Abstract
Compounds based on the pyrazoloquinazoline scaffold are of significant interest in synthetic organic chemistry owing to their potential biological activity. In this work, we describe the synthesis of a new derivative with this scaffold, 9-oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic acid, obtained by reacting 2,4-dioxo-4-(p-tolyl)butanoic acid with 2-aminobenzohydrazide in a 1:1 ratio when mixed in ethanol. The compound was characterized by 1H/13C NMR, IR, and X-ray diffraction analysis.
1. Introduction
The pyrazoloquinazoline scaffold is a promising nitrogen-containing heterocycle of significant pharmacological interest. Compounds bearing this scaffold have been explored as potential therapeutic agents with diverse activities, including antimicrobial [1,2], antioxidant [3,4], and antitumor effects [5] (Figure 1).
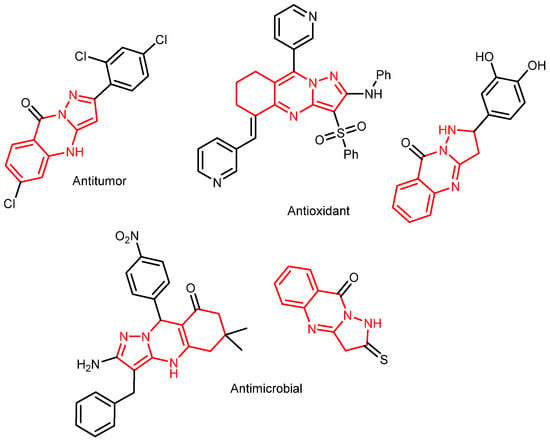
Figure 1.
Potential pharmaceutical substances bearing a pyrazoloquinazoline scaffold.
As a continuation of the development of methods for the synthesis of pyrazolines by the reaction of aroylpyruvic acids and substituted hydrazines in various proportions [6,7,8,9] (Scheme 1), we report a novel 9-oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic acid 1 obtained from the reaction 2,4-dioxo-4-(p-tolyl)butanoic acid and 2-aminobenzohydrazide. Although a similar synthetic strategy for the preparation of pyrazoloquinazolines has been reported [10], our approach utilizes a β-diketone as the starting material, unlike the β-ketoester employed previously.
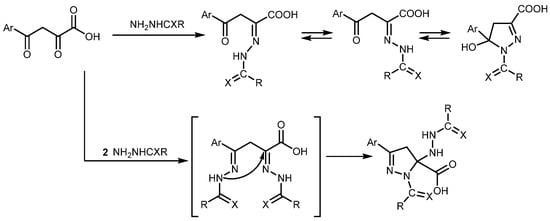
Scheme 1.
Synthesis of pyrazolines by reaction of aroylpyruvic acids and substituted hydrazines in various proportions.
2. Results and Discussion
The title compound 1 was synthesized in several steps (Scheme 2). Initially, 2,4-dioxo-4-(p-tolyl)butanoic acid 3 was obtained by the Claisen condensation of 1-(p-tolyl)ethan-1-one and diethyl oxalate. Then, as a result of the reaction of compound 3 and 2-aminobenzohydrazide 2, 9-oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic acid 1, the target compound, was obtained for the first time.
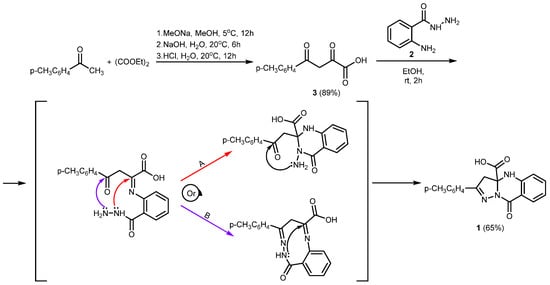
Scheme 2.
Synthesis of 9-oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic acid 1.
Our analysis suggests two plausible mechanisms. The first is derived from literature data [11], whereas the second is modeled on analogous transformations that we previously observed [8,9].
The structure of compound 1 was unambiguously confirmed by X-ray diffraction analysis of a single crystal (CCDC 2503244) (Figure 2). Compound 1 crystallizes as a racemate in the centrosymmetric space group P − 1. The asymmetric unit contains three independent molecules of 1 (only one of them is shown in Figure 2).
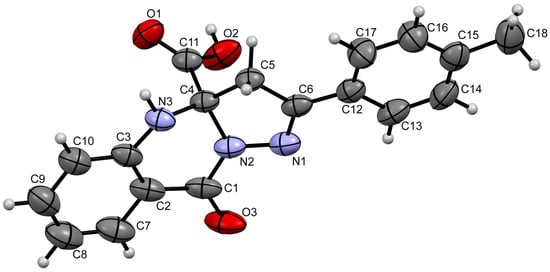
Figure 2.
Structure of compound 1, obtained by X-ray diffraction analysis.
In this work, the target compound 1 was successfully synthesized and characterized. Investigation of its biological properties constitutes the subsequent phase of our research program.
3. Materials and Methods
3.1. General Information
1H and 13C NMR spectra (Supplementary Materials) were obtained on a Bruker Avance III 400 HD spectrometer (Fällanden, Switzerland) (at 400 and 100 MHz, respectively) in DMSO-d6 using the solvent residual signal (in 1H NMR, 2.50 for DMSO-d6; in 13C NMR, 39.51 for DMSO-d6) as an internal standard. The IR spectrum was recorded on a Perkin Elmer Spectrum Two Spectrometer (Shelton, CT, USA) as mulls in mineral oil. The melting point was measured on the device of the Khimlabpribor PTP (USSR). Elemental analysis was carried out on a Vario MICRO Cube analyzer (Langenselbold, Germany). The single crystal X-ray analysis of compound 1 was performed on an Xcalibur Ruby diffractometer (Agilent Technologies, Wroclaw, Poland). An empirical absorption correction was introduced by the multi-scan method using the SCALE3 ABSPACK algorithm [12]. Using OLEX2 [13], the structure was solved with the SHELXT [14] program and refined by the full-matrix least-squares minimization in the anisotropic approximation for all non-hydrogen atoms with the SHELXL [15] program. Hydrogen atoms bound to carbon were positioned geometrically and refined using a riding model. Hydrogen atoms of OH and NH groups were refined independently with isotropic displacement parameters. Thin-layer chromatography (TLC) was performed on silica gel 60 F254 (Filter-bio, Nantong, China) plates using Toluene/EtOAc, 1:1 υ/υ, as eluents and was manifested with iodine vapor. The starting compound 3 was obtained according to the reported procedures [16] from commercially available reagents. All procedures with compound 3 were performed in oven-dried glassware. All other solvents and reagents were purchased from commercial vendors and used as received.
3.2. 9-Oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic Acid 1
A suspension of 1.364 g (6.6 mmol) 2,4-dioxo-4-(p-tolyl)butanoic acid 3 and 1 g (6.6 mmol) 2-aminobenzohydrazide 2 in 15 mL EtOH was stirred for 2 h. The formed precipitate was filtered off and recrystallized from acetic acid to yield the title compound 1. Yield: 1.39 g (65%); light yellow solid; m.p. 245–247 °C (decomp.). 1H NMR (DMSO-d6, 400 MHz): δ = 2.37 (s, 3H), 3.65 (d, 1H, J = 17.5 Hz), 3.90 (d, 1H, J = 17.5 Hz), 6.87 (t, 1H, J = 7.8 Hz), 6.97 (d, 1H, J = 8.0 Hz), 7.31 (d, 2H, J = 8.2 Hz), 7.37 (t, 1H, J = 7.8 Hz), 7.73 (d, 2H, J = 8.2 Hz), 7.77 (d, 1H, J = 8.0 Hz), 8.16 (s, 1H), 13.29 (s, 1H) ppm. 13C NMR (DMSO-d6, 100 MHz): δ = 20.9, 44.1, 78.0, 115.2, 115.7, 118.9, 126.8 (2C), 127.7, 127.8, 129.3 (2C), 133.4, 140.6, 146.1, 155.0, 156.2, 171.8 ppm. IR (mineral oil): 3355, 3332, 3274, 1747, 1726 cm−1. Anal.Calcd (%) for C18H15N3O3: C 67.28; H 4.71; N 13.08. Found: C 67.45; H 4.62; N 12.96.
Crystal Data of 1. C18H15N3O3, M = 321.33, triclinic, space group P−1, a = 11.2015(12) Å, b = 14.5766(13) Å, c = 15.9294(15) Å, α = 100.824(8)°, β = 100.914(8)°, γ = 108.031(9)°, V = 2342.4(4) Å3, T = 295(2) K, Z = 6, μ(Mo Kα) = 0.095 mm−1. The final refinement parameters: R1 = 0.0597 [for observed 5844 reflections with I > 2σ(I)]; wR2 = 0.1534 (for all independent 10888 reflections, Rint = 0.0323), S = 1.025. Largest diff. Peak and hole 0.232 and −0.232 ēÅ−3. Crystal structure of compound 1 was deposited at the Cambridge Crystallographic Data Centre with the deposition number CCDC 2503244.
Supplementary Materials
The following supporting information can be downloaded online: copies of NMR spectra for the new compound.
Author Contributions
Conceptualization, A.N.M. and Y.V.S.; methodology, A.A.A. and A.N.M.; validation, A.A.A. and A.N.M.; investigation, A.A.A. (synthetic chemistry); writing—original draft preparation, A.A.A., A.N.M. and Y.V.S.; writing—review and editing, A.A.A., A.N.M. and Y.V.S.; visualization, A.A.A.; supervision, A.N.M. and Y.V.S.; project administration, A.N.M.; funding acquisition, A.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation (Project No. FSNF-2025-0013).
Data Availability Statement
The presented data are available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanad, S.M.H.; Mekky, A.E.M.; Ahmed, A.A.M. Pyrazolo[5,1-b]quinazolines and Their Bis-analogues Linked to Different Spacers: Regioselective Synthesis, Antibacterial Screening and SwissADME Prediction Study. ChemistrySelect 2023, 8, e202300171. [Google Scholar] [CrossRef]
- Haggam, R.A.; Soylem, E.A.; Assy, M.G.; Arastiedy, M.F. Synthesis and Biological Activities of Some Condensed Oxazine and Pyrimidine Derivatives: Cyclization, Ring Transformation and Functionalization of Oxazine. Curr. Sci. 2018, 115, 1893–1903. [Google Scholar] [CrossRef]
- Hussein, M.A. Synthesis, anti-inflammatory, and structure antioxidant activity relationship of novel 4-quinazoline. Med. Chem. Res. 2013, 22, 4641–4653. [Google Scholar] [CrossRef]
- Fadda, A.A.; Refat, H.M.; Mohamed, N.A.; AbdelAal, M.T. Synthesis and Antioxidant of Some New Pyrazolo[1,5-a]pyrimidine, Pyrazolo[5,1-b]quinazoline and Imidazo[1,2-b]pyrazole Derivatives Incorporating Phenylsulfonyl Moiety. Lett. Appl. NanoBioSci 2021, 10, 2414–2428. [Google Scholar] [CrossRef]
- Kapoor, T.M. Pyrazoloquinazolinone Antitumor Agents. WO Patent WO2018213712A1, 22 November 2018. [Google Scholar]
- Igidov, S.N.; Turyshev, A.; Makhmudov, R.R.; Shipilovskikh, D.A.; Igidov, N.M.; Shipilovskikh, S.A. Synthesis, Intramolecular Cyclization, and Analgesic Activity of Substituted 2-[2-(Furancarbonyl)hydrazinylydene]-4-oxobutanoic Acids. Russ. J. Gen. Chem. 2022, 92, 1629–1636. [Google Scholar] [CrossRef]
- Lipin, D.V.; Denisova, E.I.; Devyatkin, I.O.; Okoneshnikova, E.A.; Shipilovskikh, D.A.; Makhmudov, R.R.; Igidov, N.M.; Shipilovskikh, S.A. Recyclization of 3-(Thiophen-2-yl)imino-3H-furan-2-ones under the Action of Cyanoacetic Acid Derivatives. Russ. J. Gen. Chem. 2020, 91, 809. [Google Scholar] [CrossRef]
- Andreeva, A.A.; Dmitriev, M.V.; Maslivets, A.N. 3-(4-Bromophenyl)-1-carbamothioyl-5-(2-carbamothioylhydrazinyl)-4,5-dihydro-1H-pyrazole-5-carboxylic Acid. Molbank 2024, 2024, M1757. [Google Scholar] [CrossRef]
- Andreeva, A.A.; Shklyaev, Y.V.; Maslivets, A.N. Reaction of Aroylpyruvic Acids with 3- and 4-Nitrobenzohydrazides. Synthesis of Pyrazoline-5-carboxylic Acids. Russ. J. Org. Chem. 2025, 61, 1458–1464. [Google Scholar] [CrossRef]
- Peet, N.P.; Huber, E.W. Pyrazoloquinazolines from 2-aminobenzoylhydrazine. Heterocycles 1993, 35, 315–323. [Google Scholar] [CrossRef]
- El-Shaieb, K.M.; Ameen, M.A.; Abdel-Latif, F.F.; Mohamed, A.H. Condensation reactions of 2-aminobenzohydrazide with various carbonyl compounds. Z. Für Naturforschung B 2012, 67, 1144–1150. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.42.74a; Rigaku Oxford Diffraction: Wroclaw, Poland, 2022. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 1 December 2023).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Claisen, L. Ueber die Einführung von Säureradicalen in Ketone. Ber. Dtsch. Chem. Ges. 1887, 20, 2178–2188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).