Abstract
The multicomponent synthesis of a novel and highly symmetric polyheterocycle based on the pyrrolo[3,4-b]pyridin-5-one core incorporating the privileged tetrahydroisoquinoline moiety is described. The target compound was synthesized as an inseparable mixture of stereoisomers through a pseudo-repetitive Ugi–Zhu five-component reaction (PR-UZ-5CR) coupled to a double post-transformation sequence involving an intermolecular aza Diels–Alder cycloaddition, an intramolecular N-acylation, and a final tandem aromatization step. The product was prepared in 63% overall yield, and with an excellent atom economy of 85%, within a total reaction time of 85 min, and a temperature range from 25 to 65 °C. Structural elucidation and molecular mass confirmation were successfully achieved through NMR and FT-IR spectroscopy, and high-resolution mass spectrometry (HRMS), respectively.
1. Introduction
The design of new molecules with pharmacological potential nowadays involves multiple theoretical, computational, and experimental considerations. However, the role of molecular symmetry and its potential influence on biological target recognition have been comparatively underexplored. Bai and Wang identified some guidelines to classify dimer-type molecules with high symmetry from a synthetic perspective [1]. When two chemical fragments connected by a linker are identical and share the same mode of connectivity to that linker, the resulting structure is defined as a bilaterally symmetric molecule (Figure 1A). In such cases, global symmetry becomes evident, and the molecules commonly exhibit C2, Cₛ, or C2ᵥ symmetry elements. Conversely, when the fragments are chemically identical but differ in their connectivity to the linker, the molecules are classified as non-bilaterally symmetric pseudo-dimers (Figure 1B). A relevant example of a dimer with bilateral symmetry is the commercial drug Daclatasvir (Figure 1A), an antiviral used for the treatment of hepatitis C by inhibiting its NS5A protein [2].
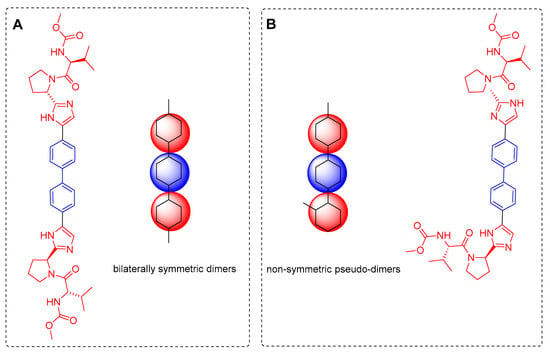
Figure 1.
Classification of molecules with evident symmetry: (A) Daclatasvir, a drug that serves as a dimer with bilateral symmetry; (B) An isomer of daclatasvir, exemplifying non-symmetric pseudo-dimers.
It is important to highlight that molecules with high symmetry exhibit exceptional stability, along in some cases with a significant degree of electronic delocalization [3]. These features are characteristic of compounds with potential antioxidant activity [4]. However, the synthesis of dimer-type molecules with bilateral symmetry may pose a synthetic challenge in the laboratory. In this context, multiple bond-forming transformations (MBFTs) offer attractive strategies to rapidly access structures with high molecular symmetry [5]. An important class of such transformations is the multicomponent reactions (MCRs), which are highly convergent one-pot processes in which the reagents undergo a sequence of elementary steps to produce a sole product with high structural complexity that retains most or all the atoms from the initial components [6]. This manuscript describes the synthesis and characterization of a new polyheterocycle bearing a pyrrolo[3,4-b]pyridin-5-one core (aza-analog of isoindolin-2-one core, a heterocycle of broad pharmacobiological relevance [7]), incorporating the privileged tetrahydroisoquinoline moiety. The target structure corresponds to a bilateral-symmetry dendrimer, obtained as an inseparable mixture of stereoisomers through the combination of two multiple bond-forming processes. These involve a pseudo-repetitive Ugi–Zhu five-component reaction (PR-UZ-5CR) [8] and a double cascade process: intermolecular aza Diels–Alder cycloaddition/intramolecular N-acylation/aromatization (decarboxylation–dehydration) steps.
2. Results and Discussion
The target polyheterocycle 17 was synthesized through two multiple-bond-forming transformations. The first one involves a PR-UZ-5CR, which produces the bis-oxazole 13. In this reaction, terephthalaldehyde 6, acting as a bi-functional reagent, 4-methoxybenzylamine 7, and an α-isocyanoacetamide 5 functionalized with the tetrahydroisoquinoline scaffold were sequentially combined. Since the latter component is not commercially available, it was synthesized through a three-step sequence involving an initial formylation, an N-acylation, and a Ugi-type dehydration, following the methodology reported by Bienaymé (Scheme 1) [9].
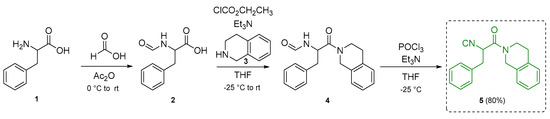
Scheme 1.
Synthesis route to access the α-isocyanoacetamide component.
The pseudo-repetitive Ugi-Zhu five-component reaction (PR-UZ-5CR) was performed by reacting terephthalaldehyde 6 with two equivalents of 4-methoxybenzylamine 7 in PhMe under continuous stirring (Scheme 2). The progress of the reaction was monitored using TLC, which indicated complete consumption of the aldehyde 6 after 30 min. Subsequently, Yb(OTf)3 (8 mol%) was added to the reaction mixture to facilitate the conversion of the imine 9 into the corresponding Lewis-type iminium cation 10. The coordination between the ytterbium center and the imine nitrogen was enhanced by microwave irradiation at 65 °C for 5 min at 65 W. The next step was to promote the reaction between the preformed intermediate 10 and the α-isocyanoacetamide 5 to yield the PR-UZ-5CR product (bis-oxazole 13) (Scheme 3). Initially, this step was performed at room temperature under constant stirring (Table 1, entry 1), resulting in a yield of 16%. To improve conversion efficiency, the effect of temperature was systematically evaluated using microwave irradiation as the heating source. At 50 °C, the yield increased to 58% (Table 1, entry 2), and further elevating the temperature to 60 °C (Table 1, entry 3) provided the optimal yield of 70% in 25 min. However, at temperatures above 60 °C, a gradual decrease in yield was observed (Table 1, entry 4).
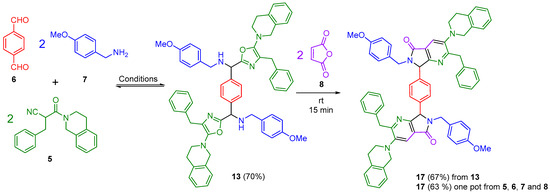
Scheme 2.
Synthetic methodology.
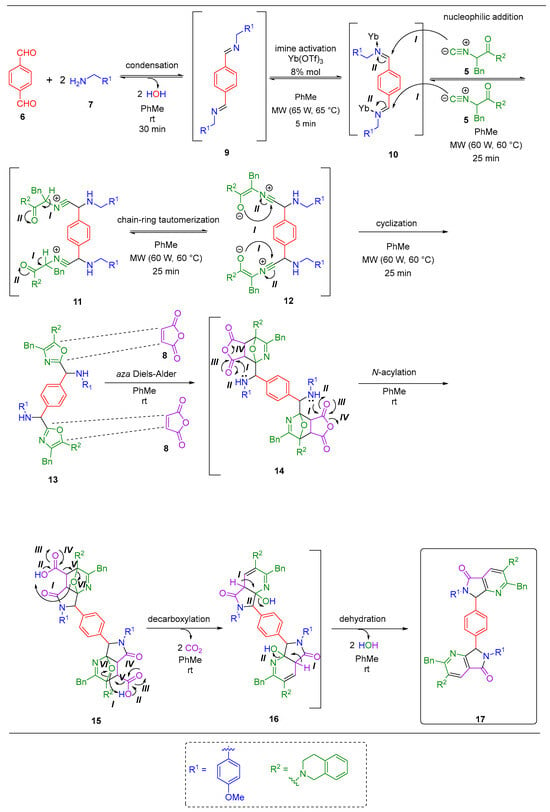
Scheme 3.
Reaction mechanism for polyheterocyclic bis-pyrrolo[3,4-b]pyridin-5-one 17.

Table 1.
Optimization of the reaction conditions.
After establishing the optimal conditions for the pseudo-repetitive Ugi-Zhu five-component reaction (PR-UZ-5CR), the focus shifted to the post-transformation step to achieve the highly symmetric polyheterocycle 17. This transformation involved a double cascade sequence: intermolecular aza Diels-Alder cycloaddition/intramolecular N-acylation/aromatization, this latter one including a tandem decarboxylation-dehydration process. Thus, the bis-oxazole 13 reacted with maleic anhydride (8), serving as the dienophile. Remarkably, with constant stirring, the reaction reached completion within 15 min, resulting in a polyheterocyclic product 17 with a yield of 67%. Alternatively, the same compound 17 was synthesized in a one-pot process, yielding a comparable result of 63%, which is considered satisfactory, especially since it only required a straightforward work-up.
The polyheterocycle 17 was characterized by 1D NMR (1H, 13C) and 2D NMR (COSY, HMBC, and HSQC) spectroscopy, allowing for the unequivocal assignment of each proton and carbon signal (see the Electronic Supplementary Material and Materials and Methods section for further details). The molecular weight was confirmed by high-resolution mass spectrometry (HRMS), with the molecular cation (C68H61N6O4)+ detected within an error margin of 0.1 ppm.
The proposed reaction mechanism leading to polyheterocycle 17 begins with the condensation of aldehyde 6 and benzylamine 7, resulting in the formation of bis-imine 9. This intermediate is then converted into the bis-iminium cation 10 through coordination with the ytterbium catalyst. This activation promotes the nucleophilic addition of the divalent carbon from α-isocyanoacetamide 5 to the electrophilic carbon of intermediate 10, which generates a bis-nitrilium cation 11. This one subsequently undergoes intramolecular tautomerization and cyclization to yield the bis-oxazole 13, which then reacts with maleic anhydride 8 via a double aza Diels–Alder cycloaddition, resulting in the formation of the oxa-bridged intermediate 14. This intermediate undergoes a rapid intramolecular acylation, followed by a tandem decarboxylation-dehydration step, which ultimately produces the polyheterocyclic bis-pyrrolo[3,4-b]pyridine-5-one 17 (Scheme 3).
It is important to highlight that the polyheterocycle 17 was obtained as an inseparable mixture of four stereoisomers due to the presence of two stereogenic centers. The PR-UZ-5CR coupled to the further cascade sequence [intermolecular aza Diels-Alder cycloaddition/intramolecular N-acylation/aromatization] did not include any stereocontrolled strategy. In addition, it is worth noting that, probably due to the high symmetry degree of compound 17, only one highly fluorescent purple spot was visualized by TLC under UV light at 254 nm and 365 nm. The presence of diastereomers is also not evident in the NMR spectra of compound 17 (See the Electronic Supplementary Material for further details).
3. Materials and Methods
3.1. General Information, Instrumentation, Sofware, and Chemicals
Hydrogen-1 (1H) and Carbon-13 (13C) Nuclear Magnetic Resonance (NMR) spectra were acquired on a Bruker Avance III 500 MHz spectrometer (Fällande, Uster, Switzerland), using deuterated chloroform (CDCl3) as the solvent. Chemical shifts (δ) are reported in parts per million (ppm) relative to the internal standard tetramethylsilane (TMS). Coupling constants (J) are expressed in Hertz (Hz), and signal multiplicities are described using the conventional abbreviations: s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). NMR data were processed and analyzed using MestReNova software (version 14.2.0-26256). Reaction progress was monitored by Thin-Layer Chromatography (TLC) on plastic plates pre-coated with silica gel F254, with spot visualization under UV light at 254 nm and 365 nm. Flash column chromatography was carried out on silica gel (230–400 mesh), employing mixtures of hexane and ethyl acetate as the eluent system. These solvent mixtures were also used for TLC development and for the determination of retention factors (Rf). All chemicals and reagents were purchased from Sigma-Aldrich-Merck (Toluca, Estado de México, Mexico) and used as received, without further purification and/or dehydration. Chemical structures were generated using ChemDraw Professional software (version 15.0.0.106, PerkinElmer Informatics, Cambridge, MA, USA).
3.2. One Pot Synthesis and Characterization of 7,7′-(1,4-Phenylene)bis(2-benzyl-3-(3,4-dihydroisoquinolin-2(1H)-yl)-6-(4-methoxybenzyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one) 17
In a 10 mL microwave reactor tube (CEM Discover SP system, Matthews, NC, USA) equipped with a magnetic stirrer, 3 mL of toluene (PhMe) was added, followed by the addition of 0.5 mmol (1 equiv.) of benzene-1,4-dicarboxaldehyde and 1 mmol (2 equiv.) of 4-methoxy benzylamine. The reaction mixture was continuously stirred at room temperature for 30 min. Subsequently, 0.04 mmol (8% mol) of ytterbium triflate Yb(OTf)3 was added, and the mixture was subjected to microwave heating at 65 °C and 65 W for 5 min. Then, 1 mmol (2 equiv.) of 1-(3,4-dihydroisoquinolin-2(1H)-yl)-2-isocyano-3-phenylpropan-1-one was added, and the reaction was continued at 60 °C and 60 W for an additional 25 min. Finally, 1 mmol (2 equiv.) of maleic anhydride was introduced, and the mixture was continuously stirred at room temperature for 15 min. Upon completion of the reaction, the crude mixture was transferred to a 100 mL separatory funnel, followed by the addition of 15 mL of saturated K2CO3 solution and 15 mL of ethyl acetate (EtOAc). The organic layer was separated, and liquid–liquid extractions were performed. The organic phase was evaporated to dryness under reduced pressure. The crude product was purified by column chromatography using silica gel as the stationary phase and mixtures of hexane and ethyl acetate as eluents. Final purification was carried out by preparative chromatography, also using silica-gel as the stationary phase and a hexane/ethyl acetate mixture (3:1 υ/υ) as the eluent. A slightly yellow solid 0.321 g of the compound 17 (Figure 2) was obtained (63% yield).
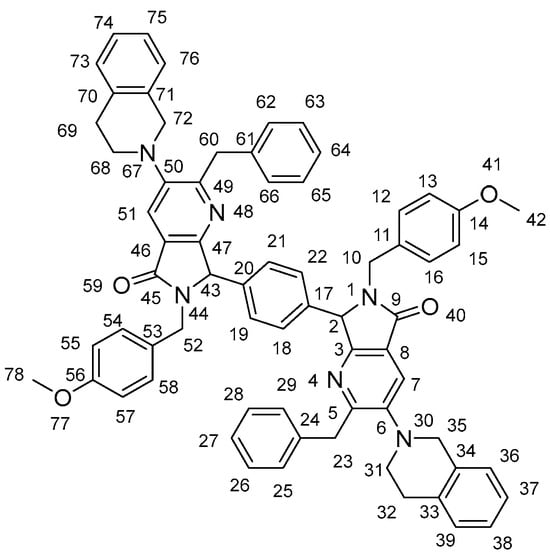
Figure 2.
Structure of compound 17.
1H NMR (500 MHz, CDCl3): δ 7.96 (s, 2H, H-7, H-51), 7.20–7.06 (m, 24H, aromatics), 7.00–6.95 (m, 2H, H-27, H-64), 6.85 (d, J = 8.8 Hz, 4H, H-13, H-15, H-55, H-57), 5.36 (d, J = 14.7 Hz, 2H, H-10, H-52), 5.28 (s, 2H, H-2, H-43), 4.28 (d, J = 14.0 Hz, 2H, H-23, H-60), 4.21 (d, J = 14.0 Hz, 2H, H-23′, H-60′), 4.08–3.99 (m, 4H, H-35, H-72), 3.78 (s, 6H, H-42, H-78), 3.37 (d, J = 14.7 Hz, 2H, H-10′, H-52′), 3.22–3.13 (m, 4H, H-31, H-68), 3.04–2.96 (m, 4H, H-32, H-69). 13C NMR (125 MHz, CDCl3): δ 167.0 (C-9, C-45), 161.9 (C-5, C-49), 159.9 (C-3, C-47), 159.2 (C-14, C-56), 148.2 (C-6, C-50), 139.2 (C-24, C-61), 136.0 (C-17, C-20), 134.3 (C-33, C-70), 133.9 (C-34, C-71), 129.8 (C-12, C-16, C-54, C-58), 128.9 (C-11, C-53), 128.8 (C-25, C-29, C-62, C-66), 128.7 (C-36, C-76), 128.1 (C-26, C-28, C-62, C-65, C-18, C-19, C-21, C-22), 126.3 (C-37, C-75), 126.1 (C-27, C-64), 125.9 (C-38, C-74), 123.9 (C-8, C-46), 123.6 (C-7, C-51), 114.2 (C-13, C-15, C-55, C-57), 64.0 (C-2, C-43), 55.4 (C-35, C-72), 50.9 (C-31, C-68), 43.4 (C-10, C-52), 39.7 (C-23, C-60), 29.5 (C-32, C-69); HRMS (ESI+): m/z calcd for C68H61N6O4 [M + H]+ 1025.4749, found 1025.4748 (error = 0.1 ppm); IR (υ, cm−1): 3429 (N-H), 1709 (C=O), 1430 (C-C), 1357 (C-O), 1220 (C=C).
4. Conclusions
The one-pot synthesis of the highly symmetric polyheterocycle 17 as an inseparable mixture of stereoisomers exemplifies the strategic coupling of multiple bond-forming transformations (MBFTs). Specifically, the current one that involves a pseudo-repetitive Ugi–Zhu 5C reaction (PR-UZ-5CR) followed by an intermolecular aza Diels–Alder/intramolecular N-acylation/tandem aromatization process, leading to the rapid formation of a bilaterally symmetric dimer that incorporates the privileged tetrahydroisoquinoline moiety. This methodology demonstrates impressive atom economy (85%), producing only four molecules of water and two molecules of carbon dioxide as byproducts. Furthermore, the reaction is conducted under mild reaction conditions, eliminating the need for elevated temperatures and highlighting the advantages of pseudo-repetitive-type multicomponent reactions over their non-pseudo-repetitive counterparts. Lastly, the inherent symmetry and the presence of privileged heterocyclic motifs within the final structure suggest that the resulting polyheterocycle may possess significant potential for biological activity against specific molecular targets.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1. 1H-NMR spectrum (CDCl3, 500 MHz) of compound 17; Figure S2. 13C-NMR spectrum (CDCl3, 125 MHz) of compound 17; Figure S3. 2D-NMR (COSY) spectrum of compound 17; Figure S4. 2D-NMR (HSQC-part I) spectrum of compound 17; Figure S5. 2D-NMR (HSQC-part II) spectrum of compound 17; Figure S6. 2D-NMR (HMBC-part I) spectrum of compound 17; Figure S7. 2D-NMR (HMBC-part II) spectrum of compound 17; Figure S8. 2D-NMR (HMBC-part III) spectrum of compound 17; Figure S9. HRMS (ESI+-TOF) spectrum of compound 17; Figure S10. FT-IR (ATR) spectrum of compound 17.
Author Contributions
Synthesis and characterization, R.E.B.-C.; data curation, A.I.-J.; investigation, E.G.-Z.; writing—original draft preparation, R.E.B.-C. and A.I.-J.; funding acquisition and writing—review and editing, A.I.-J. All authors have read and agreed to the published version of the manuscript.
Funding
A.I.-J. thanks “Proyecto de Investigación por Personal Académico de Ingreso Reciente 2024 UAM” for financial support. E.G.-Z. acknowledges DCBI-UAMI/PEAPDI 2024 and SECIHTI CBF-2025-I-3043 for financial support.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
R.E.B.C. gratefully acknowledges the support of SECIHTI-México through a postdoctoral scholarship (815447). Authors acknowledge Atilano Gutiérrez-Carrillo and Mónica A. Rincón-Guevara for NMR and HRMS acquisitions, respectively.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bai, W.; Wang, X. Appreciation of symmetry in natural product synthesis. Nat. Prod. Rep. 2017, 34, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Jayakumar, S.; Huang, W.-C.; Leyssen, P.; Neyts, J.; Bachurin, S.O.; Hwu, J.R.; Tsay, S.-C. Bis(Benzofuran–1,3-N,N-heterocycle)s as Symmetric and Synthetic Drug Leads against Yellow Fever Virus. Int. J. Mol. Sci. 2022, 23, 12675. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Almazán, S.; Navarrete-Vázquez, G.; Padilla-Martínez, I.I.; Correa-Basurto, J.; Alemán-González-duhart, D.; Tamay-Cach, F.; Mendieta-Wejebe, J.E. A new symmetrical thiazolidinedione derivative: In silico design, synthesis, and in vivo evaluation on a streptozotocin-induced rat model of diabetes. Processes 2021, 9, 1294. [Google Scholar] [CrossRef]
- Nejo, A.A.; Kolawole, G.A.; Opoku, A.R.; Wolowska, J.; O’Brien, P. Synthesis, characterization and preliminary insulin-enhancing studies of symmetrical tetradentate Schiff base complexes of oxovanadium(IV). Inorganica Chim. Acta 2009, 362, 3993–4001. [Google Scholar] [CrossRef]
- Bonne, D.; Constantieux, T.; Coquerel, Y.; Rodriguez, J. Stereoselective Multiple Bond-Forming Transformations (MBFTs): The Power of 1,2- and 1,3-Dicarbonyl Compounds. Chem. Eur. J. 2013, 19, 2218–2231. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.A.; Islas-Jacome, A.; Gonzalez-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.P.; Thapa, P.; Sharma, R.; Sharma, M. 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity. Fitoterapia 2020, 146, 104722. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Janvier, P.; Zhao, G.; Bienayme, H.; Zhu, J. A Novel Multicomponent Synthesis of Polysubstituted 5-Aminooxazole and its New Scaffold-Generating Reaction to Pyrrolo[3,4-b]pyridine. Org. Lett. 2001, 3, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Fayol, A.; Housseman, C.; Sun, X.; Janvier, P.; Bienayme, H.; Zhu, J. Synthesis of α-Isocyano-α-alkyl(aryl)acetamides and their Use in the Multicomponent Synthesis of 5-Aminooxazole, Pyrrolo [3,4-b]pyridin-5-one and 4,5,6,7-Tetrahydrofuro [2,3-c]pyridine. Synthesis 2005, 1, 161–165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).