
The diseases with the greatest diversity of species and families of transmitting molluscs are: ‘Dicroceliosis’ (15 species and eight families), ‘Echinostomiasis’ (five species and two families), ‘Fasciolosis’ (seven species and one family) and ‘Cercarial dermatitis’ (six species and two families) (Figure 9).
Figure 9.
Diversity of species (yellow) and families of molluscs (blue) transmitters of diseases produced by digenean trematodes.
Native mollusc species are the ones that transmit the greatest number of species of parasites (12 parasites and five diseases) with a large number of hosts (21 species). However, introduced species, represented by five host species, transmit a total of 10 parasites species (almost the same as native species) and more diseases (9 vs 5 from native species). The mollusc species with a cryptogenic character are the ones that transmit the least species of parasites (five parasites and five diseases) with a total of three host species (Figure 10).
Figure 10.
Diversity of parasites, transmitted diseases and hosts depending on the status of the hosts: cryptogenic (grey), introduced (red) and native (green).
Terrestrial molluscs, despite having twice as many intermediate hosts as freshwater molluscs (19 and 10 respectively), transmit three parasites and three diseases in contrast to freshwater ones, which transmit a total of 18 parasites and eight diseases (Figure 11).
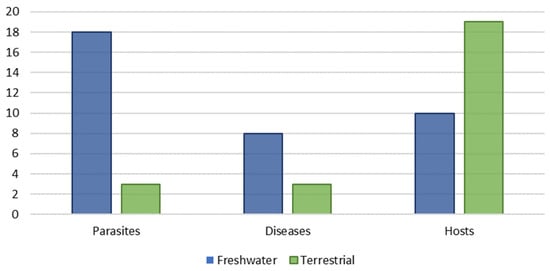
Figure 11.
Diversity of parasites, transmitted diseases and hosts depending on the biology of the hosts, freshwater molluscs (blue) and terrestrial (green).
3.5. Distribution of Parasite-Transmitting Molluscs
From the 29 parasite-transmitting molluscs whose trematodes can parasitise humans, 16 taxa are distributed throughout the entire Asturian region: 11 terrestrial and five freshwater species.
The land species are: A. ater, Deroceras spp. (two taxa), C. bidentata abietina, C. lubrica, M. obscura, C. barbara, H. itala itala, C. aspersum, C. nemoralis and V. costata. In the case of freshwater molluscs, the five species are also distributed throughout all territory: Radix spp. (both species), A. balthica, G. truncatula and B. tentaculata. The remaing 13 mollusc species are found in more restricted areas. The five freshwater species (Figure 12a) (i.e., O. glabra, L. stagnalis, S. cf. palustris, M. tuberculata and S. cf. quadrata) have very restricted distributions, limited to one or two localities in Asturias. The eight remaining terrestrial species have restricted distributions (Figure 12b), such as Theba pisana and Cochlicella acuta, (generally distributed along the coastal fringe), Xerotricha corderoi (distributed throughout the mountainous areas of the central and south of the region), Monacha cartusiana (extended by the eastern half) and C. virgata and X. cespitum (distributed throughout the central area). In addition, R. decollata and C. hortensis are limited to a single locality until now.
Figure 12.
Distribution of parasite-transmitters freshwater molluscs in Asturias. (a) Freshwater molluscs: Melanoides tuberculata & Sinoataia cf. quadrata (●), Stagnicola cf. palustris (⋆), Omphiscola glabra (▲), Lymnaea stagnalis (■) and (b) terrestrial molluscs: Cochlicella acuta & Theba pisana (orange), Cernuella virgata (green), Xerosecta cespitum (red), Xerotricha corderoi (blue), Monacha cartusiana (stripes), Rumina decollata (●) and Cepaea hortensis (▲). The municipalities (Avilés, Gijón and Oviedo) with the majority of parasite-transmitters molluscs are indicated in yellow in (a).
4. Discussion
Currently, biological invasions and emerging infectious diseases are two of the main study subjects of the scientific community []. The alarming increase of emerging zoonotic diseases has been linked, to a large extent, to changes in the geographic distribution of the parasite hosts due to climate change []. However, this is only one of the factors, the globalization and the high traffic and trade of ornamental species of plants and animals also enhance the number of introductions vias and vectors and facilitate the dispersal of zoonotic agents worldwide [,]. The introduction of pathogens into new systems can present negative consequences for human health and ecosystems [,] especially for the capacity of trematodes of evolve quickly, infect new species and create new hosts within the native fauna [].
In this work, we report ten species of continental gastropods (snails) that constitute new records from the Principality of Asturias and many of them from northern Spain. From them, six species correspond to introduced species. All of them have been found in anthropized environments and the majority found near ornamental plant species. Records of these introduced species have a great importance. For example, M. tuberculata and P. cf. maculata are included in the Spanish Catalogue of Invasive Alien Species (RD 630/2013) due to the incalculable damage they cause in other regions of Spain such as the Ebro River Basin []. Also important is P. duryi, which have developed an invasive behavior in other countries []. Of the remaining new species, O. glabra and S. cf. palustris have been classified as cryptogenic due to little information about their origin in the Principality of Asturias and their behavior outside the original distributions. On the other hand, terrestrial species are common species in the Iberian Peninsula (e.g., O. lactea and R. decollata) or Europe (e.g., H. pomatia and C. hortensis) and its presence in Asturias is probably consequence of several introductions due to people and vehicle movements and the terrestrial plants transport for gardening and forestry. It is important to note that most trematodes families have aquatic life cycles [], and only two families are adapted to terrestrial habits []. Thus, the importance of molluscs as host is biased to aquatic species that can concentrate more trematodes. In fact, the Asturian catalogue of malacofauna has 126 terrestrial species in front of 39 aquatic molluscs. However, only 19 species of terrestrial molluscs are supposed to be able to transmit parasites according to literature (only a 15.1% of the total terrestrial species of Asturias). On the contrary, 10 species of aquatic snails can transmit trematodiasis (a total of 25.6% of the total aquatic species of Asturias). This highlights the importance of freshwater species and its parasites comparing with terrestrial ones. Dicrocoelidae and Brachylaemidae are the only families of trematodes well adapted to terrestrial environments []. Nineteen mollusc taxa in Asturias can act as intermediate hosts of trematodes that can parasite humans. It is interesting to note that the majority has only one parasite (or disease) comparing with the aquatic snails and the number of parasites and diseases they can transmit. On the contrary, slugs are not such important vectors as snails in terrestrial environments. Although less is known about the relationship between slugs and trematodes [,,], the most recent works did not find trematodes in most of studied slugs [,,]. However, more studies are needed to reach the real importance of slugs as hosts for trematodes.
Regarding the features of the intermediate hosts, freshwater species, despite having half as intermediate hosts as terrestrial species, can transmit up to six times more parasites, and therefore a higher number of diseases than terrestrial gastropods. The most dangerous families potentially for humans in Asturias would be the Thiaridae and Lymnaeidae. The only representative of the Thiaridae family (M. tuberculata), as well as a large part of the species of the family Lymnaeidae, correspond to introduced or cryptogenic species, and many of them were not known to be present in Asturias. In this study, the introduced species, despite being four times smaller in number than the native ones, transmit the same number of parasites and twice the number of diseases. On the contrary, although native species supposed the major percentage of molluscs of Asturias (141 native species to 17 non-indigenous ones, excluding cryptogenic species), only 21 native molluscs species (14.8% of total native species) can transmit trematodiasis. However, the 29.4% of all introduced and invasive species in Asturias has the potential to transmit parasites (5 of 17 species). Thus, the data confirms the danger that introduced and invasive species pose to human health, since the potential to transmit parasites and to act as an intermediate host are higher in non-indigenous species comparing to native ones. It is interesting to remark that there are certain areas where there seems to be a greater presence of species of parasite-transmitting molluscs. These areas correspond to the municipalities of Oviedo, Avilés and Gijón, the main urbanized areas of the region, the biggest cities and therefore with those with the higher anthropic pressure. These crowded areas, where the non-native species pathways of introductions (gardening, aquarium hobby and plant or pet trade, tourism, among others) are accentuated, could suffer a greater risk of bioinvasions in the future. The three main cities of Asturias concentrate the highest number of parasite-transmitting molluscs (both native species and practically all introduced or invasive species). In addition to being the areas with the highest potential bio-sanitary risk, they are also ideal for monitoring introduced mollusc populations and for detecting new bioinvasions in their initial stages.
To estimate the risk of these parasites, it is important to know the status of the diseases in the country. Thus, the diseases with the greatest diversity of transmitting gastropod species in Asturias are Dicroceliosis, Echinostomiasis, Fasciolosis and Cercarial dermatitis. The most frequent trematodiasis in Spain is fasciolosis produced by Fasciola hepatica due to the intake of vegetables infected with metacercariae [,,,]. Most of the described cases are from the north coast of the Iberian Peninsula and generally affect adults []. However, the causes are decreasing since the 1990s []. In addition, there is evidence of the presence of Fasciola gigantica in Spain, specifically in the vicinity of the Guadalquivir valley and in Ciudad Real, due to the importation of livestock from its endemic north African and Arabian regions []. Some freshwater species as R. auricularia and probably G. truncatula act as intermediate hosts here in the Iberian Peninsula. These hosts are widely distributed throughout Asturias, where they inhabit all kinds of bodies of water frequented by livestock. Thus, the trade in aquatic and ornamental plant species together with the movements and transport of cattle to the region, could suppose a health risk in Asturias by helping the dispersal of these gastropods and its parasites. On the other hand, the cases described of Dicroceliosis in Spain generally correspond to people of sub-Saharan and Maghreb origin [,] being a very exceptional parasitosis in Spain. Infection in humans occurs by ingestion of raw or undercooked liver or by infected water []. Dicrocoelium dendriticum, the parasite that transmits Dicroceliosis, uses terrestrial gastropods (generally Geomitridae and Helicidae families) as intermediate hosts. These families are widely distributed in the Peninsula and particularly, in the Principality of Asturias []. Snails of this families are very common in meadows frequented by cattle increasing the risks of infections particularly in livestock areas. Furthermore, Dicroceliosis is an important veterinary disease that can infect livestock species, causing great losses in the sector [,]. Echinostomiasis infections are endemic from Asia, where it presents a high mortality and morbidity due to the ingestion of fish, crustaceans or snails infected by Echinostoma spp. trematodes [,]. Three species of Schistosoma (Schistosoma mansoni Sambon, 1907, S. japonicum (Katsurada, 1904) and S. haematobium Bilharz, 1892) are responsible for more than 24,000 deaths annually [,]. However, no published data about cases of Echinostomiasis have been found in Spain, probably due to the geographical separation between the Iberian Peninsula and Asia. It is important to note that the presence in Asturias of the Asian snail S. quadrata [] may play a critical role in the transmission of Echinostomiasis in the future. Another common trematodiasis is the Cercarial dermatitis. It consists of a skin rash due to an allergic reaction caused by flukes of the Schistosomatidae family present in bodies of water. It parasitizes ruminant mammals and waterfowl, but which repeatedly cause damage to agricultural workers, fishermen and bathers [,]. The cases have increased in Europe recently, being considered an emerging disease and affecting more than 10 countries []. In the Iberian Peninsula, cases of infected people have been reported after travels to Central Europe and tropical areas []. Due to the low specificity host of its parasites, it has a high number of host species, all of them from freshwater environments. Among those present in the Principality of Asturias we can highlight the native species belonging to the genus Radix and Ampullaceana. They are very common species in all rivers and small bodies of water and can serve as a vector for spreading this trematodiasis in Asturias.
The rest of the diseases that can be transmitted have very low prevalence worldwide and some of them have no published cases in Spain. Paragonimiasis, rare in Spain, is normally imported from tropical and subtropical countries and the trematodiasis caused by eastern flukes (e.g., opistorchiasis, clonorchiasis, hetophyasis) appear exceptionally in Spain in people coming from Southeast Asia []. The same can be attribute to another intestinal heminthiasis (e.g., the produced by the family Brachylaimidae) but, in the way that intermediate hosts occur in Asturias, the risk always exists even whether it is low. In this context, future biological invasions could increase the risk of trematodiasis in Asturias and the Iberian Peninsula due to the non-indigenous mollusc species.
5. Conclusions
A total of ten species of land snails are recorded for the first time in the Principality of Asturias, seven of them are also new records for northern Spain. The introduced/invasive status of seventeen reported taxa evidence the increase of the bioinvasion rate in continental molluscs, as well as their potential bio-sanitary risk for human and other animals’ health. In addition, the first detection of some snails, such as M. tuberculata, P. cf. maculata, and P. duryi is of great interest to be able to start a monitoring program of their populations and the environments in which they have been found, since they have already demonstrated an invasive behavior in other localities of Spain. It should be noted that the terrestrial host gastropods, despite being very abundant and common species in Asturias, present a low bio-sanitary risk (particularly, slugs), since they are only capable of transmitting D. dendriticum and Brachylaima cribbi. It would be advisable to be extremely careful in areas frequented by livestock since their movements are one of the main entry routes for parasites worldwide. It is important to avoid eating collected by oneself snails or, at least, ensure that they are well cooked. By contrast, freshwater snail hosts present a very high bio-sanitary risk, and many of them, such as G. truncatula, B. tentaculata and Radix/Ampullaceana spp., are very common species in all types of freshwater bodies in northern Spain. In these cases, it is highly important to avoid exposure in environments where these molluscs are abundant, as well as to exercise extreme care when ingesting water from sources and springs with their presence. The provided data strongly suggest that the increase of invasive freshwater snail species subsequently lead to an increase in parasitic infections, and this is a crucial point that transcends the merely scientific to the political-social sphere.
Author Contributions
Conceptualization, A.A., O.S. and J.R.; methodology, O.S., J.R. and A.A.; formal analysis, O.S., J.R. and A.A.; investigation, O.S., J.R. and A.A.; resources, O.S., J.R. and A.A.; writing—original draft preparation, O.S., J.R. and A.A.; writing—review and editing, O.S., J.R. and A.A.; supervision, A.A.; project administration, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We would express our gratitude to Alejandro Pérez, Miguel Carrillo, Álvaro Alonso and Han Raven for providing grey references and for their valuable comments on the manuscript. We are also grateful to Guillermo García-Saúco for his help with the English editing. We thank three anonymous referees for their careful reviews and helpful suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Diversity of freshwater molluscs of the Principality of Asturias. NEW: New record. ‘?’: needs confirmation.
Table A1.
Diversity of freshwater molluscs of the Principality of Asturias. NEW: New record. ‘?’: needs confirmation.
| Family | Species | Status |
|---|---|---|
| Sphaeriidae | Euglesa casertana (Poli, 1791) | Native |
| Margaritiferidae | Margaritifera margaritifera (Linnæus, 1758) | Native |
| Ampullariidae | Marisa cornuarietis (Linnaeus, 1758) | Introduced |
| Ampullariidae | Pomacea cf. maculata Perry, 1810 (NEW) | Introduced |
| Thiaridae | Melanoides tuberculata (O. F. Müller, 1774) (NEW) | Introduced |
| Ellobiidae | Leucophytia bidentata (Montagu, 1808) | Native |
| Ellobiidae | Myosotella denticulata (Montagu, 1803) | Native |
| Ellobiidae | Myosotella myosotis (Draparnaud, 1801) | Native |
| Ellobiidae | Ovatella aequalis (Lowe, 1832) | Native |
| Ellobiidae | Pseudomelampus exiguus (Lowe, 1832) | Native |
| Lymnaeidae | Ampullaceana balthica (Linnaeus, 1758) | Native |
| Lymnaeidae | Galba truncatula (O.F. Müller, 1774) | Native |
| Lymnaeidae | Lymnaea stagnalis (Linnaeus, 1758) (NEW) | Native |
| Lymnaeidae | Omphiscola glabra (O.F. Müller, 1774) (NEW) | Cryptogenic |
| Lymnaeidae | Radix auricularia (Linnaeus, 1758) | Native |
| Lymnaeidae | Radix labiata (Rossmässler, 1835) (?) | Native |
| Lymnaeidae | Stagnicola cf. palustris (O. F. Müller, 1774) (NEW) | Cryptogenic |
| Physidae | Physella acuta (Draparnaud, 1805) | Invasive |
| Planorbidae | Ancylus fluviatilis O.F. Müller, 1774 | Native |
| Planorbidae | Gyraulus albus (O.F. Müller, 1774) | Native |
| Planorbidae | Planorbella duryi (Wetherby, 1879) (NEW) | Introduced |
| Neritidae | Theodoxus fluviatilis fluviatilis (Linnaeus, 1758) | Native |
| Bithyniidae | Bithynia tentaculata (Linnaeus, 1758) | Native |
| Hydrobiidae | Alzoniella asturica (Boeters & Rolán, 1988) | Native |
| Hydrobiidae | Alzoniella camocaensis Rolán & Boeters, 2015 | Native |
| Hydrobiidae | Alzoniella cantabrica (Boeters, 1983) | Native |
| Hydrobiidae | Alzoniella lucensis (Rolán, 1992) | Native |
| Hydrobiidae | Alzoniella marianae Arconada, Rolán & Boeters, 2007 | Native |
| Hydrobiidae | Alzoniella montana (Rolán, 1993) | Native |
| Hydrobiidae | Alzoniella ovetensis (Rolán, 1993) | Native |
| Hydrobiidae | Alzoniella somiedoensis Rolán, Arconada & Boeters, 2009 | Native |
| Hydrobiidae | Deganta azarum (Boeters & Rolán, 1988) | Native |
| Hydrobiidae | Mercuria tachoensis (Frauenfeld, 1865) | Native |
| Hydrobiidae | Islamia ayalga Alonso, Quiñonero-Salgado & Rolán, 2018 | Native |
| Moitessieriidae | Spiralix asturica Quiñonero-Salgado, Ruiz-Cobo & Rolán, 2017 | Native |
| Moitessieriidae | Spiralix vetusta Quiñonero-Salgado, Alonso & Rolán, 2018 | Native |
| Tateidae | Potamopyrgus antipodarum (Gray, 1843) | Invasive |
| Truncatellidae | Truncatella subcylindrica (Linnaeus, 1767) | Native |
| Viviparidae | Sinotaia cf. quadrata (Benson, 1842) | Introduced |

Table A2.
Diversity of terrestrial molluscs of the Principality of Asturias. NEW: New record. ‘?’: needs confirmation.
Table A2.
Diversity of terrestrial molluscs of the Principality of Asturias. NEW: New record. ‘?’: needs confirmation.
| Family | Species | Status |
|---|---|---|
| Achatinidae | Rumina decollata (Linnaeus, 1758) (NEW) | Introduced |
| Ferussaciidae | Cecilioides acicula (O. F. Müller, 1774) | Native |
| Arionidae | Arion ater (Linnaeus, 1758) | Native |
| Arionidae | Arion hipanicus Simroth, 1886 | Native |
| Arionidae | Arion hortensis Férussac, 1819 (?) | Native |
| Arionidae | Arion intermedius Normand, 1852 | Native |
| Arionidae | Arion rufus (Linnaeus, 1758) | Native |
| Arionidae | Arion vulgaris Moquin-Tandon, 1855 | Cryptogenic |
| Arionidae | Geomalacus maculosus Allman, 1843 | Native |
| Azecidae | Azeca goodalli (A. Férussac, 1821) | Native |
| Azecidae | Cryptazeca subcylindrica Folin & Bérillon, 1877 | Native |
| Chondrinidae | Abida vasconica (Kobelt, 1882) | Native |
| Chondrinidae | Chondrina avenacea (Bruguière, 1792) | Native |
| Chondrinidae | Chondrina cantabroccidentalis Somoza-Valdeolmillos & Vázquez-Sanz, 2021 | Native |
| Chondrinidae | Chondrina cliendentata E. Gittenberger, 1973 | Native |
| Chondrinidae | Chondrina kobelti kobelti (Westerlund, 1887) | Native |
| Chondrinidae | Chondrina kobelti ordunensis Pilsbry, 1918 | Native |
| Chondrinidae | Chondrina kobeltoides E. Gittenberger, 1973 | Native |
| Truncatellinidae | Columella aspera Waldén, 1966 | Native |
| Truncatellinidae | Columella edentula (Draparnaud, 1805) | Native |
| Truncatellinidae | Truncatellina callicratis (Scacchi, 1833) | Native |
| Clausiliidae | Balea heydeni Maltzan, 1881 | Native |
| Clausiliidae | Clausilia bidentata abietina Dupuy, 1849 | Native |
| Aciculidae | Acicula fusca (Montagu, 1803) | Native |
| Aciculidae | Menkia horsti Boeters, E. Gittenberger & Subai, 1985 | Native |
| Aciculidae | Menkia rolani E. Gittenberger, 1991 | Native |
| Aciculidae | Platyla callostoma (Clessin, 1911) | Native |
| Megalomastomatidae | Obscurella asturica Raven, 1990 | Native |
| Megalomastomatidae | Obscurella bicostulata (Gofas, 1989) | Native |
| Megalomastomatidae | Obscurella hidalgoi (Crosse, 1864) | Native |
| Ellobiidae | Carychium minimum O.F. Müller, 1774 | Native |
| Ellobiidae | Carychium tridentatum (Risso, 1826) | Native |
| Ellobiidae | Zospeum gittenbergeri Jochum, Prieto & De Winter, 2019 | Native |
| Ellobiidae | Zospeum percostulatum Alonso, Prieto, Quiñonero-Salgado & Rolán, 2018 | Native |
| Ellobiidae | Zospeum praetermissum Jochum, Prieto & De Winter, 2019 | Native |
| Gastrodontidae | Aegopinella nitidula (Draparnaud, 1805) | Native |
| Gastrodontidae | Aegopinella pura (Alder, 1830) | Native |
| Gastrodontidae | Zonitoides nitidus (O. F. Müller, 1774) | Native |
| Oxychilidae | Morlina glabra (Rossmässler, 1835) | Native |
| Oxychilidae | Oxychilus alliarius (J. S. Miller, 1822) | Native |
| Oxychilidae | Oxychilus anjana Altonaga, 1986 | Native |
| Oxychilidae | Oxychilus cellarius (O. F. Müller, 1774) | Native |
| Oxychilidae | Oxychilus draparnaudi (H. Beck, 1837) | Native |
| Oxychilidae | Oxychilus navarricus (Bourguignat, 1870) | Native |
| Oxychilidae | Perpolita hammonis (Strøm, 1765) | Native |
| Oxychilidae | Retinella incerta (Draparnaud, 1805) | Introduced |
| Pristilomatidae | Vitrea contracta (Westerlund, 1871) | Native |
| Pristilomatidae | Vitrea subrimata (Reinhardt, 1871) | Native |
| Elonidae | Elona quimperiana (Blainville, 1821) | Native |
| Geomitridae | Cernuella virgata (Da Costa, 1778) | Introduced |
| Geomitridae | Cochlicella acuta (O. F. Müller, 1774) | Native |
| Geomitridae | Cochlicella barbara (Linnaeus, 1758) | Native |
| Geomitridae | Helicella itala itala (Linnaeus, 1758) | Native |
| Geomitridae | Plentuisa vendia Puente & Prieto, 1992 | Native |
| Geomitridae | Ponentina revelata (Michaud, 1831) | Native |
| Geomitridae | Xerosecta cespitum (Draparnaud, 1801) | Introduced |
| Geomitridae | Xerotricha apicina (Lamarck, 1822) | Native |
| Geomitridae | Xerotricha corderoi (E. Gittenberger & Manga, 1977) | Native |
| Geomitridae | Xeroplexa intersecta (Poiret, 1801) | Native |
| Helicidae | Cepaea hortensis (O. F. Müller, 1774) (NEW) | Cryptogenic |
| Helicidae | Cepaea nemoralis (Linnaeus, 1758) | Native |
| Helicidae | Cornu aspersum (O. F. Müller, 1774) | Native |
| Helicidae | Eobania vermiculata (O. F. Müller, 1774) | Introduced |
| Helicidae | Helix pomatia Linnaeus, 1758 (NEW) | Introduced |
| Helicidae | Otala lactea (O. F. Müller, 1774) (NEW) | Introduced |
| Helicidae | Otala punctata (O. F. Müller, 1774) | Introduced |
| Helicidae | Theba pisana pisana (O. F. Müller, 1774) | Native |
| Hygromiidae | Ashfordia granulata (Alder, 1830) | Native |
| Hygromiidae | Cryptosaccus asturiensis Prieto & Puente, 1994 | Native |
| Hygromiidae | Mengoana jeschaui (Kobelt, 1878) | Native |
| Hygromiidae | Monacha cartusiana (O. F. Müller, 1774) | Native |
| Hygromiidae | Portugala inchoata (Morelet, 1854) | Native |
| Hygromiidae | Pyrenaearia cantabrica (Hidalgo, 1873) | Native |
| Hygromiidae | Zenobiellina graminicola Holyoak, D. T. & Holyoak, G. A., 2018 | Native |
| Hygromiidae | Zenobiellina subrufescens (J. S. Miller, 1822) (?) | Native |
| Trissexodontidae | Oestophora barbella (Servain, 1880) | Native |
| Trissexodontidae | Oestophora lusitanica (L. Pfeiffer, 1841) | Native |
| Trissexodontidae | Oestophora silvae Ortiz de Zárate López, 1962 | Native |
| Trissexodontidae | Oestophorella buvinieri (Michaud, 1841) | Native |
| Limacidae | Ambigolimax valentiana (Férussac, 1822) | Native |
| Limacidae | Lehmannia marginata (O.F. Müller, 1774) | Native |
| Limacidae | Limacus flavus (Linnaeus, 1758) | Native |
| Limacidae | Limax maximus Linnaeus, 1758 | Native |
| Vitrinidae | Vitrina pellucida (O. F. Müller, 1774) | Native |
| Agriolimacidae | Deroceras agreste (Linnaeus, 1758) | Native |
| Agriolimacidae | Deroceras ercinae De Winter, 1985 | Native |
| Agriolimacidae | Deroceras invadens Reise, Hutchinson, Schunack & Schlitt, 2011 | Introduced |
| Agriolimacidae | Deroceras laeve (O.F. Müller, 1774) | Native |
| Agriolimacidae | Deroceras lombricoides (Morelet, 1845) | Native |
| Agriolimacidae | Deroceras reticulatum (O.F. Müller, 1774) | Native |
| Agriolimacidae | Deroceras rodnae Grossu & Lupu, 1965 | Native |
| Agriolimacidae | Furcopenis darioi Castillejo & Wiktor, 1983 | Native |
| Pomatiidae | Leonia mammillaris (Lamarck, 1822) | Introduced |
| Pomatiidae | Pomatias elegans (O. F. Müller, 1774) | Native |
| Papillodermatidae | Papilloderma altonagai Wiktor, Martín & Castillejo, 1990 | Native |
| Milacidae | Milax gagates (Draparnaud, 1801) | Native |
| Milacidae | Milax nigricans (Philippi, 1836) | Native |
| Discidae | Discus rotundatus (O. F. Müller, 1774) | Native |
| Helicodiscidae | Lucilla scintilla (Lowe, 1852) | Cryptogenic |
| Helicodiscidae | Lucilla singleyana (Pilsbry, 1890) (?) | Cryptogenic |
| Punctidae | Paralaoma servilis (Shuttleworth, 1852) | Native |
| Punctidae | Punctum pygmaeum (Draparnaud, 1801) | Native |
| Cochlicopidae | Cochlicopa lubrica (O. F. Müller, 1774) | Native |
| Cochlicopidae | Cochlicopa lubricella (Porro, 1838) | Native |
| Enidae | Merdigera obscura (O. F. Müller, 1774) | Native |
| Enidae | Jaminia quadridens (O. F. Müller, 1774) | Native |
| Lauriidae | Lauria cylindracea (Da Costa, 1778) | Native |
| Lauriidae | Lauria sempronii (Charpentier, 1837) | Native |
| Lauriidae | Leiostyla anglica (Férussac, 1821) | Native |
| Pupillidae | Pupilla muscorum (Linnaeus, 1758) | Native |
| Pyramidulidae | Pyramidula rupestris (Draparnaud, 1801) | Native |
| Pyramidulidae | Pyramidula umbilicata (Montagu, 1803) | Native |
| Valloniidae | Acanthinula aculeata (O. F. Müller, 1774) | Native |
| Valloniidae | Vallonia costata (O. F. Müller, 1774) | Native |
| Valloniidae | Vallonia excentrica Sterki, 1893 (?) | Native |
| Valloniidae | Vallonia pulchella (O. F. Müller, 1774) | Native |
| Vertiginidae | Vertigo antivertigo (Draparnaud, 1801) | Native |
| Vertiginidae | Vertigo pusilla O. F. Müller, 1774 | Native |
| Vertiginidae | Vertigo pygmaea (Draparnaud, 1801) | Native |
| Vertiginidae | Vertigo substriata (Jeffreys, 1833) | Native |
| Succineidae | Oxyloma elegans (Risso, 1826) | Native |
| Succineidae | Quickella arenaria (Potiez & Michaud, 1838) | Native |
| Testacellidae | Testacella haliotidea Draparnaud, 1801 | Native |
| Testacellidae | Testacella maugei Férussac, 1819 | Native |
| Testacellidae | Testacella scutulum G. B. Sowerby I, 1821 | Cryptogenic |
| Euconulidae | Euconulus fulvus (O. F. Müller, 1774) | Native |
Appendix B

Table A3.
Taxonomic list of the diversity of parasites and their intermediate hosts. ND: No data.
Table A3.
Taxonomic list of the diversity of parasites and their intermediate hosts. ND: No data.
| Family | Species | Author | 1st Intermediate Host | 2nd Intermediate Host/Definitive Host | Reference |
|---|---|---|---|---|---|
| Aporocotylidae | Sanguinicola inermis | Plehn, 1905 | Lymnaea stagnalis | Fishes | [] |
| Aporocotylidae | Sanguinicola inermis | Plehn, 1905 | Radix auricularia | Fishes | [] |
| Aporocotylidae | Sanguinicola inermis | Plehn, 1905 | Radix peregra group | Fishes | [] |
| Aporocotylidae | Sanguinicola inermis | Plehn, 1905 | Stagnicola palustris | Fishes | [] |
| Aporocotylidae | Sanguinicola intermedius | Ejsmont, 1926 | Lymnaea stagnalis | Fishes | [] |
| Aporocotylidae | Sanguinicola intermedius | Ejsmont, 1926 | Radix auricularia | Fishes | [] |
| Aspidogastridae | Aspidogaster conchicola | Baer, 1827 | Bithynia tentaculata | Fishes; Reptiles (Turtles); Mollusc | [] |
| Aspidogastridae | Aspidogaster conchicola | Baer, 1827 | Sinotaia quadrata | Fishes; Reptiles (Turtles); Mollusc | [] |
| Aspidogastridae | Aspidogaster limacoides | Diesing, 1834 | Radix balthica | Fishes; Reptiles (Turtles); Mollusc | [] |
| Azygiidae | Azygia lucii | (Müller, 1776) | Stagnicola palustris | Fishes (salmonids) | [] |
| Brachylaimidae | Brachycoelium obesum | Nicoll, 1914 | Deroceras reticulatum | Birds and mammals | [] |
| Brachylaimidae | Brachylaema nicolli | (Witenberg) | Deroceras agreste | Birds and mammals | [] |
| Brachylaimidae | Brachylaema sp. | ND | Deroceras reticulatum | Birds and mammals | [] |
| Brachylaimidae | Brachylaemus spp. | ND | Arion lusitanicus = A. vulgaris | Birds and mammals | [] |
| Brachylaimidae | Brachylaemus spp. | ND | Deroceras reticulatum | Birds and mammals | [] |
| Brachylaimidae | Brachylaima aspersae | Segade et al., 2011 | Cornu aspersum | Mammals (Rodents) | [] |
| Brachylaimidae | Brachylaima cf. mesostoma | (Rudolphi, 1803) | Cornu aspersum | Birds and mammals | [] |
| Brachylaimidae | Brachylaima cribbi | Butcher & Grove, 2001 | Cernuella virgata | Mammals (Humans) | [,] |
| Brachylaimidae | Brachylaima cribbi | Butcher & Grove, 2001 | Rumina decollata | Mammals (Humans) | [] |
| Brachylaimidae | Brachylaima cribbi | Butcher & Grove, 2001 | Cochlicella acuta | Mammals (Humans) | [] |
| Brachylaimidae | Brachylaima cribbi | Butcher & Grove, 2001 | Cochlicella barbara | Mammals (Humans) | [,] |
| Brachylaimidae | Brachylaima cribbi | Butcher & Grove, 2001 | Cornu aspersum | Mammals (Humans) Birds and reptiles | [,,] |
| Brachylaimidae | Brachylaima cribbi | Butcher & Grove, 2001 | Deroceras spp. | Mammals (Humans) | [] |
| Brachylaimidae | Brachylaima cribbi | Butcher & Grove, 2001 | Theba pisana | Mammals (Humans) | [,] |
| Brachylaimidae | Brachylaima erinacei | Blanchard, 1847 | Arion spp. | Birds and mammals | [] |
| Brachylaimidae | Brachylaima llobregatensis | González-Moreno & Gracenea, 2006 | Cornu aspersum | Mammals (Rodents) | [] |
| Brachylaimidae | Brachylaima mascomai | Gracenea & González-Moreno, 2002 | Cornu aspersum | Mammals (Rodents) | [] |
| Brachylaimidae | Brachylaima mesostoma | (Rudolphi, 1803) | Arion ater | Birds and mammals | [] |
| Brachylaimidae | Brachylaima mesostoma | (Rudolphi, 1803) | Arion vulgaris | Birds and mammals | [] |
| Brachylaimidae | Brachylaima mesostoma | (Rudolphi, 1803) | Cepaea hortensis | Birds and mammals | [] |
| Brachylaimidae | Brachylaima mesostoma | (Rudolphi, 1803) | Cepaea nemoralis | Birds and mammals | [] |
| Brachylaimidae | Brachylaima ruminae | Mas-Coma & Montoliu, 1986 | Rumina decollata | Mammals (Rodents) | [] |
| Brachylaimidae | Brachylaima sp. | ND | Cornu aspersum | Birds and mammals | [] |
| Brachylaimidae | Brachylecithum orfi | Kingston & Freeman, 1959 | Deroceras laeve | Birds | [] |
| Brachylaimidae | Brachylecithum orfi | Kingston & Freeman, 1959 | Deroceras reticulatum | Birds | [] |
| Brachylaimidae | Brachylecithum orfi | Kingston & Freeman, 1959 | Cochlicopa lubrica | Birds | [] |
| Brachylaimidae | Brachylecithum sp. | ND | Cepaea nemoralis | Birds | [] |
| Brachylaimidae | Panopistus pricei | Sinitsin, 1931 | Deroceras agreste | Birds and mammals | [] |
| Cryptogonimidae | Acanthostomum burminis | (Bhalerao, 1926) | Melanoides tuberculata | Amphibians | [] |
| Cyathocotylidae | Holostephanus cobitidis | Opravilova, 1968 | Bithynia tentaculata | Fishes | [] |
| Cyathocotylidae | Holostephanus curonensis | (Szidat, 1933) | Bithynia tentaculata | Fishes | [] |
| Cyathocotylidae | Holostephanus dubinini | Vojtek & Vojtkova, 196 | Bithynia tentaculata | Birds | [] |
| Cyathocotylidae | Holostephanus luehei | Szidat, 1936 | Bithynia tentaculata | ND | [] |
| Cyathocotylidae | Holostephanus volgensis | (Sudarikov, 1962) | Bithynia tentaculata | ND | [] |
| Cyathocotylidae | Cyathocotyle bithyniae | Saad, 1994 | Bithynia tentaculata | ND | [] |
| Cyathocotylidae | Cyathocotyle bushiensis | Khan, 1962 | Bithynia tentaculata | Birds (Anatids) | [] |
| Cyathocotylidae | Cyathocotyle opaca | Wisniewski, 1934 | Bithynia tentaculata | Annelids (Hirudinea) | [] |
| Cyathocotylidae | Cyathocotyle prussica | Mühling, 1898 | Bithynia tentaculata | Fishes | [] |
| Cyathocotylidae | Mesostephanus haliasturis | Tubangui & Masilungan, 1941 | Melanoides tuberculata | Birds | [] |
| Cyclocoelidae | Cyclocoelum microstomum | Creplin, 1829 | Lymnaea stagnalis | Birds | [] |
| Cyclocoelidae | Cyclocoelum microstomum | Creplin, 1829 | Radix peregra group | Birds | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Cepaea hortensis | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Cepaea nemoralis | Mammals (Human) | [,] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Cernuella virgata | Mammals (Human) | [,] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Clausilia bidentata | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Cochlicella acuta | Mammals (Human ) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Cochlicella barbara | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Cochlicopa lubrica | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Deroceras reticulatum | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Helicella itala | Mammals (Human) | [,] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Merdigera obscura | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Monacha cartusiana | Mammals (Human) | [,] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Vallonia costata | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Xerosecta cespitum | Mammals (Human) | [] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Xerotricha corderoi | Mammals (Human) | [,] |
| Dicrocoeliidae | Dicrocoelium dendriticum | (Rudolphi, 1819) | Cornu aspersum | Mammals (Human) | [] |
| Dicrocoeliidae | Eurytrema sp. | ND | Arion ater | Mammals (Humans) | [] |
| Dicrocoeliidae | Orthetrotrema monostomum | Macy & Basch, 1972 | Melanoides tuberculata | Odonata | [] |
| Diplostomidae | Tylodelphys clavata | (Nordmann, 1832) | Lymnaea stagnalis | Fishes | [] |
| Diplostomidae | Tylodelphys clavata | (Nordmann, 1832) | Radix auricularia | Fishes | [] |
| Diplostomidae | Tylodelphys clavata | (Nordmann, 1832) | Radix peregra group | Fishes | [] |
| Diplostomidae | Tylodelphys clavata | (Nordmann, 1832) | Stagnicola palustris | Fishes | [] |
| Diplostomidae | Diplostomum baeri | Dubois, 1937 | Radix auricularia | Fishes | [] |
| Diplostomidae | Diplostomum baeri | Dubois, 1937 | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum commutatum | (Diesing, 1850) | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum gobiorum | Shigin, 1965 | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum mergi | Dubois, 1932 | Radix auricularia | Fishes; Birds | [] |
| Diplostomidae | Diplostomum mergi | Dubois, 1932 | Radix peregra group | Fishes; Birds | [] |
| Diplostomidae | Diplostomum paracaudum | Iles, 1959 | Lymnaea stagnalis | Fishes | [] |
| Diplostomidae | Diplostomum paracaudum | Iles, 1959 | Radix auricularia | Fishes | [] |
| Diplostomidae | Diplostomum paracaudum | Iles, 1959 | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum paracaudum | Iles, 1959 | Stagnicola palustris | Fishes | [] |
| Diplostomidae | Diplostomum parviventosum | Dubois, 1932 | Radix auricularia | Fishes | [] |
| Diplostomidae | Diplostomum parviventosum | Dubois, 1932 | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum parviventosum | Dubois, 1932 | Stagnicola palustris | Fishes | [] |
| Diplostomidae | Diplostomum petromyzifluviatilis | Diesing, 1850 | Bithynia tentaculata | Fishes | [] |
| Diplostomidae | Diplostomum phoxini | (Faust, 1918) | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum pseudospathaceum | Niewiadomska, 1984 | Lymnaea stagnalis | Fishes | [] |
| Diplostomidae | Diplostomum pseudospathaceum | Niewiadomska, 1984 | Radix auricularia | Fishes | [] |
| Diplostomidae | Diplostomum pseudospathaceum | Niewiadomska, 1984 | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum pseudospathaceum | Niewiadomska, 1984 | Stagnicola palustris | Fishes | [] |
| Diplostomidae | Diplostomum spathaceum | Rudolphi, 1809 | Lymnaea stagnalis | Fishes | [] |
| Diplostomidae | Diplostomum spathaceum | Rudolphi, 1809 | Radix auricularia | Fishes | [] |
| Diplostomidae | Diplostomum spathaceum | Rudolphi, 1809 | Radix peregra group | Fishes | [] |
| Diplostomidae | Diplostomum spathaceum | Rudolphi, 1809 | Stagnicola palustris | Fishes | [] |
| Echinochasmidae | Echinochasmus bagulai | Verma, 1935 | Melanoides tuberculata | Birds | [] |
| Echinochasmidae | Echinochasmus japonicus | Tanabe, 1926 | Melanoides tuberculata | Mammals (Human) | [] |
| Echinochasmidae | Echinochasmus milvi | Yamaguti, 1939 | Melanoides tuberculata | Fishes | [] |
| Echinostomatidae | Echinoparyphium aconiatum | Dietz, 1909 | Lymnaea stagnalis | ND | [] |
| Echinostomatidae | Echinoparyphium aconiatum | Dietz, 1909 | Radix auricularia | ND | [] |
| Echinostomatidae | Echinoparyphium aconiatum | Dietz, 1909 | Radix peregra group | ND | [] |
| Echinostomatidae | Echinoparyphium aconiatum | Dietz, 1909 | Stagnicola palustris | ND | [] |
| Echinostomatidae | Echinoparyphium recurvatum | (Linstow, 1873) | Lymnaea stagnalis | Mammals (Rodents) | [] |
| Echinostomatidae | Echinoparyphium recurvatum | (Linstow, 1873) | Radix auricularia | Mammals (Rodents) | [] |
| Echinostomatidae | Echinoparyphium recurvatum | (Linstow, 1873) | Radix peregra group | Mammals (Rodents) | [] |
| Echinostomatidae | Echinoparyphium recurvatum | (Linstow, 1873) | Stagnicola palustris | Mammals (Rodents) | [] |
| Echinostomatidae | Echinostoma bolschewense | Kotova, 1939 | Lymnaea stagnalis | Mammals (Human) | [] |
| Echinostomatidae | Echinostoma bolschewense | Kotova, 1939 | Radix peregra group | Mammals (Human) | [] |
| Echinostomatidae | Echinostoma revolutum | (Fröhlich, 1802) | Lymnaea stagnalis | Mammals (Human) | [] |
| Echinostomatidae | Echinostoma revolutum | (Fröhlich, 1802) | Radix peregra group | Mammals (Human) | [] |
| Echinostomatidae | Echinostoma revolutum | (Fröhlich, 1802) | Stagnicola palustris | Mammals (Human) | [] |
| Echinostomatidae | Echinostoma sp. | ND | Sinotaia quadrata | Mammals (Human) | [,] |
| Echinostomatidae | Hypoderaeum conoideum | (Block, 1872) | Lymnaea stagnalis | Mammals (Human) | [] |
| Echinostomatidae | Hypoderaeum conoideum | (Block, 1872) | Radix auricularia | Mammals (Human) | [] |
| Echinostomatidae | Hypoderaeum conoideum | (Block, 1872) | Radix peregra group | Mammals (Human) | [] |
| Echinostomatidae | Hypoderaeum conoideum | (Block, 1872) | Stagnicola palustris | Mammals (Human) | [] |
| Echinostomatidae | Isthmiophora melis | (Schrank, 1788) | Lymnaea stagnalis | ND | [] |
| Echinostomatidae | Moliniella anceps | (Molin, 1859) | Lymnaea stagnalis | ND | [] |
| Echinostomatidae | Moliniella anceps | (Molin, 1859) | Radix auricularia | ND | [] |
| Echinostomatidae | Moliniella anceps | (Molin, 1859) | Radix peregra group | ND | [] |
| Echinostomatidae | Moliniella anceps | (Molin, 1859) | Stagnicola palustris | ND | [] |
| Echinostomatidae | Petasiger radiatus | (Dujardin, 1845) | Radix auricularia | Birds | [] |
| Eumegacetidae | Eumegacetes artamii | Mehra, 1935 | Melanoides tuberculata | ND | [] |
| Eumegacetidae | Eumegacetes spinosus | Fahmy, Khalifa & Abdel-Rahman, 1981 | Melanoides tuberculata | ND | [] |
| Fasciolidae | Fasciola gigantica | Cobbold, 1855 | Galba truncatula | Mammals (Human) | [] |
| Fasciolidae | Fasciola gigantica | Cobbold, 1855 | Radix auricularia | Mammals (Human) | [] |
| Fasciolidae | Fasciola gigantica | Cobbold, 1855 | Radix peregra group | Mammals (Human) | [] |
| Fasciolidae | Fasciola hepatica | (Linnaeus, 1758) | Galba truncatula | Mammals (Human) | [,,] |
| Fasciolidae | Fasciola hepatica | (Linnaeus, 1758) | Lymnaea stagnalis | Mammals (Human) | [] |
| Fasciolidae | Fasciola hepatica | (Linnaeus, 1758) | Omphiscola glabra | Mammals (Human) | [,] |
| Fasciolidae | Fasciola hepatica | (Linnaeus, 1758) | Radix auricularia | Mammals (Human) | [] |
| Fasciolidae | Fasciola hepatica | (Linnaeus, 1758) | Radix peregra group | Mammals (Human) | [] |
| Fasciolidae | Fasciola hepatica | (Linnaeus, 1758) | Stagnicola palustris | Mammals (Human) | [] |
| Fasciolidae | Fascioloides magna | Bassi, 1875 | Radix peregra group | Mammals (Ruminants) | [] |
| Haematoloechidae | Haematoloechus similis? | Looss, 1899 | Radix peregra group | Amphibians | [] |
| Heterophyidae | Centrocestus formosanus | (Nishigori, 1924) | Melanoides tuberculata | Fishes | [,] |
| Heterophyidae | Centrocestus unequiorchalis | Saad, 1994 | Melanoides tuberculata | Fishes; Birds (Anatids); Mammals (Rodents) | [] |
| Heterophyidae | Haplorchis pleurolophocerca | (Sonsino, 1892) | Melanoides tuberculata | Fishes | [] |
| Heterophyidae | Haplorchis pumilio | (Looss, 1896) | Melanoides tuberculata | Fishes | [] |
| Heterophyidae | Haplorchis taichui | (Nishigori, 1924) | Melanoides tuberculata | Mammals (Human) | [] |
| Heterophyidae | Haplorchis yokogawai | (Katsuta, 1932) | Melanoides tuberculata | Mammals (Human) | [] |
| Heterophyidae | Haplorchoides cahirinus | (Looss, 1896) | Melanoides tuberculata | Fishes | [] |
| Heterophyidae | Haplorchoides mehrai | Pande & Shukla, 1976 | Melanoides tuberculata | Fishes | [] |
| Heterophyidae | Heterophyes heterophyes | (Siebold, 1853) | Melanoides tuberculata | Mammals (Human) | [] |
| Heterophyidae | Heterophyes pumilio | (Looss, 1896) | Melanoides tuberculata | Mammals (Cannids); Fishes | [] |
| Heterophyidae | Stellantchasmus falcatus | Onji & Nishio, 1916 | Melanoides tuberculata | Mammals (Rodents) | [,] |
| Heterophyidae | Procerovum cheni | Hsü, 1950 | Melanoides tuberculata | Fishes (Eels) | [] |
| Heterophyidae | Procerovum varium | Onji & Nishio, 1916 | Melanoides tuberculata | Mammals (Human); Fishes | [] |
| Heterophyidae | Pygidiopsis genata | Looss, 1907 | Melanoides tuberculata | Mammals (Rodents); Fishes | [] |
| Lecithodendriidae | Loxogenoides bicolor | (Krull, 1933) | Melanoides tuberculata | Amphibians | [] |
| Lecithodendriidae | Paralecithodendrium pyramidium | Azim, 1936 | Melanoides tuberculata | Mammals (Quiroptera) | [] |
| Lecithodendriidae | Pleurogenoides medians | (Olsson, 1876) | Bithynia tentaculata | Amphibians | [] |
| Lepocreadiidae | Stegodexamene anguillae | MacFarlane, 1951 | Potamopyrgus antipodarum | Fish (Eels) | [] |
| Lissorchiidae | Asymphylodora tincae | (Modeer, 1790) | Bithynia tentaculata | Fishes | [] |
| Lissorchiidae | Asymphylodora tincae | (Modeer, 1790) | Radix auricularia | Fishes | [] |
| Lissorchiidae | Asymphylodora tincae | (Modeer, 1790) | Radix peregra group | Fishes | [] |
| Lissorchiidae | Asymphylodora tincae | (Modeer, 1790) | Stagnicola palustris | Fishes | [] |
| Notocotylidae | Catatropis verrucosa | (Frölich, 1789) | Bithynia tentaculata | Birds (Anatids) | [] |
| Notocotylidae | Notocotylus attenuatus | (Rudolphi, 1809) | Bithynia tentaculata | ND | [] |
| Notocotylidae | Notocotylus attenuatus | (Rudolphi, 1809) | Lymnaea stagnalis | Birds | [] |
| Notocotylidae | Notocotylus attenuatus | (Rudolphi, 1809) | Radix auricularia | Birds | [] |
| Notocotylidae | Notocotylus attenuatus | (Rudolphi, 1809) | Radix peregra group | Birds | [] |
| Notocotylidae | Notocotylus attenuatus | (Rudolphi, 1809) | Stagnicola palustris | Birds | [] |
| Notocotylidae | Notocotylus imbricatus | (Looss, 1893) | Bithynia tentaculata | Birds (Anatids) | [] |
| Notocotylidae | Notocotylus mamii | Hsti, 1954 | Melanoides tuberculata | Birds (Anatids) | [] |
| Notocotylidae | Notocotylus ralli | Baylis, 1936 | Stagnicola palustris | ND | [] |
| Omphalometridae | Omphalometra flexuosa | (Rudolphi, 1809) | Stagnicola palustris | Mammals (Rodents) | [] |
| Omphalornetridae | Neoglyphe sobolevi | (Shaldybin, 1953) | Lymnaea stagnalis | Reptiles | [] |
| Opecoelidae | Sphaerostoma bramae | (Müller, 1776) | Bithynia tentaculata | Fishes | [] |
| Opisthorchiidae | Clonorchis sinensis | Looss, 1907 | Melanoides tuberculata | Mammals (Human) | [] |
| Opisthorchiidae | Metorchis intermedius | Heinemann, 1937 | Bithynia tentaculata | Birds | [] |
| Opisthorchiidae | Metorchis xanthosomus | (Creplin, 1846) | Bithynia tentaculata | Fishes | [] |
| Opisthorchiidae | Ophisthorchis felineus | Rivolta, 1884 | Bithynia tentaculata | Mammals (Human) | [] |
| Paramphistomatidae | Gastrodiscus aegyptiacus | (Cobbold, 1876) | Melanoides tuberculata | Mammals (Equidae) | [] |
| Paramphistomidae | Calicophoron daubneyi | Dinnik, 1962 | Galba truncatula | Mammals (Ruminants) | [] |
| Paramphistomidae | Calicophoron daubneyi | Dinnik, 1962 | Omphiscola glabra | Mammals (Ruminants) | [] |
| Paramphistomidae | Calicophoron microbothrium | (Fischoeder, 1901) | Melanoides tuberculata | Mammals (Ruminants) | [] |
| Philophthalmidae | Philophthalmus distomatosa | (Looss, 1896) | Melanoides tuberculata | Birds | [] |
| Philophthalmidae | Philophthalmus gralli | Mathis & Leger, 1910 | Melanoides tuberculata | Birds | [] |
| Philophthalmidae | Philophthalmus nocturnus | Looss, 1907 | Melanoides tuberculata | Birds | [] |
| Plagiorchiidae | Haplometra cylindracea | (Zeder, 1800) | Galba truncatula | Amphibians | [] |
| Plagiorchiidae | Haplometra cylindracea | (Zeder, 1800) | Galba truncatula | Amphibians | [] |
| Plagiorchiidae | Haplometra cylindracea | (Zeder, 1800) | Lymnaea stagnalis | Amphibians | [] |
| Plagiorchiidae | Haplometra cylindracea | (Zeder, 1800) | Omphiscola glabra | Amphibians | [] |
| Plagiorchiidae | Haplometra cylindracea | (Zeder, 1800) | Radix peregra group | Amphibians | [] |
| Plagiorchiidae | Haplometra cylindracea | (Zeder, 1800) | Stagnicola palustris | Amphibians | [] |
| Plagiorchiidae | Leptophallus nigrovenosus | (Bellingham, 1844) | Radix peregra group | Reptiles (Snakes) | [] |
| Plagiorchiidae | Ophisthioglyphe ranae | (Frölich, 1971) | Lymnaea stagnalis | Fishes | [] |
| Plagiorchiidae | Ophisthioglyphe ranae | (Frölich, 1971) | Radix auricularia | Fishes | [] |
| Plagiorchiidae | Ophisthioglyphe ranae | (Frölich, 1971) | Radix peregra group | Fishes | [] |
| Plagiorchiidae | Ophisthioglyphe ranae | (Frölich, 1971) | Stagnicola palustris | Fishes | [] |
| Plagiorchiidae | Plagiorchis elegans | (Rudolphi, 1802) | Lymnaea stagnalis | Diptera | [] |
| Plagiorchiidae | Plagiorchis elegans | (Rudolphi, 1802) | Radix peregra group | Diptera | [] |
| Plagiorchiidae | Plagiorchis maculosus | (Rudolphi, 1802) | Lymnaea stagnalis | Annelids (Hirudinea) | [] |
| Plagiorchiidae | Plagiorchis maculosus | (Rudolphi, 1802) | Radix auricularia | Annelids (Hirudinea) | [] |
| Plagiorchiidae | Plagiorchis neomidis | Brendow, 1970 | Radix peregra group | ND | [] |
| Pronocephalidae | Neopronocephalus triangularis | Mehra, 1932 | Melanoides tuberculata | ND | [] |
| Prosthogonimidae | Prosthogonimus ovatus | (Rudolphi, 1803) | Bithynia tentaculata | Birds | [] |
| Psilostomatidae | Psilotrema simillinum | (Mühling, 1898) | Bithynia tentaculata | Mammals (Rodents) | [] |
| Psilostomatidae | Psilotrema spiculigerum | (Muehling, 1898) | Bithynia tentaculata | Mammals (Rodents) | [] |
| Psilostomatidae | Psilotrema tuberculata | de Filippi, 1857 | Bithynia tentaculata | ND | [] |
| Psilostomatidae | Sphaeridiotrema globulus | (Rudolphi, 1814) | Bithynia tentaculata | Birds | [] |
| Psilostomidae | Grysoma indica | Umadevi & Madhavi, 1995 | Melanoides tuberculata | Birds (Anatids) | [] |
| Schistosomatidae | Dendritobilharzia sp. | --- | Melanoides tuberculata | Birds | [] |
| Schistosomatidae | Ornithobilharzia turkestanicum | Skrjabin in 191 | Radix auricularia | Mammals | [] |
| Schistosomatidae | Trichobilharzia franki | Müller & Kimmig, 199 | Radix auricularia | Mammals (Human) | [,,] |
| Schistosomatidae | Trichobilharzia ocellata | (La Valette, 1855) | Lymnaea stagnalis | Mammals (Human) | [] |
| Schistosomatidae | Trichobilharzia sp. | --- | Lymnaea stagnalis | Mammals (Human) | [] |
| Schistosomatidae | Trichobilharzia szidati | Neuhaus, 1952 | Lymnaea stagnalis | Mammals (Human) | [,] |
| Schistosomatidae | Trichobilharzia szidati | Neuhaus, 1952 | Radix auricularia | Mammals (Human ) | [,] |
| Schistosomatidae | Trichobilharzia szidati | Neuhaus, 1952 | Radix peregra group | Mammals (Human) | [] |
| Schistosomatidae | Trichobilharzia szidati | Neuhaus, 1952 | Stagnicola palustris | Mammals (Human) | [] |
| Schistosomatidae | Gigantobilharzia sp. | ND | Melanoides tuberculata | Mammals (Human) | [,] |
| Schistosomatidae | Gigantobilharzia sp. | ND | Radix auricularia | Mammals (Human) | [] |
| Strigeidae | Apatemon gracilis | (Rudolphi, 1819) | Lymnaea stagnalis | Fishes; Birds (Anatids) | [] |
| Strigeidae | Apatemon gracilis | (Rudolphi, 1819) | Radix auricularia | Fishes; Birds (Anatids) | [] |
| Strigeidae | Apatemon gracilis | (Rudolphi, 1819) | Radix peregra group | Fishes; Birds (Anatids) | [] |
| Strigeidae | Apatemon gracilis | (Rudolphi, 1819) | Stagnicola palustris | Fishes; Birds (Anatids) | [] |
| Strigeidae | Apharyngostrigea cornu | (Zeder, 1800) | Stagnicola palustris | Birds | [] |
| Strigeidae | Australapatemon burti | (Miller, 1923) | Lymnaea stagnalis | Birds | [] |
| Strigeidae | Australapatemon burti | (Miller, 1923) | Radix peregra group | Birds | [] |
| Strigeidae | Australapatemon burti | (Miller, 1923) | Stagnicola palustris | Birds | [] |
| Strigeidae | Australapatemon minor | (Yamaguti, 1933) | Lymnaea stagnalis | Annelids (Hirudinea); Birds | [] |
| Strigeidae | Australapatemon minor | (Yamaguti, 1933) | Radix peregra group | Annelids (Hirudinea); Birds | [] |
| Strigeidae | Australapatemon minor | (Yamaguti, 1933) | Stagnicola palustris | Annelids (Hirudinea); Birds | [] |
| Strigeidae | Cotylurus brevis | Dubois & Rausch, 1950 | Lymnaea stagnalis | Birds | [] |
| Strigeidae | Cotylurus cornutus | (Rudolphi, 1809) | Lymnaea stagnalis | Birds | [] |
| Strigeidae | Cotylurus cornutus | (Rudolphi, 1809) | Radix auricularia | Birds | [] |
| Strigeidae | Cotylurus cornutus | (Rudolphi, 1809) | Radix peregra group | Birds | [] |
| Strigeidae | Cotylurus cornutus | (Rudolphi, 1809) | Stagnicola palustris | Birds | [] |
| Strigeidae | Strigea tarda | (Steenstrup, 1842) | Lymnaea stagnalis | ND | [] |
| Strigeidae | Strigea tarda | (Steenstrup, 1842) | Stagnicola palustris | ND | [] |
| Transversotrematidae | Transversotrema patialense | (Soparkar, 1924) | Melanoides tuberculata | Fishes | [] |
| Troglotrematidae | Paragonimus westermani | Kerbert, 1878 | Melanoides tuberculata | Mammals (Human) | [] |
References
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. In Freshwater Animal Diversity Assessment; Balian, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 149–166. [Google Scholar]
- Welter-Schultes, F.W. European Non-Marine Molluscs, a Guide for Species Identification: Bestimmungsbuch für Europäische Land-und Süsswassermollusken; Planet Poster Editions: Göttingen, Germany, 2012. [Google Scholar]
- Cadevall, J.; Orozco, A. (Eds.) Caracoles y Babosas de la Península Ibérica y Baleares; Omega: Barcelona, Spain, 2016; p. 817. [Google Scholar]
- Neiber, M.T.; Hausdorf, B. Molecular phylogeny reveals the polyphyly of the snail genus Cepaea (Gastropoda: Helicidae). Mol. Phylogenet. Evol. 2015, 93, 143–149. [Google Scholar] [CrossRef]
- Chueca, L.J.; Gómez-Moliner, B.J.; Madeira, M.J.; Pfenninger, M. Molecular phylogeny of Candidula (Geomitridae) land snails inferred from mitochondrial and nuclear markers reveals the polyphyly of the genus. Mol. Phylogenet. Evol. 2018, 118, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.R.; Capowiez, Y.; Mazzia, C.; Eckstein, H.; Kaczmarek, N.; Bilton, M.C.; Burmester, J.K.Y.; Capowiez, L.; Chueca, L.J.; Favilli, L.; et al. Experimental simulation of environmental warming selects against pigmented morphs of land snails. Ecol. Evol. 2021, 11, 1111–1130. [Google Scholar] [CrossRef]
- Silvertown, J.; Cook, L.; Cameron, R.; Dodd, M.; McConway, K.; Worthington, J.; Skelton, P.; Anton, C.; Bossdorf, O.; Baur, B.; et al. Citizen science reveals unexpected continental-scale evolutionary change in a model organism. PLoS ONE 2011, 6, e18927. [Google Scholar] [CrossRef]
- Guiller, A.; Martin, M.C.; Hiraux, C.; Madec, L. Tracing the invasion of the Mediterranean land snail Cornu aspersum aspersum becoming an agricultural and garden pest in areas recently introduced. PLoS ONE 2012, 7, e49674. [Google Scholar] [CrossRef] [PubMed]
- Zając, K.S.; Gawel, M.; Filipiak, A.; Kramarz, P. Arion vulgaris Moquin-Tandon, 1855—The aetiology of an invasive species. Folia Malacol. 2017, 25, 81–93. [Google Scholar] [CrossRef]
- Mc Donnell, R.; Santangelo, R.; Paine, T.; Hoddle, M. The feeding behaviour of Rumina decollata (Subulinidae: Gastropoda) raises questions about its efficacy as a biological control agent for Cornu aspersum (Helicidae: Gastropoda). Biocontrol. Sci. Technol. 2016, 26, 331–336. [Google Scholar] [CrossRef]
- Robla, J.; Alonso, Á. Notas biológicas y de distribución sobre el género Testacella en Asturias (NO de España), con la primera cita de Testacella scutulum G. B. Sowerby, 1821 para la región. Elona 2020, 2, 57–66. [Google Scholar]
- Grewal, P.S.; Grewal, S.K.; Tan, L.; Adams, B.J. Parasitism of molluscs by nematodes: Types of associations and evolutionary trends. J. Nematol. 2003, 35, 146–156. [Google Scholar]
- Lockyer, A.E.; Jones, C.S.; Noble, L.R.; Rollinson, D. Trematodes and snails: An intimate association. Can. J. Zool. 2004, 82, 251–269. [Google Scholar] [CrossRef]
- Antzée-Hyllseth, H.; Trandem, N.; Torp, T.; Haukeland, S. Prevalence and parasite load of nematodes and trematodes in an invasive slug and its susceptibility to a slug parasitic nematode compared to native gastropods. J. Invertebr. Pathol. 2020, 173, 107372. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Matthews, B.E. An Introduction to Parasitology; Cambridge University Press: Cambridge, UK, 1998; p. 192. [Google Scholar]
- Banerjee, A.; Barquera, S.; Blyth, F.; Cowie, B.; Gunnell, D.; Lan, Q.; McGrath, J.; Patton, G.C.; Woolf, A.; Lucas, R. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Quiroz, H. Parasitología y Enfermedades Parasitarias de Animales Domésticos; UTEHA-Editorial Noriega SA: Ciudad de Mexico, Mexico, 1994; p. 876. [Google Scholar]
- Huson, K.M.; Oliver, N.A.; Robinson, M.W. Paramphistomosis of ruminants: An emerging parasitic disease in Europe. Trends Parasitol. 2017, 33, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.A. Relationships between Trematodes and Molluscs. Ann. Trop. Med. Parasit. 1959, 54, 1–7. [Google Scholar] [CrossRef]
- Dodangeh, S.; Daryani, A.; Sharif, M.; Gholami, S.; Kialashaki, E.; Moosazadeh, M.; Sarvi, S. Freshwater snails as the intermediate host of trematodes in Iran: A systematic review. Epidemiol. Health 2019, 41, e2019001. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Dobrovolskij, A. The Biology and Evolution of Trematodes: An Essay on the Biology, Morphology, Life Cycles, Transmissions, and Evolution of Digenetic Trematodes; Springer Science & Business Media: Secaucus, NJ, USA, 2003. [Google Scholar]
- Cowie, R.H.; Robinson, D.G. Pathways of introduction of nonindigenous land and freshwater snails and slugs. In Invasive Species: Vectors and Management Strategies; Ruiz, G.M., Carlton, J.T., Eds.; Island Press: Washington, DC, USA, 2003; pp. 93–122. [Google Scholar]
- Vendetti, J.E.; Sandig, K.; Sahakyan, A.; Granados, A. Multiple introductions of the pestiferous land snail Theba pisana (Müller, 1774) (Gastropoda: Helicidae) in Southern California. Insects 2021, 12, 662. [Google Scholar] [CrossRef]
- Roll, U.; Dayan, T.; Simberloff, D.; Mienis, H.K. Non-indigenous land and freshwater gastropods in Israel. Biol. Invasions 2009, 11, 1963–1972. [Google Scholar] [CrossRef]
- García, M.A. Atlas Climático Ibérico. In Agencia Estatal de Meteorología—Instituto de Meteorología de Portugal; Closas-Orcoyen, S.L., Ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2011; pp. 1–8. [Google Scholar]
- Anadón, N.; Anadón, E. Estudios sobre los efectos del aislamiento en poblaciones de gasterópodos terrestres asturianos. I° Composición específica de las poblaciones. Bol. Inst. Estud. Asturianos 1978, 23, 121–143. [Google Scholar]
- Álvarez-Cuesta, D.; Anadón, N.; Ocharan-Larrondo, F.J.; Anadón, A. Malacofauna terrestre de la Reserva Natural Integral de Muniellos. In I Congreso de Estudios Asturianos; RIDEA: Oviedo, Spain, 2007; pp. 331–350. [Google Scholar]
- Ocharan-Larrondo, F.J.; Anadón, M.A.; Melero-Cimas, V.X.; Monteserín-Real, S.; Ocharan-Ibarrar, R.; García, R.; Vázquez-Felechosa, M.T. Invertebrados de la Reserva Natural Integral de Muniellos, Asturias; KRK Ediciones; Consejería de Medio Ambiente del Principado de Asturias: Oviedo, Spain, 2003; p. 357. [Google Scholar]
- Carlton, J.T. Biological invasions and cryptogenic species. Ecology 1996, 77, 1653–1655. [Google Scholar] [CrossRef]
- Vidal-Abarca, C.; Suárez, M.L. Lista Faunística y Bibliográfica de Los Moluscos (Gastropoda & Bivalvia) de Las Aguas Continentales de la Península Ibérica e Islas Baleares; Publicación N°2; Asociación Española de Limnología; Universitat Barcelona Editions: Madrid, Spain, 1985; p. 191. [Google Scholar]
- Bofill, A.; Haas, F. Molluscos recollits en Asturias en 1918 per en Josep Maluquer. Butll. Inst. Catalana. Hist. 1919, 3, 25–34. [Google Scholar]
- Cowie, R.H.; Thiengo, S.C. The apple snails of the Americas (Mollusca: Gastropoda: Ampullariidae: Asolene, Felipponea, Marisa, Pomacea, Pomella): A nomenclatural and type catalog. Malacologia 2003, 45, 41–100. [Google Scholar]
- Arias, A.; Torralba-Burrial, A. First European record of the giant ramshorn snail Marisa cornuarietis (Linnaeus, 1758) (Gastropoda: Ampullariidae) from northern Spain. Limnetica 2014, 33, 65–72. [Google Scholar]
- Quiñonero-Salgado, S.; López Soriano, J. Moluscos dulceacuícolas invasores del Delta del Ebro (Cataluña, España). Spira 2013, 5, 59–71. [Google Scholar]
- Burks, R.L.; Kyle, C.H.; Trawick, M.K. Pink eggs and snails: Field ovoposition patterns of an invasive snail, Pomacea insularum, indicate a preference for an invasive macrophyte. Hydrobiology 2010, 646, 243–251. [Google Scholar] [CrossRef]
- Raven, J.G.M. A revision of Obscurella Clessin, 1889 (Gastropoda Prosobranchia: Cyclophoridae). Basteria 1990, 54, 17–62. [Google Scholar]
- Boeters, H.D.; Gittenberger, E.; Subai, P. Eine neue Gattung der Aciculidae (Gastropoda: Prosobranchia) mit zwei neuen Arten. Basteria 1985, 49, 59–63. [Google Scholar]
- Ruiz-Cobo, J.; Vázquez-Toro, F. Caracoles continentales de Cantabria. In Atlas de Especies y Ambientes; Tantín: Santander, Spain, 2019; p. 284. [Google Scholar]
- Gittenberger, E. Two more Menkia species (Mollusca: Gastropoda Prosobranchia: Aciculidae). Zool. Meded. 1991, 65, 251–255. [Google Scholar]
- Boeters, H.D.; Gittenberger, E.; Subai, P. Die Aciculidae (Mollusca: Gastropoda Prosobranchia); Rijksmuseum van Natuurlijke Historie: Leiden, The Netherlands, 1989; p. 230. [Google Scholar]
- Lee, J.S. Rediscovery of Sinotaia quadrata (Architaenioglossa: Viviparidae) of Kumpung Reservoir in the Jellabuk-do, Korea. Korean J. Malacol. 2009, 25, 243–245. [Google Scholar]
- Ovando, X.M.C.; Cuezzo, M.G. Discovery of an established population of a non-native species of Viviparidae (Caenogastropoda) in Argentina. Molluscan Res. 2012, 32, 121–131. [Google Scholar]
- Cianfanelli, S.; Stasolla, G.; Inghilesi, A.F.; Tricarico, E.; Goti, E.; Strangi, A.; Bodon, M. First European record of Sinotaia quadrata (Benson, 1842), an alien invasive freshwater species: Accidental or voluntary introduction? (Caenogastropoda: Viviparidae). Boll. Malacol. 2017, 53, 150–160. [Google Scholar]
- Arias, A.; Fernández-Rodríguez, I.; Sánchez, O.; Borrell, Y.J. Integrative taxonomy reveals the occurrence of the Asian freshwater snail Sinotaia cf. quadrata in inland waters of SW Europe. Aquat. Invasions 2020, 15, 616–632. [Google Scholar] [CrossRef]
- Alonso, Á. Sobre Retinella incerta (Draparnaud, 1805) y otras especies de gasterópodos terrestres introducidas en Asturias (NO de España). Elona 2020, 2, 43–48. [Google Scholar]
- Jarillo, R.; Quiñonero-Salgado, S. Presencia de Melanoides tuberculatus (OF Müller, 1774) (Gastropoda: Thiaridae) en l’Aldea (el Baix Ebre, Cataluña, España). Spira 2010, 3, 141–147. [Google Scholar]
- Gutiérrez-Gregoric, D.E.; Nuñez, M.V.; Ferrando, N.S.; Rumi, A.M.Z. First records of invasive snail Melanoides tuberculatus (Gastropoda: Prosobranchia: Thiaridae) to the Iguazú River basin, Argentina–Brazil. Comun. Soc. Malacol. Urug. 2007, 9, 109–112. [Google Scholar]
- Groh, K.; García, A. Mollusca. In Lista de Especies Silvestres de Canarias (Hongos, Plantas y Animales Terrestres); Izquierdo, I., Martín, J.L., Zurita, N., Rechavaleta, A., Eds.; Consejería de Medio Ambiente y Ordenación Territorial; Gobierno de Canarias: Canarias, Spain, 2004; pp. 149–154. [Google Scholar]
- Boeters, H.D.; Rolán, E. Unknown west European prosobranchs, 9. Some new Spanish freshwater prosobranchs. Basteria 1988, 52, 197–202. [Google Scholar]
- Arconada, B.; Rolán, E.; Boeters, H.D. A revision of the genus Alzoniella Giusti & Bodon, 1984 (Gastropoda, Caenogastropoda, Hydrobiidae) on the Iberian Peninsula and its implications for the systematics of the European hydrobiid fauna. Basteria 2007, 71, 113–156. [Google Scholar]
- Boeters, H.D. Unbekannte westeuropäische Prosobranchia. V. Arch. Molluskenkd. Senckenbergischen Nat. Ges. 1983, 114, 17–24. [Google Scholar]
- Rolán, E.; Boeters, H.D. The genus Alzoniella Giusti & Bodon, 1984 (Gastropoda, Hydrobiidae) in Asturias (northern Spain), with the description of a new species. Basteria 2015, 79, 48–54. [Google Scholar]
- Rolán, E. El género Belgrandiella Wagner, 1927 en el norte de la Península Ibérica con descripción de tres especies nuevas. Thalassas 1991, 9, 99–122. [Google Scholar]
- Ojea, M.; Anadón, N. Estudio faunístico de los gasterópodos de las vertientes sur y oeste del monte Naranco (Oviedo, Asturias). Boletín Cienc. Nat. IDEA 1983, 32, 69–90. [Google Scholar]
- Boeters, H.D. Old and new taxa of Bythinella Moquin-Tandon, 1856 (Gastropoda: Caenogastropoda: Truncatelloidea) in Spain and adjacent France. Arch. Molluskenkd. Int. J. Malacol. 2019, 148, 161–183. [Google Scholar] [CrossRef]
- Rolán, E.; Arconada, B.; Boeters, H.D. A new species of Alzoniella Giusti & Bodon, 1984 (Gastropoda, Caenogastropoda, Hydrobiidae) from northern Spain. Basteria 2009, 73, 117–121. [Google Scholar]
- Alonso, Á.; Quiñonero-Salgado, S.; Raven, J.H.M. Nuevos datos sobre Deganta azarum (Boeters & Rolán, 1988) (Gastropoda: Hydrobiidae) en el NO de España. Elona 2019, 1, 21–28. [Google Scholar]
- Ruiz-Cobo, J.; Alonso, Á.; Quiñonero-Salgado, S.; Rolán, E. Two new species of the genus Islamia Radoman, 1973 (Gastropoda: Hydrobiidae) from the north of Spain. Nemus 2018, 8, 85–93. [Google Scholar]
- Quiñonero-Salgado, S.; Ruiz-Cobo, J.; Rolán, E. Three new species of Spiralix (Burgosia) (Gastropoda, Moitessieriidae) from the northern Iberian Peninsula. Iberus 2017, 35, 59–70. [Google Scholar]
- Rolán, E.; Ramos, M.A. Una nueva especie de Hydrobiidae (Mollusca, Prosobranchia) del norte de la Península Ibérica. Iberus 1995, 13, 119–127. [Google Scholar]
- Rolán, E.; Arconada, B. Nueva información sobre Paladilhiopsis septentrionalis (Molusca, Prosobranchia). Iberus 2003, 21, 141–143. [Google Scholar]
- Quiñonero-Salgado, S.; Alonso, Á.; Rolán, E. Spiralix (Burgosia) vetusta (Gastropoda: Moitessieriidae) a new species from Asturias (North of Spain). Nemus 2018, 8, 95–100. [Google Scholar]
- Alonso, Á.; Castro-Díez, P. The exotic aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca): State of the art of a worldwide invasion. Aquat. Sci. 2012, 74, 375–383. [Google Scholar] [CrossRef]
- Arconada, B.; de Andrés, J.; Araujo, R. Omphiscola glabra (Müller, 1774) (Gastropoda: Lymnaeidae) en la Península Ibérica. Iberus 2019, 37, 209–217. [Google Scholar]
- Dillon, R.T., Jr.; Wethington, A.R.; Rhett, J.M.; Smith, T.P. Populations of the European freshwater pulmonate Physa acuta are not reproductively isolated from American Physa heterostropha or Physa integra. Invertebr. Biol. 2002, 121, 226–234. [Google Scholar] [CrossRef]
- Vinarski, M.V. The history of an invasion: Phases of the explosive spread of the physid snail Physella acuta through Europe, Transcaucasia and Central Asia. Biol. Invasions 2017, 19, 1299–1314. [Google Scholar] [CrossRef]
- Larraz, M.L.; Equisoain, J.J.; Agorreta, A.; Oscoz, J. Physa acuta Draparnaud, 1805 (Mollusca Gastropoda) en plantas depuradoras de agua. Not. SEM 2007, 47, 47–49. [Google Scholar]
- Quiñonero-Salgado, S.; López-Soriano, J.; Glöer, P. First report of Radix labiata (Rossmässler, 1835) (Gastropoda: Lymnaeidae) in Aragon (NE Spain). Spira 2016, 6, 85–86. [Google Scholar]
- Hermida, J. Estudios Faunísticos y Ecológicos de Los Moluscos Gasterópodos Terrestres de Asturias, León, Zamora y Salamanca. Ph.D. Thesis, Facultad de Bioloxia, Departamento de Bioloxia Animal, Universidad de Santiago de Compostela, Santiago, Spain, 1992. [Google Scholar]
- Beran, L.; Glöer, L. Gyraulus chinensis (Dunker, 1848)—A new greenhouse species for the Czech Republic (Gastropoda: Planorbidae). Malacol. Bohemoslov. 2006, 5, 25–28. [Google Scholar] [CrossRef]
- Cejka, T.; Dvorák, L.; Horsák, M.; Steffek, J. Checklist of the molluscs (Mollusca) of the Slovak Republic. Folia Malacol. 2007, 15, 49–58. [Google Scholar] [CrossRef]
- Salgado-Quiñonero, S.; Soriano-López, J.; Jarillo, R.R.; Alabau, A.L.; Ferrer, A.P. Nuevas citas de Planorbella duryi (Wetherby, 1879) (Gastropoda: Planorbidae) para España. Spira 2014, 5, 133–135. [Google Scholar]
- Soria, J.M.; Sahuquillo, M. 1150 Lagunas costeras. In Bases Ecológicas Preliminares Para la Conservación de Los Tipos de Hábitat de Interés Comunitario en España; Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2009. [Google Scholar]
- Bank, R.A.; Groh, K.; Ripken, T.E. Catalogue and bibliography of the non-marine Mollusca of Macaronesia. Collect. Malacol.–Festschr. Gerhard Falkner 2002, 20, 89–235. [Google Scholar]
- Alonso, Á.; Ruiz-Cobo, J.; Raven, J.H.M.; Quiñonero-Salgado, S. En tierra de nadie: Nuevos datos de las familias Truncatellidae Gray, 1840 y Ellobiidae, L. Pfeiffer, 1854 (Gastropoda) en el NO de España. Elona 2019, 1, 45–51. [Google Scholar]
- Jochum, A.; Prieto, C.E.; Kampschulte, M.; Martels, G.; Ruthensteiner, B.; Vrabec, M.; Dörge, D.D.; de Winter, A.J. Re-evaluation of Zospeum schaufussi von Frauenfeld, 1862 and Z. suarezi Gittenberger, 1980, including the description of two new Iberian species using Computer Tomography (CT) (Eupulmonata, Ellobioidea, Carychiidae). ZooKeys 2019, 835, 65–86. [Google Scholar] [CrossRef]
- Alonso, Á.; Prieto, C.E.; Quiñonero-Salgado, S.; Rolán, E. A morphological gap for Iberian Zospeum filled: Zospeum percostulatum sp. n. (Gastropoda, Eupulmonata, Carychiidae) a new species from Asturias (Spain). Subterr. Biol. 2018, 25, 35–48. [Google Scholar] [CrossRef]
- Prieto, C.E.; Quiñonero-Salgado, S.; Ruiz-Cobo, J.; Alonso, Á. Shells of Papilloderma Wiktor, Martín and Castillejo, 1999 (Gastropoda, Eupulmonata, Papillodermatoidea) found in caves reveal an unknown dwarf hypogean species. J. Conchol. 2020, 43, 641–653. [Google Scholar]
- Castillejo, J. Babosas del Noroeste Ibérico; Universidad de Santiago de Compostela: Santiago, Spain, 1997; p. 192. [Google Scholar]
- Horsák, M.; Šteffek, J.; Čejka, T.; Ložek, V.; Juřičková, L. Occurrence of Lucilla scintilla (RT Lowe, 1852) and Lucilla singleyana (Pilsbry, 1890) in the Czech and Slovak Republics-with remarks how to distinguish these two non-native minute snails. Malacol. Bohemoslov. 2009, 8, 24–27. [Google Scholar]
- Quiñonero-Salgado, S.; Alonso, Á.; López-Soriano, J. First records for Lucilla scintilla (Lowe, 1852) (Gastropoda: Helicodiscidae) in the Iberian Peninsula. Elona 2019, 1, 16–20. [Google Scholar]
- Schikov, E.V. Lucilla singleyana (Pilsbry, 1890) and L. scintilla (RT Lowe, 1852) (Gastropoda, Pulmonata, Endodontidae) in the Caucasus and in Russia. Folia Malacol. 2017, 25, 165–174. [Google Scholar] [CrossRef][Green Version]
- Holyoak, G.A.; Holyoak, D.T. Quickella arenaria (Gastropoda: Succineidae) living in the Cantabrian mountains, NW. Spain. Not. SEM 2009, 52, 26–27. [Google Scholar]
- Armbruster, G. Univariate and multivariate analyses of shell variables within the genus Cochlicopa (Gastropoda: Pulmonata: Cochlicopidae). J. Molluscan Stud. 1995, 61, 225–235. [Google Scholar] [CrossRef]
- Larraz-Azcárate, M.L.; Rolán, E. Distribución de Lauria sempronii (Gastropoda, Pupillidae) en la Península Ibérica. Not. SEM 2003, 39, 69–71. [Google Scholar]
- Rolán, E. Leiostyla anglica (Pulmonata, Pupillidae) en Asturias. Not. SEM 2003, 39, 67–68. [Google Scholar]
- Altonaga, K.; Gómez, B.; Martín, R.; Prieto, C.E.; Puente, A.I.; Rallo, A. Estudio Faunístico y BiogeográFico de los Moluscos Terrestres del Norte de la Península Ibérica; Eusko Legebiltzarra, Parlamento Vasco: Vitoria-Gasteiz, Spain, 1994; p. 505. [Google Scholar]
- Rolán, E.; Gómez, B. El género Vertigo en Asturias (Gastropoda, Pulmonata). Not. SEM 2004, 39, 54–58. [Google Scholar]
- Raven, J.G.M. Notes on Spanish non-marine molluscs. 2. New data on the distribution of some species. Basteria 1984, 48, 17–21. [Google Scholar]
- Hidalgo, J.G. Catálogo Iconográfico y Descriptivo de Los Moluscos Terrestres de España, Portugal y las Baleares; Imprenta de Segundo Martínez: Madrid, Spain, 1875; Volume 1. [Google Scholar]
- Gittenberger, E.; Preece, R.C.; Ripken, T.E. Balea heydeni von Maltzan, 1881 (Pulmonata: Clausiliidae): An overlooked but widely distributed European species. J. Conchol. 2006, 39, 145–150. [Google Scholar]
- Martínez-Ortí, A. Balea heydeni Von Maltzan, 1881 (Gastropoda, Clausilidae) en España: Características conquiológicas y distribución. Not. SEM 2006, 45, 30–37. [Google Scholar]
- Ruiz-Cobo, J.R.; Quiñonero-Salgado, S. Presencia de Balea heydeni von Maltzan, 1881 (Gastropoda: Clausiliidae) en Cantabria. Spira 2016, 6, 91–93. [Google Scholar]
- Hatteland, B.A.; Solhoy, T.; Schander, C.; Skage, M.; von Proschwitz, T.; Noble, L.R. Introgression and differentiation of the invasive slug Arion vulgaris from native A. ater. Malacologia 2015, 58, 303–321. [Google Scholar] [CrossRef]
- Burnet, B. Enzyme protein polymorphism in the slug Arion ater. Genet. Res. 1972, 20, 161–173. [Google Scholar] [CrossRef]
- Evans, N.J. An investigation of the status of the terrestrial slugs Arion ater ater (l.) and Arion ater rufus (l.) (Mollusca, Gastropoda, Pulmonata) in Britain. Zool. Scr. 1986, 15, 313–322. [Google Scholar] [CrossRef]
- Quinteiro, J.; Rodríguez-Castro, J.; Castillejo, J.; Iglesias-Piñeiro, J.; Rey-Méndez, M. Phylogeny of slug species of the genus Arion: Evidence of monophyly of Iberian endemics and of the existence of relict species in Pyrenean refuges. J. Zool. Syst. Evol. Res. 2005, 43, 139–148. [Google Scholar] [CrossRef]
- Bank, R.A.; Falkner, G.; Proschwitz, T. A revised checklist of the non-marine Mollusca of Britain and Ireland. Heldia 2007, 5, 41–72. [Google Scholar]
- Rowson, B.; Anderson, R.; Turner, J.A.; Symondson, W.O. The slugs of Britain and Ireland: Undetected and undescribed species increase a well-studied, economically important fauna by more than 20%. PLoS ONE 2014, 9, e91907. [Google Scholar] [CrossRef] [PubMed]
- Peláez, M.L.; Valdecasas, A.G.; Martínez, D.; Horreo, J.L. Towards the unravelling of the slug A. ater–A. rufus complex (Gastropoda Arionidae): New genetic approaches. Web Ecol. 2018, 18, 115–119. [Google Scholar] [CrossRef]
- Castillejo, J. Guía de las babosas Ibéricas. In Real Academia Galega de Ciencias; Graficolor Minerva S.L.: Galicia, Spain, 1998; p. 151. [Google Scholar]
- Castillejo, J. Las babosas de la familia Arionidae Gray, 1840 en la Península Ibérica e Islas Baleares. Morfología y distribución. (Gastropoda, Pulmonata, Terrestria Nuda). R. Acad. Gal. Cienc Nat. 1997, 16, 51–118. [Google Scholar]
- Zając, K.S.; Hatteland, B.A.; Feldmeyer, B.; Pfenninger, M.; Filipiak, A.; Noble, L.R.; Lachowska-Cierlik, D. A comprehensive phylogeographic study of Arion vulgaris Moquin-Tandon, 1855 (Gastropoda: Pulmonata: Arionidae) in Europe. Org. Divers. Evol. 2020, 20, 37–50. [Google Scholar] [CrossRef]
- Pfenninger, M.; Weigand, A.; Bálint, M.; Klussmann-Kolb, A. Misperceived invasion: The Lusitanian slugs (Arion lusitanicus auct. non-Mabille or Arion vulgaris Moquin-Tandon, 1855) is native to Central Europe. Evol. Appl. 2014, 7, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Mc Donnell, R.; O’Meara, K.; Nelson, B.; Marnell, F.; Gormally, M. Revised distribution and habitat associations for the protected slug Geomalacus maculosus (Gastropoda, Arionidae) in Ireland. Basteria 2013, 77, 33–37. [Google Scholar]
- Castillejo, J.; Iglesias, J.; Gómez-Rodríguez, C.; Baselga, A. El estatus de las especies del género Geomalacus Allman, 1843 en la península ibérica (Gastropoda, Pulmonata, Arionidae). Nova Acta Científica Compostel. 2019, 26, 65–108. [Google Scholar]
- Verdú, J.R.; Galante, E. (Eds.) Geomalacus (Geomalacus) maculosus Allman, 1843. In Libro Rojo de Los Invertebrados de España; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2006; pp. 848–851. [Google Scholar]
- Greenwood, J.J.D. A preliminary note on aspects of the variation of Lehmannia marginata. J. Molluscan Stud. 1966, 37, 119–125. [Google Scholar] [CrossRef]
- Castillejo, J. Los pulmonados desnudos de Galicia II. Género Lehmannia Heynemann, 1862. (Pulmonata: Limacidae). Iberus 1982, 2, 19–28. [Google Scholar]
- Torres-Alba, J.S.; López-García, J.C.; Vázquez-Toro, F.; Ripoll, J. Malacofauna del Jardín Botánico Histórico de la Concepción (Málaga, España). Elona 2019, 1, 34–44. [Google Scholar]
- Reise, H.; Hutchinson, J.M.; Schunack, S.; Schlitt, B. Deroceras panormitanum and congeners from Malta and Sicily, with a redescription of the widespread pest slug as Deroceras invadens n. sp. Folia Malacol. 2011, 19, 201–223. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Reise, H.; Robinson, D. A biography of an invasive terrestrial slug: The spread, distribution and habitat of Deroceras invadens. NeoBiota 2014, 23, 17–64. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Schlitt, B.; Kořínková, T.; Reise, H.; Barker, G.M. Genetic evidence illuminates the origin and global spread of the slug Deroceras invadens. J. Molluscan Stud. 2020, 86, 306–322. [Google Scholar] [CrossRef]
- Pesqueira, R.; López, M.T.R.; Rodríguez, A.M.O. La familia Agriolimacidae Wagner, 1935 en la Provincia de Lugo (Noroeste de la Peninsula Ibérica): (Gastropoda, Pulmonata). Boll. Malacol. 2003, 39, 43–48. [Google Scholar]
- Altonaga, K. A new Oxychilus (Gastropoda, Stylommatophora, Zonitidae) from N Iberian Peninsula. Iberus 1986, 6, 237–244. [Google Scholar]
- Altonaga, K. Nuevos datos sobre Oxychilus helveticus (Blum, 1881) Pulmonata, Zonitidae) en la Peninsula Ibérica. Iberus 1991, 10, 1–26. [Google Scholar]
- Bech, M. Nuevas aportaciones al conocimiento de la Malacofauna Ibérica. Iberus 1986, 6, 289–291. [Google Scholar]
- Altonaga, K. Nuevos datos sobre Oxychilus (Morlina) glaber (Rossmaessler 1835) (Pulmonata: Stylommatophora: Zonitidae) en la Península Ibérica. Iberus 1988, 8, 39–45. [Google Scholar]
- Horsáková, V.; Nekola, J.C.; Horsák, M. Integrative taxonomic consideration of the Holarctic Euconulus fulvus group of land snails (Gastropoda, Stylommatophora). Syst. Biodivers. 2020, 18, 142–160. [Google Scholar] [CrossRef]
- Prieto, C. Sobre la distribución geográfica de los géneros “Monacha” y “Helicigona” “(Mollusca: Pulmonata: Helicidae)” en la Península Ibérica. Butll. Inst. Catalana. Hist. Nat. 1985, 52, 73–82. [Google Scholar]
- Manga-González, M.Y. Notas sobre Ponentina ponentina (Morelet, 1845) y Euomphalia (Mengoana) brigantina (Da Silva Mengo, 1867) (Gastropoda, Helicidae), en la provincia de León. In Comunicaciones del Primer Congreso Nacional de Malacología; Museo Nacional de Ciencias Naturales. Grupo de Trabajo de Malacología: Madrid, Spain, 1980; pp. 41–45. [Google Scholar]
- Puente, A.I. Estudio Taxonómico y Biogeográfico de la Superfamilia Helicoidea Rafinesque, 1815 (Gastropoda: Pulmonata: Stylommatophora) de la Península Ibérica e Islas. Ph.D. Thesis, Universidad del País Vasco-Euskal Herriko Unibertsitatea, Leioa, Spain, 1994. [Google Scholar]
- Rolán, E. Columella aspera en Asturias. Not. SEM 2004, 41, 33–34. [Google Scholar]
- Holyoak, D.T.; Holyoak, G.A. A review of the genus Ponentina Hesse 1921 with descriptions of seven new species from Portugal and Spain (Gastropoda, Pulmonata: Hygromiidae). J. Conchol. 2012, 41, 173–238. [Google Scholar]
- Alonso, Á. Presencia de Cernuella virgata (Da Costa, 1778) en la cornisa cantábrica (noroeste de España) y su posible carácter de especie introducida. Elona 2020, 2, 49–56. [Google Scholar]
- Altimira, C. Moluscos terrestres y de agua dulce en la provincia de Lugo (Galicia) y Asturias. Publ. Inst. Biol. Api. Barcelona 1969, 46, 107–113. [Google Scholar]
- Puente, A.I.; Prieto, C.E. Plentuisa vendia, a new genus and species from the Picos de Europa (North of the Iberian Peninsula) (Gastropoda: Helicoidea: Hygromiidae). J. Conchol. 1992, 34, 159–168. [Google Scholar]
- Alonso, Á.; Raven, J.H.M. New records of Xerotricha corderoi (Gittenberger & Manga, 1977) and Plentuisa vendia Puente & Prieto, 1992 (Gastropoda: Helicoidea: Geomitridae) of the Cantabrian Mountains (NW Spain). Elona 2019, 1, 14–15. [Google Scholar]
- Prieto, C.E.; Puente, A.I. Un nuevo Hygromiinae del Norte de la Península Ibérica, Cryptosaccus asturiensis n. gen., n. sp. (Pulmonata: Helicoidea: Hygromiidae). Arch. Molluskenkd. 1994, 123, 109–122. [Google Scholar] [CrossRef]
- Prieto, C.E.; Puente, A. El género Hygromia Risso, 1826 en la Península Ibérica, con descripción de Hygromia gofasi sp. nov., y consideraciones sobre la interpretación funcional del aparato estimulador de Hygromiidae. Bull. Mus. Natl. Hist. Nat. 1992, 14, 383–404. [Google Scholar]
- Elejalde, M.A. Sistema Molecular, Taxonomía y Evolución de Los Géneros Iberus y Pyrenaearia (Gastropoda, Helicoidea). Ph.D. Thesis, Universidad del País Vasco (Facultad de Ciencias), Leioa, Spain, 2008. [Google Scholar]
- Elejalde, M.A.; Madeira, M.J.; Prieto, C.E.; Backeljau, T.; Gómez-Moliner, B.J. Molecular phylogeny, taxonomy, and evolution of the land snail genus Pyrenaearia (Gastropoda, Helicoidea). Am. Malacol. Bull. 2009, 27, 69–81. [Google Scholar] [CrossRef]
- Alonso, Á.; Raven, J.G.M.; Quiñonero-Salgado, S. The Iberian Pyrenaearia cantabrica (Hidalgo, 1873) clade: Elucidation of the type localities of its taxa (Gastropoda, Pulmonata, Hygromiidae). Basteria 2019, 83, 169–180. [Google Scholar]
- Holyoak, D.T.; Holyoak, G.A. A new genus Zenobiellina for Helix subrufescens Miller, 1822 (Hygromiidae), with description of a new congeneric species from northern Spain. Iberus 2018, 36, 133–147. [Google Scholar]
- Holyoak, D.T.; Holyoak, G.A. A taxonomic revision of Oestophora barbula (Rossmässler, 1838) and O. barbella (Servain, 1880), two Iberian endemic land-snail species (Gastropoda: Trissexodontidae). Iberus 2012, 31, 15–40. [Google Scholar]
- Holyoak, D.T.; Holyoak, G.A. A review of species-limits in some Cryptazeca (Gastropoda: Azecidae). Iberus 2012, 30, 91–102. [Google Scholar]
- Raven, J.G.M. Notes on Spanish non-marine molluscs: 3. Chondrinidae from the Cantabrian Mountains (Gastropoda: Pulmonata). Zool. Meded. 1986, 60, 27–37. [Google Scholar]
- Somoza-Valdeolmillos, E.; Vázquez-Sanz, J.; Gómez-Moliner, B.J.; Caro, A.; Madeira, M.J. Phylogenetic study and taxonomic update of Chondrina (Gastropoda, Pulmonata, Chondrinidae) in the Cantabrian Mountain region (Iberian Peninsula). Syst. Biodivers. 2021, 19, 218–236. [Google Scholar] [CrossRef]
- Gittenberger, E. Beiträge zur Kenntnis der Pupillacea III. Chondrininae; EJ Brill: Leiden, The Netherlands, 1973; Volume 3. [Google Scholar]
- Holyoak, D.T.; Holyoak, G.A.; Alba, J. A reassessment of the species of Truncatellina (Gastropoda: Vertiginidae) in the Iberian Peninsula and North-west Africa. Iberus 2012, 30, 7–33. [Google Scholar]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, K.; Bespalaya, Y.; Bodis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 272–607. [Google Scholar] [CrossRef] [PubMed]
- Verdú, J.R.; Galante, E. (Eds.) Margaritifera margaritifera (Linné, 1758). In Libro Rojo de los Invertebrados de España; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2006; pp. 246–253. [Google Scholar]
- Gofas, S. The systematics of Pyrenean and Cantabrian Cochlostoma (Gastropoda, Cyclophoroidea) revisited. J. Nat. Hist. 2001, 35, 1277–1369. [Google Scholar] [CrossRef]
- Drake, J.M. Risk analysis for invasive species and emerging infectious diseases: Concepts and applications. Am. Midl. Nat. 2005, 153, 4–19. [Google Scholar] [CrossRef]
- Hulme, P.E. Invasive species challenge the global response to emerging diseases. Trends Parasitol. 2014, 30, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.; Tan, S.K.; Wong, W.H.; Meier, R.; Chan, S.Y.; Tan, H.H.; Yeo, D.C. Molluscs for sale: Assessment of freshwater gastropods and bivalves in the ornamental pet trade. PLoS ONE 2016, 11, e0161130. [Google Scholar] [CrossRef]
- Yamaguti, S. A Synoptical Review of Life Histories of Digenetic Trematodes of Vertebrates; Keigaku Publishing Co.: Tokyo, Japan, 1975; p. 590. [Google Scholar]
- Sirgel, W.F.; Artigas, P.; Bargues, M.D.; Mas-Coma, S. Life cycle of Renylaima capensis, a brachylaimid trematode of shrews and slugs in South Africa: Two-host and three-host transmission modalities suggested by epizootiology and DNA sequencing. Parasites Vectors 2012, 5, 1–18. [Google Scholar] [CrossRef]
- Stephenson, J.W.; Knutson, L.V. A resume of recent studies of invertebrates associated with slugs. J. Econ. Entomol. 1966, 59, 356–360. [Google Scholar] [CrossRef]
- Filipiak, A.; Haukeland, S.; Zając, K.; Lachowska-Cierlik, D.; Hatteland, B.A. Helminths associated with terrestrial slugs in some parts of Europe. Bonn. Zool. Bull. 2020, 69, 11–26. [Google Scholar]
- Barger, M.A.; Hnida, J.A. Survey of trematodes from terrestrial gastropods and small mammals in southeastern Nebraska, USA. Comp. Parasitol. 2008, 75, 308–314. [Google Scholar] [CrossRef]
- Waki, T. Diversity of terrestrial mollusks and their helminths in artificial environments in Yoyogi Park, Tokyo, Japan. J. Asia Pac. Biodivers. 2017, 10, 254–256. [Google Scholar] [CrossRef]
- Ivanova, E.; Clausi, M.; Sparacio, I.; Spiridonov, S. Preliminary data on the parasite survey of terrestrial gastropods of Sicily. Russ. J. Nematol. 2019, 27, 37–45. [Google Scholar]
- Llorente, J.G.; Domingo, A.H.; González, P.C. Subcapsular abscess: An unusual CT finding in hepatic fascioliasis. Am. J. Roentgenol. 2002, 178, 514–515. [Google Scholar] [CrossRef]
- Fernández, M.J.N.; Anibarro, L.; Gómez-Durán, L.P. Fasciolasis en el sur de Galicia: Presentación de dos casos. An. Med. Inter. 2001, 18, 280–281. [Google Scholar] [CrossRef][Green Version]
- Carranza-Rodríguez, C.; Escamilla-González, M.; Fuentes-Corripio, I.; Perteguer-Prieto, M.J.; Gárate-Ormaechea, T.; Pérez-Arellano, J.L. Helmintosis y eosinofilia en España (1990–2015). Enferm. Infecc. Microbiol. Clin. 2018, 36, 120–136. [Google Scholar] [CrossRef]
- Cilla, G.; Serrano-Bengoechea, E.; Cosme, A.; Abadía, L.; Pérez-Trallero, E. Decrease in human fascioliasis in Gipuzkoa (Spain). Eur. J. Epidemiol. 2001, 17, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Robles-Pérez, D. Nuevas Técnicas Para el Estudio de Cepas Ovinas de Fasciola hepatica con Diferente Origen y Grado de Resistencia a Fármacos Antihelmínticos. Ph.D. Thesis, Facultad de veterinaria—Universidad de León, León, Spain, 2015. [Google Scholar]
- Martín-Gil, I.Z.; Pérez, J.P.J.; González, J. Seudoparasitismo por Dicrocoelium dendriticum. Atención Primaria 2007, 39, 379–380. [Google Scholar] [CrossRef]
- Cabeza-Barrera, I.; Cabezas-Fernández, T.; Salas Coronas, J.; Vázquez Villegas, J.; Cobo, F. Dicrocoelium dendriticum: An emerging spurious infection in a geographic area with a high level of immigration. Ann. Trop. Med. Parasit. 2011, 105, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Fos-Claver, S.; Vendrell, B.E.; Minardi, M.R.; Suárez-Varela, M.M.; Llopis, A.G. Enfermedades parasitarias de origen alimentario más frecuentes en España: Incidencia y comparación con las de origen vírico y bacteriano. Ars Pharm. 2000, 41, 293–305. [Google Scholar]
- Manga-González, M.Y.; González-Lanza, C.; Cabanas, E.; Campo, R. Contributions to and review of dicrocoeliosis, with special reference to the intermediate hosts of Dicrocoelium dendriticum. Parasitology 2001, 123, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Carney, W.P. Echinostomiasis–a snail-borne intestinal trematode zoonosis. SE Asian J. Trop. Med. Public 1991, 22, 206–211. [Google Scholar]
- Fried, B.; Graczyk, T.K.; Tamang, L. Food-borne intestinal trematodiases in humans. Parasitol. Res. 2004, 93, 159–170. [Google Scholar] [CrossRef]
- World Health Organization Organización (WHO). Schistosomiasis: Progress Report 2001–2011 and Strategic Plan 2012–2020; World Health Organization: Geneva, Switzerland, 2013; p. 74. [Google Scholar]
- World Health Organization Organización (WHO). Informe Mundial Sobre el Paludismo. 2018. Available online: http://www.who.int/malaria/publications/world-malaria-report-2018/report/es/ (accessed on 20 May 2021).
- Liberato, C.D.; Berrilli, F.; Bossù, T.; Magliano, A.; Filippo, M.M.D.; Cave, D.D.; Sigismondi, M.; Cannavacciuolo, A.; Scaramozzino, P. Outbreak of swimmer’s itch in Central Italy: Description, causative agent and preventive measures. Zoonoses. Public Health 2019, 66, 377–381. [Google Scholar] [CrossRef]
- Bou, A.; Gascon, J.; Eugenia, M.V.; Corachan, M. Katayama fever in Spanish tourists: Analysis of 25 cases. Med. Clin. 2001, 116, 220–222. [Google Scholar] [CrossRef]
- Cichy, A.; Faltynkova, A.; Zbikowska, E. Cercariae (Trematoda, Digenea) in European freshwater snails-a checklist of records from over one hundred years. Folia Malacol. 2011, 19, 165–189. [Google Scholar] [CrossRef]
- Alves, P.V.; Vieira, F.M.; Santos, C.P.; Scholz, T.; Luque, J.L. A checklist of the Aspidogastrea (Platyhelminthes: Trematoda) of the World. Zootaxa 2015, 3918, 339–396. [Google Scholar] [CrossRef]
- Cragg, J.B.; Foster, R.; Vincent, M. Larval trematodes (Brachylaemidae) from the slugs Milax sowerbii (Férussac), Agriolimax reticulatus (Müller) and Arion lusitanicus Mabille. Parasitology 1957, 47, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Ansart, A.; Decanter, N.; Martin, M.C.; Dahirel, M. Brachylaima spp. (Trematoda) parasitizing Cornu aspersum (Gastropoda) in France with potential risk of human consumption. Parasite 2020, 27, 15. [Google Scholar] [CrossRef]
- Butcher, A.R.; Grove, D.I. Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human faeces in Australia. Syst. Parasitol. 2001, 49, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Butcher, A.R.; Grove, D.I. Second intermediate host land snails and definitive host animals of Brachylaima cribbi in southern Australia. Parasite 2005, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, E.; Marszewska, A.; Cichy, A.; Templin, J.; Smorąg, A.; Strzała, T. Cepaea spp. as a source of Brachylaima mesostoma (Digenea: Brachylaimidae) and Brachylecithum sp. (Digenea: Dicrocoeliidae) larvae in Poland. Parasitol. Res. 2020, 119, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kingston, N.; Freeman, R.S. On the trematodes Brachylecithum orfi sp. nov. (Dicrocoeliidae) and Tanaisia sp. (Eucotylidae) from the Ruffed Grouse, Bonasa umbellus L. Can. J. Zool. 1959, 37, 121–127. [Google Scholar] [CrossRef]
- Kingston, N. Comparison of the Rate of Development of Brachylecithum orfi (Trematoda: Dicrocoeliidae) in the Land Snails, Zonitoides Arboreus and Cionella Lubrica; Pennsylvania Academy of Science: Pennsylvania, PA, USA, 1963; pp. 151–155. [Google Scholar]
- Pinto, H.A.; de Melo, A.L. A checklist of trematodes (Platyhelminthes) transmitted by Melanoides tuberculata (Mollusca: Thiaridae). Zootaxa 2011, 2799, 15–28. [Google Scholar] [CrossRef]
- Alunda, J.M. Ecología de Dicrocoelium dendriticum en León. Institución <<Fray Bernardino de Sahagún>>; Excma. Diputación de León; Consejo Superior de Investigaciones Científicas (CECEL): León, Spain, 1984. [Google Scholar]
- Martínez-Ibeas, A.M.; Martínez-Valladares, M.; González-Lanza, C.; Miñambres, B.; Manga-González, M.Y. Detection of Dicrocoelium dendriticum larval stages in mollusc and ant intermediate hosts by PCR, using mitochondrial and ribosomal internal transcribed spacer (ITS-2) sequences. Parasitology 2011, 138, 1916–1923. [Google Scholar] [CrossRef]
- Gürelli, G.; Göçmen, B. Natural infection of Helix aspersa (Mollusca: Pulmonata) by Dicrocoeliidae (Digenea) larval stages in Izmir, Turkey. Turkiye Parazitol. Derg. 2007, 31, 150–153. [Google Scholar]
- Graczick, T.; Fried, B. Echinostomiasis: A common but forgotten food-borne disease. Am. J. Trop. Med. Hyg. 1998, 58, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.C.; Escobar, J.S.; Durand, P.; Renaud, F.; David, P.; Jarne, P.; Pointier, J.P.; Hurtrez-Boussès, S. Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of Fascioliasis. BMC Evol. Biol. 2010, 10, 381. [Google Scholar] [CrossRef]
- Iglesias-Piñeiro, J.; González-Warleta, M.; Castro-Hermida, J.A.; Córdoba, M.; González-Lanza, C.; Manga-González, M.Y.; Mezo, M. Transmission of Calicophoron daubneyi and Fasciola hepatica in Galicia (Spain): Temporal follow-up in the intermediate and definitive hosts. Parasites Vectors 2016, 9, 610. [Google Scholar] [CrossRef]
- Abrous, M.; Rondelaud, D.; Dreyfuss, G.; Cabaret, J. Infection of Lymnaea truncatula and Lymnaea glabra by Fasciola hepatica and Paramphistomum daubneyi in farms of central France. Vet. Res. 1999, 30, 113–118. [Google Scholar]
- Jokela, J.; Lively, C.M. Spatial variation in infection by digenetic trematodes in a population of freshwater snails (Potamopyrgus antipodarum). Oecologia 1995, 103, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Goumghar, M.D.; Abrous, M.; Ferdonnet, D.; Dreyfuss, G.; Rondelaud, D. Prevalence of Haplometra cylindracea infection in three species of Lymnaea snails in central France. Parasitol. Res. 2000, 86, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Joosse, J.; Van Elk, R. Trichobilharzia ocellata: Physiological characterization of giant growth, glycogen depletion, and absence of reproductive activity in the intermediate snail host, Lymnaea stagnalis. Exp. Parasitol. 1986, 62, 1–13. [Google Scholar] [CrossRef]
- Skala, V.; Walker, A.J.; Horak, P. Snail defence responses to parasite infection: The Lymnaea stagnalis-Trichobilharzia szidati model. Dev. Comp. Immunol. 2020, 102, 103464. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).