Abstract
1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide is a natural eudesmane sesquiterpene lactone isolated from the aerial parts of Inula nervosa Wall. In this paper, we report the X-ray crystallography of the compound 1 for the first time, along with the 1D/2D NMR spectra.
1. Introduction
Natural products have long been a vital source for drug discovery and development, offering unique advantages for creating innovative therapeutics due to their structural diversity and remarkable biological activities [1]. Particularly, Chinese herbal medicines, widely used in traditional medical practices, represent a valuable reservoir for identifying bioactive lead compounds [2]. In this context, plants from the genus Inula have attracted considerable interest owing to their applications in both traditional and modern research [3]. For instance, Inula nervosa Wall., commonly known as “Xiǎo Hēi Yào” (Little Black Medicine) in Chinese folk medicine, particularly in regions like Yunnan, is used for a variety of purposes based on traditional experience, including wind-cold cough, phlegm-fluid retention, chest and diaphragmatic fullness, and vomiting with belching. Modern pharmacological studies further indicate that this genus exhibits promising activities, including antitumor [4], anti-inflammatory [5], and antioxidant effects [6], primarily attributed to its abundant secondary metabolites such as sesquiterpenes, diterpenes, and triterpenes. To further elucidate its pharmacologically active constituents, we conducted a systematic phytochemical investigation on I. nervosa, leading to the isolation of a sesquiterpene compound identified as 1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide (1). This paper details the isolation and purification process of this compound and confirms its absolute configuration via X-ray single-crystal diffraction analysis.
2. Results and Discussion
A systematic phytochemical investigation of the ethanolic extract from the aerial parts of Inula nervosa led to the isolation of an eudesmane-type sesquiterpene lactone (1) shown in Figure 1. The structure was elucidated as 1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide through comprehensive spectroscopic analyses, including high-resolution electrospray ionization mass spectrometry (HRESIMS), nuclear magnetic resonance (NMR) spectroscopy, and X-ray single-crystal diffraction. Its spectral data showed high consistency with those reported for 1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide [7]. The detailed process of structural elucidation will be discussed in the following section.
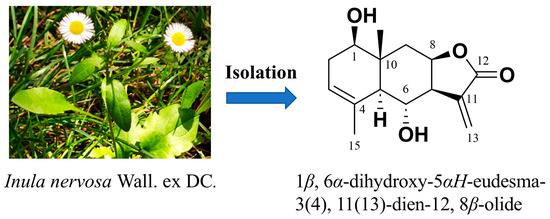
Figure 1.
Structure of 1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide (1) isolated from Inula nervosa Wall.
2.1. Determination of Molecular Formula and Basic Physical Properties
Compound 1 was obtained as colorless needle-shaped crystals and was characterized as a eudesmane-type sesquiterpene. Its molecular formula was determined to be C15H20O4 from the quasimolecular ion peak observed at m/z 265.1434 [M + H]+ (calcd for C15H21O4, 265.1434) in the HRESIMS analysis, corresponding to six degrees of unsaturation.
2.2. Structural Elucidation Based on NMR Spectroscopic Data
The 1H NMR spectrum displayed an olefinic proton signal [δH 5.37 (1H, s, H-3)]; a terminal methylene group [δH 6.16 (1H, s, H-13α), 5.85 (1H, s, H-13β)]; and two methyl singlets [δH 0.83 (3H, s, H-14), 1.88 (3H, s, H-15)]. The 1H and 13C NMR spectra of compound 1 were analyzed in detail (data provided in Materials and Methods). The 13C NMR spectra revealed the presence of 15 carbon signals, comprising a lactone carbonyl carbon (δC 172.6, C-12); two pairs of olefinic carbons [δC 135.6 (C-4), 123.7 (C-3); δC 141.7 (C-11), 122.9 (C-13)]; two oxygenated carbons [δC 76.4 (C-1), 71.2 (C-6)]; one oxygenated methine carbon (δC 79.7, C-8); along with two methyl carbons [δC 12.1 (C-14), 25.3 (C-15)]; two methylene carbons [δC 32.6 (C-2), 36.9 (C-9)]; two methine carbons [δC 51.1 (C-5), 51.1 (C-7)]; and one quaternary carbon [δC 40.0 (C-10)]. All proton and carbon signals were unambiguously assigned with the aid of HSQC, 1H—1H COSY, and HMBC experiments. The spectral data of compound 1 closely matched those reported for the known compound 1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide [7].
2.3. Structural Confirmation by X-Ray Diffraction
The structure of compound 1 was initially deduced from spectroscopic data, while its complete relative configuration, precise molecular geometry, crystal system, and unit cell parameters were unambiguously confirmed by X-ray single-crystal diffraction analysis (Figure 2). Specifically, compound 1 crystallizes in the orthorhombic system with unit cell parameters: a = 6.9234(3) Å, b = 10.0147(4) Å, c = 20.6838(8) Å, unit cell volume V = 1434.39(9) Å3, Z = 4, and T = 293.81(10) K; the diffraction data were collected with Cu Kα radiation (μ = 0.804 mm−1), yielding 7004 measured reflections and 2714 unique reflections (Rint = 0.0216, Rsigma = 0.0217), which were used for structure calculations with final refinement parameters R1 = 0.0309 (I > 2σ(I)), wR2 = 0.0862 (all data), and Flack parameter = −0.02 (9) (data from Table S1). Its core structure consists of two fused six-membered rings (Ring A: C1-C2-C3-C4-C5-C10; Ring B: C5-C6-C7-C8-C9-C10) and one γ-lactone ring (Ring C: C7-C8-O1-C11-C12), where Ring A adopts a half-chair conformation, Ring B exhibits a chair conformation, and lactone Ring C is a nearly planar five-membered ring. Detailed geometric parameters of compound 1, including all bond lengths and bond angles with their corresponding estimated standard deviations (ESDs), are provided in Table S2. These parameters are consistent with the typical structural characteristics of eudesmane-type sesquiterpene lactones, further verifying the accuracy of the structure elucidation. This X-ray diffraction analysis not only validated the initially deduced structure but also established the relative configurations of all chiral centers (1β, 5α, 6α, 8β), thereby fully confirming the structure of compound 1 as depicted in Figure 2. Crystallographic data for compound 1 have been deposited at the Cambridge Crystallographic Data Centre.
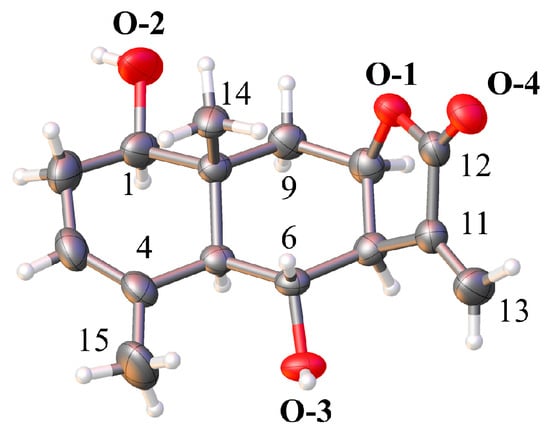
Figure 2.
X-ray crystal structure of 1β, 6α-dihydroxy-5αH-eudesma-3(4),11(13)-dien-12, 8β-olide (CCDC: 2408916).
3. Materials and Methods
3.1. Plant Materials
The aerial parts of I. nervosa Wall. were collected in October 2022 from Kunming City of Yunnan province, China. The plant was authenticated by Professor Jun-Mian Tian, kept dry and ventilated. A voucher specimen (TJJ-20221011) was stored in a cool and dry environment of Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University.
3.2. Extraction and Isolation
The dried aerial parts of I. nervosa Wall. (135 kg) were extracted with 95% EtOH (200 L × 2 h × 3). The combined extracts were then concentrated under reduced pressure to obtain a residual extract, which was further suspended in water and partitioned with petroleum ether, CH2Cl2, and ethyl acetate, successively. The EtOAc-soluble fraction (2 kg) was chromatographed on a silica gel column eluting with a CH2Cl2/MeOH (100: 0–1: 1 v/v) gradient to obtain fourteen fractions. After repeated column chromatography of fraction Fr. 3, subfraction Fr.3.7.1.9.5 (259.5 mg) was purified by semipreparative HPLC (CH3CN-H2O, 20%, 2 mL/min) to afford this natural product (5.6 mg, tR = 33.6 min).
1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide (1): white amorphous powder. The physicochemical properties were consistent with those reported in the literature for the same compound [7]: white amorphous powder, m.p. 155.7–158.2 °C; [α]20_D –21.5 (c 0.1, MeOH); IR (KBr) νmax 3423, 2922, 1757, 1635, 1400, 1130 cm−1. The compound was soluble in common organic solvents such as methanol and dimethyl sulfoxide. 1H NMR (400 MHz, CD3OD, J/Hz) δ 3.46 (1H, m, H-1), 2.25 (1H, m, H-2α), 1.93 (1H, m, H-2β), 5.37 (1H, s, H-3), 2.00 (1H, m, H-5), 3.46 (1H, m, H-6), 2.82 (1H, t, J = 7.2, H-7), 4.74 (1H, m, H-8), 2.48 (1H, dd, J = 15.6, 1.6, Hα-9), 1.51 (1H, dd, J = 16.0, 5.2, Hβ-9), 6.16 (1H, s, H-13α), 5.85 (1H, s, H-13β), 0.83 (3H, s, H-14), 1.88 (3H, s, H-15); 13C NMR (100 MHz, CD3OD, J/Hz) δ 76.4 (C-1), 32.6 (C-2), 123.7 (C-3), 135.6 (C-4), 51.1 (C-5), 71.2 (C-6), 51.1 (C-7), 79.7 (C-8), 36.9 (C-9), 40.0 (C-10), 141.7 (C-11), 172.6 (C-12), 122.9 (C-13), 12.1 (C-14), 25.3 (C-15). Colorless X-ray-quality crystals of this natural product were grown by the slow cooling of a sample dissolved in methanol.
3.3. X-Ray Structure of 1β, 6α-Dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide (1)
Suitable crystals were selected and analyzed on a XtaLAB Synergy R, DW system, HyPix diffractometer (Rigaku, Tokyo, Japan). The crystal was kept at 296(4) K during data collection. Using Olex2, the structure was solved with the ShelXS structure solution program using Direct Methods and refined with the ShelXL refinement package using Least Squares minimization. The crystal data are as follows: C15H22O5 (M = 282.32 g/mol): orthorhombic, space group P212121 (no. 19), a = 6.9234(3) Å, b = 10.0147(4) Å, c = 20.6873(8) Å, V = 1434.39(9) Å3, Z = 4, T = 293.81(10) K, μ(Cu Kα) = 0.804 mm−1, Dcalc = 1.307 g/cm3, 7004 reflections measured (8.548° ≤ 2Θ ≤ 139.74°), 2714 unique (Rint = 0.0216, Rsigma = 0.0217) which were used in all calculations. The final R1 was 0.0309 (I > 2σ(I)) and wR2 was 0.0862 (all data). Flack parameter = −0.02 (9). Crystallographic data for compound 1 have been deposited at the Cambridge Crystallographic Data Center with the number CCDC-2408916.
4. Conclusions
This study led to the isolation of an eudesmane-type sesquiterpene lactone from the medicinal plant Inula nervosa Wall. Its structure was unequivocally identified as 1β, 6α-dihydroxy-5αH-eudesma-3(4), 11(13)-dien-12, 8β-olide through a combination of spectroscopic techniques (HRESIMS and NMR) and X-ray crystallographic analysis. The X-ray diffraction study confirmed the molecular structure and definitively established the absolute configuration of all chiral centers. The successful isolation and structural elucidation of this compound enrich the chemical database of the Inula genus and lay a foundation for further investigation into its biological activities.
Supplementary Materials
The following supporting information can be downloaded online: Figure S1: 1H-NMR spectrum of compound 1; Figure S2: 13C DEPT spectrum of compound 1; Figure S3: 1H-1H COSY spectrum of compound 1; Figure S4: HSQC spectrum of compound 1; Figure S5: HMBC spectrum of compound 1; Figure S6: 1H-1H NOESY spectrum of compound 1; Figure S7: ESI mass spectrum of compound 1; Figure S8: HPLC chromatogram of compound 1; CIF file for compound 1; Table S1: Crystal data and structure refinement for compound 1; Table S2: The key bond distance and bond angle of compound 1.
Author Contributions
Conceptualization, J.-J.T.; methodology and investigation, D.-D.X. and J.-Y.R.; software, Z.-N.W.; validation, J.-Y.R. and Z.-N.W.; formal analysis, D.-D.X. and Y.-Y.Q.; sources and data curation, D.-D.X. and J.-J.T.; writing—original draft preparation, Z.-N.W.; writing—review and editing, J.-J.T.; supervision, J.-J.T.; project administration, J.-Y.R.; funding acquisition, J.-J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Tibet Autonomous Region (XZ202401ZR0068) and Natural Science Foundation of Guangdong Province (2024A1515011616).
Data Availability Statement
CCDC 2408916 contains the Supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (10 December 2024).
Acknowledgments
The authors express gratitude to Ping Xiang from the State Key Laboratory of Crop Stress Biology for Arid Areas, NWAFU, for NMR analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, M.X.; Lv, H.Z.; Chen, H.Y.; Dong, S.T.; Zhang, J.H.; Liu, W.J.; He, L.; Ma, Y.M.; Yu, H.; Chen, S.L.; et al. Strategies on biosynthesis and production of bioactive compounds in medicinal plants. Chin. Herb. Med. 2023, 16, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L. Biosynthesis of natural products from medicinal plants: Challenges, progress and prospects. Chin. Herb. Med. 2024, 16, 1–2. [Google Scholar] [CrossRef]
- Seca, A.M.; Grigore, A.; Pinto, D.C.; Silva, A.M. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Guo, L.; Luo, D.; Lou, S.Q.; Liang, D.E.; Xu, X.Y.; Xi, Z.F.; Zhan, Z.J.; Ma, L.F. Sesquiterpene Monomers and Dimers From the Flower of Inula japonica Thunb. With Selective Antiproliferation Against Breast Cancer Cells. Chem. Biodivers. 2025, 22, e202402492. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.A.; Lee, J.Y.; Kim, Y.J.; Ji, K.Y.; Le, M.H.; Jung, D.H.; Kim, Y.H.; Kim, W.J.; Moon, B.C.; Kim, B.Y.; et al. Inula japonica Thunb. and its active compounds ameliorate airway inflammation by suppressing JAK-STAT signaling. Biomed. Pharmacother. 2025, 183, 117852. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.X.; Luo, M.F.; Guo, W.J.; He, X.; Zhou, J.; Qiu, X.Y.; Gong, J.P.; Li, M.C.; Chen, X.T.; Wu, D.; et al. Quality Assessment and Antioxidant Activities of the Blossoms of Inula nervosa Wall. J. AOAC Int. 2021, 104, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Du, J.L.; Ren, J.; Ye, F.M.; Xie, Y.G.; Cheng, X.R.; Yan, S.K.; Jin, H.Z. Three new sesquiterpene lactones from Inula britannica. Arch. Pharm. Res. 2015, 38, 666–672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).