Abstract
The reaction of a 2-naphthol-derived Mannich base with the push-pull 5-morpholinopenta-2,4-dienal under acidic conditions unexpectedly afforded (7aR*,7bR*)-7a,7b-dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene. The structure of this product was unambiguously confirmed by NMR spectroscopy and X-ray diffraction analysis. A plausible mechanism involves the in situ generation of 1,2-naphthoquinone-1-methide, followed by a [4 + 2] cycloaddition and a subsequent interrupted iso-Nazarov cyclization. In this process, the enol tautomer of the resulting fused cyclopentenone is trapped by a second equivalent of the 1,2-naphthoquinone-1-methide, leading to the observed polycyclic framework.
1. Introduction
4H-Chromenes and their benzo analogues are privileged structural motifs, fragments of which are present in a large number of natural compounds, pharmaceuticals, and functional organic materials [1,2,3,4,5,6]. Of particular interest are electron-deficient 4H-chromenes bearing an electron-withdrawing group at the β-position relative to the pyran oxygen atom [7]. The high degree of polarization of the double bond in the pyran ring makes it susceptible to attack by nucleophiles, ambiphilic reagents, and 1,3-dipoles, making such heterocycles valuable substrates for the Michael reaction and 1,3-dipolar cycloaddition. Finally, a large number of compounds with proven antitumor activity have been found among this type of chromenes. For example, crolibulin is a low-molecular-weight inhibitor of tubulin polymerization with an antineoplastic effect and an acceptable side effect profile [8]. Chromeceptin selectively reduces the viability and growth of hepatocellular carcinoma cells overexpressing IGF2 (insulin-like growth factor-2) and binds multifunctional protein 2 (MFP-2), which is involved in peroxisomal oxidation [9]. Compound MX58151 showed growth inhibition in highly drug-resistant MES-SA/DX-5 tumor cells [10]. Chromene LY294002 is a selective, cell-permeable, potent, and specific inhibitor of phosphatidylinositol 3-kinase PI3K [11]. Increased levels of PI3K products were observed in colorectal tumors and breast cancer. Additional examples of push-pull chromenes with anticancer activity include compounds SP-6-27 [12], LY290181 [13], and HA 14-1 [14] (Figure 1).
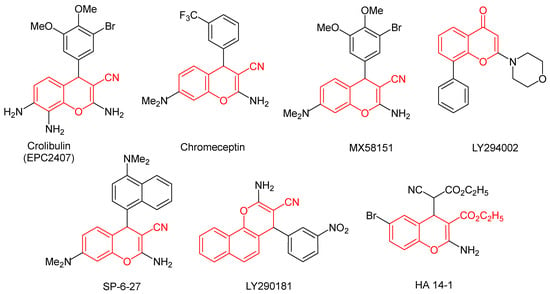
Figure 1.
Highly polarized 4H-chromenes with anticancer activity.
One of the effective approaches to the synthesis of electron-deficient 4H-chromenes is the Diels-Alder reaction between ortho-quinone methides (o-QM) and push-pull olefins, for which we have previously studied a number of enaminoketones, enaminals, nitroenamines and some others [15,16].
2. Results and Discussion
2.1. Synthesis and Spectroscopy
Continuing our research into developing methods for the preparation of electron-deficient 4H-chromenes [15,16], we studied the interaction of 2-naphthol Mannich base 1 with push-pull dienal 2 (Zincke aldehyde), which contains a donor (morpholine fragment) and an acceptor (aldehyde) group at opposite ends of the conjugated 1,3-diene system. This resulted in a complex mixture of products, from which dichromene 3 and the literature-described bis(2-hydroxy-1-naphthyl)methane 4 were isolated in pure form and identified (Scheme 1). The reaction was carried out by refluxing compounds 1 and 2 in a 2:1 ratio in acetic acid for 2.5 h. The products were isolated by column chromatography on silica gel.
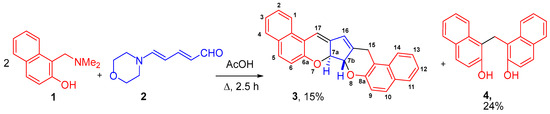
Scheme 1.
Reaction of Mannich base 1 with Zincke aldehyde 2.
In the 1H NMR spectrum of compound 3, the diastereotopic protons of the methylene group (H-15) appear as two doublet signals at 4.06 and 4.14 ppm with a geminal coupling constant 2J = 20.8 Hz. The protons H-7a and H-7b are observed in the region of 5.08–5.25 ppm, with a spin-spin coupling constant between them of 3.7 Hz. Two singlet signals in the region of 6.60–7.04 ppm correspond to the olefinic protons at positions 16 and 17 (Figure S1). In the 13C NMR spectrum, the methylene carbon atom resonates at 25.3 ppm. The sp3-hybridized carbon atoms bonded to oxygen (C-7a, C-7b) appear at 81.5 and 83.4 ppm. The most deshielded signals belong to the aromatic carbon atoms attached to oxygen (C-6a, C-8a), which resonate at 151.2 and 152.0 ppm (Figure S2). In the DEPT spectra, the number of carbon atoms directly bonded to protons is consistent with the considered structure (Figure S3).
In addition, the structure of dichromene 3 was confirmed by X-ray diffraction data, according to which the protons at positions 7a and 7b occupy a trans-arrangement with respect to each other in the five-membered carbocycle (Figure 2). The torsion angle between protons 7a and 7b is 136.91°. In structure 3, the five-membered carbocycle is nearly planar, with torsion angles not exceeding 13.3°. The O1C1C10C11 torsion angle (according to the numbering in Figure 2) is 4.70°, and the C1C10C11C12 angle is 11.74°, indicating effective conjugation in this structural fragment.
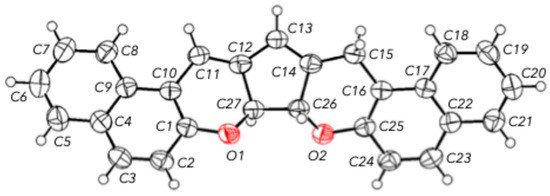
Figure 2.
Molecular structure of compound 3 shown with thermal ellipsoids of atomic displacement parameters at the 50% probability level.
2.2. Proposed Mechanism
We initially hypothesized that the reaction of Mannich base 1 with dienal 2 would yield aldehyde B in the E-configuration via a Diels-Alder reaction between in situ-generated 1,2-naphthoquinone 1-methide A and the most electron-rich double bond of dienal 2, followed by elimination of morpholine. Furthermore, due to the lower energy barrier of E/Z isomerization, especially in acidic media, we might expect the formation of a cycloaddition product C with the Z-configuration of the exocyclic double bond, which could isomerize to 7aH,12H-benzo[f]pyrano[2,3-b]chromene D via 6π-oxa-electrocyclization [17]. However, this reaction pathway was also not realized. Apparently, the aldehyde undergoes cyclization in an acidic medium to the enol E, followed by the addition of another 1 equivalent of o-QM A to the electron-rich double bond of the enol form of the ketone E (the intermolecular interrupted iso-Nazarov reaction) [18,19,20,21,22]. Subsequent dehydration and a 1,5-sigmatropic hydrogen shift lead to the formation of dichromene 3 (Scheme 2).
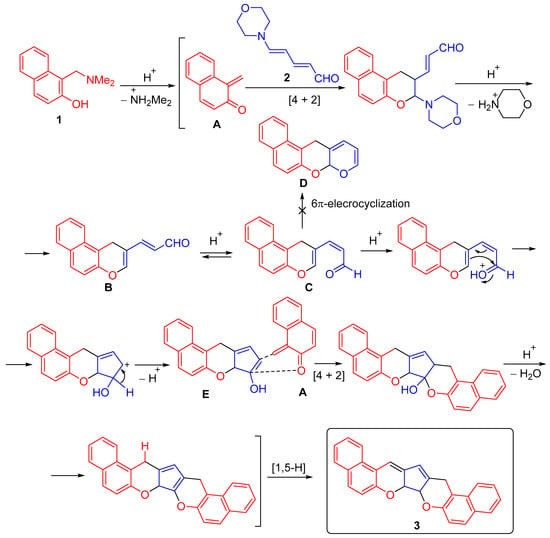
Scheme 2.
Proposed mechanism for the formation of (7aR*,7bR*)-7a,7b-dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene 3.
The formation of 1,1′-methylenebis(naphthalen-2-ol) 4 apparently occurs via deamination of Mannich base 1 under acidic conditions through a retro-Mannich reaction, followed by addition of the resulting 2-naphthol to o-QM A (Scheme 3).
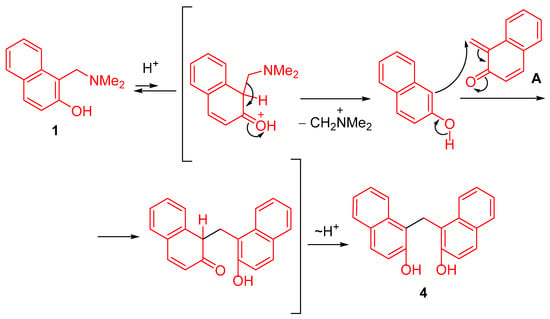
Scheme 3.
Proposed mechanism for the formation of 1,1′-methylenebis(naphthalen-2-ol) 4.
3. Materials and Methods
All synthetic manipulations were performed in air. All reagents and solvents were purchased from commercial vendors and used as received. 1H and 13C (proton-decoupled) NMR spectra (at 400 and 100 MHz, respectively), as well as DEPT-135 spectrum, were registered on a JEOL JNM-ECX400 spectrometer (Tokyo, Japan) in CDCl3. Chemical shifts were referenced internally to the residual solvent signal (CDCl3: 7.26 ppm for 1H nuclei, 77.2 ppm for 13C nuclei). Elemental analysis was performed on an automated Euro Vector EA-3000 CHNS analyzer (Milan, Italy) using L-cystine as a standard. Melting point was determined by the capillary method on an SRS OptiMelt MPA100 instrument (Sunnyvale, CA, USA). Reaction progress and purity of the obtained compounds were monitored by TLC on Merck Silica gel 60 F254 plates (eluent–CH2Cl2). The starting dienal 2 was obtained by a method published previously [23].
3.1. Synthesis and Characterization of (7aR*,7bR*)-7a,7b-Dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene (3)
A solution of 0.36 g (1.8 mmol) of 1-[(dimethylamino)methyl]naphthalen-2-ol 1 and 0.15 g (0.9 mmol) of (2E,4E)-5-morpholinopenta-2,4-dienal 2 in 10 mL of acetic acid was refluxed for 2.5 h. The mixture was cooled and poured with stirring into 30 mL of ice-cold water. The precipitated solid was filtered off, and the crude product was purified by column chromatography on silica gel (eluent–CH2Cl2). For further purification, the product was dissolved in CH2Cl2 upon heating, three volumes of methanol were added, and CH2Cl2 was evaporated until the onset of product crystallization. The mixture was then kept at −30 °C for 2 h. The precipitate was filtered off, washed with ice-cold methanol, and dried in air to afford 3 (50 mg, 15%).
Colorless crystals, mp 243–245 °C. 1H NMR (400 MHz, CDCl3) δ, ppm: 8.03 (d, J = 8.2 Hz, 1H), 7.83–7.78 (m, 3H), 7.690 (d, J = 8.9 Hz, 1H), 7.685 (d, J = 8.9 Hz, 1H), 7.56–7.49 (m, 2H), 7.42–7.37 (m, 2H), 7.30 (d, J = 8.9 Hz, 1H), 7.21 (d, J = 8.9 Hz, 1H), 7.04 (s, 1H), 6.60 (br. s, 1H), 5.25 (d, J = 3.7 Hz, 1H), 5.08 (dd, J = 3.7, 2.3 Hz, 1H), 4.14 (d, J = 20.8 Hz, 1H), 4.06 (d, J = 20.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ, ppm: 152.0 (C), 151.2 (C), 142.4 (C), 136.0 (C), 132.6 (C), 130.4 (C), 130.0 (C), 129.5 (C), 128.74 (CH), 128.69 (CH), 128.67 (CH), 128.5 (CH), 126.8 (CH), 126.7 (CH), 125.0 (CH), 124.2 (CH), 123.9 (CH), 122.1 (CH), 121.8 (CH), 119.5 (CH), 118.1 (CH), 117.7 (C), 111.6 (C), 109.6 (CH), 83.4 (CH), 81.5 (CH), 25.3 (CH2). Calc. for C27H18O2: C 86.61; H 4.85. Found: C 86.56; H 4.82.
3.2. X-Ray Crystallography
X-ray structural analysis of compound 3 was carried out on an Agilent SuperNova diffractometer (Agilent Technologies, Santa Clara, CA, USA) equipped with a microfocus copper-anode X-ray source with Cu Kα radiation (λ = 1.54184 Å) and an Atlas S2 CCD detector. Crystals suitable for X-ray diffraction were grown by slow evaporation from a CH2Cl2–MeOH mixture at room temperature. Data collection, as well as determination and refinement of unit-cell parameters, were performed using the CrysAlisPro software package, version 1.171.38.41 [24]. The structure was solved with ShelXT [25] and refined using ShelXL [26]. Molecular graphics and preparation of the manuscript were performed with the Olex2 software package, version 1.2.10 [27].
Selected crystallographic data: C27H18O2, M = 374.41, monoclinic, a = 6.8568(1), b = 7.6873(1), c = 34.9443(6) Å, α = 90°, β = 92.552(2)°, γ = 90°, V = 1840.10(5) Å3, T = 293 K, space group P21/c, Z = 4, dcalc = 1.352 g/cm3. A colorless plate-shaped single crystal with dimensions of 0.05 × 0.16 × 0.37 mm was selected for data collection. A total of 19,725 reflections were measured (μ = 0.662 mm−1, θmax = 76.220°). After merging symmetry-equivalent reflections and applying empirical absorption corrections, 3837 independent reflections were obtained (Rint = 0.0315). Final refinement converged at R1 = 0.0535 [for 3268 reflections with I > 2σ(I)], wR2 = 0.1363 (for all 3837 reflections), S = 1.073. The largest differences in electron density peak and hole were 0.284 and −0.207 e/Å3, respectively. All non-hydrogen atoms were refined anisotropically; hydrogen atoms were placed in calculated positions and refined with riding models. CCDC 2256754 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
4. Conclusions
In this work, we described the preparation of a new heterocyclic system, (7aR*,7bR*)-7a,7b-dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene. Structural elucidation by NMR spectroscopy and X-ray diffraction revealed a unique fused heptacyclic framework with trans-arrangement of hydrogen atoms in a five-membered carbocycle.
Supplementary Materials
Figure S1: 1H NMR spectrum of 3; Figure S2: 13C NMR spectrum of 3; Figure S3: DEPT-135 spectrum of 3.
Author Contributions
D.V.O.—funding acquisition, supervision, writing (original draft, review and editing); O.P.D.—investigation, data analysis; V.A.O.—conceptualization, supervision, data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation No. 22-13-00253-П, https://rscf.ru/project/22-13-00253/.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
This work was performed using the equipment of the Center for Collective Use «Investigation of Physicochemical Properties of Substances and Materials» of the Samara State Technical University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NMR | Nuclear Magnetic Resonance |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| o-QM | ortho-Quinone Methide |
References
- Wen, Z.; Yang, K.-C.; Deng, J.-F.; Chen, L. Advancements in the Preparation of 4H-Chromenes: An Overview. Adv. Synth. Catal. 2023, 365, 1290–1331. [Google Scholar] [CrossRef]
- Chaudhary, A.; Singh, K.; Verma, N.; Kumar, S.; Kumar, D.; Sharma, P.P. Chromenes—A Novel Class of Heterocyclic Compounds: Recent Advancements and Future Directions. Mini Rev. Med. Chem. 2022, 22, 2736–2751. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Lee, J. 2H/4H-Chromenes—A Versatile Biologically Attractive Scaffold. Front. Chem. 2020, 8, 623. [Google Scholar] [CrossRef]
- Katiyar, M.K.; Dhakad, G.K.; Shivani; Arora, S.; Bhagat, S.; Arora, T.; Kumar, R. Synthetic strategies and pharmacological activities of chromene and its derivatives: An overview. J. Mol. Struct. 2022, 1263, 133012. [Google Scholar] [CrossRef]
- Sharon, K.N.; Padmaja, P.; Reddy, P.N. A Brief Review on the Synthesis of 4H-Chromene-Embedded Heterocycles. ChemistrySelect 2024, 9, e202400565. [Google Scholar] [CrossRef]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Osipov, D.V.; Osyanin, V.A.; Klimochkin, Y.N. Reactions of 5-formyl- and 5-acyl-3,4-dihydro-2H-pyrans and their annelated analogs with nucleophiles. Targets Heterocycl. Syst. 2019, 22, 436–467. [Google Scholar] [CrossRef]
- Lorza, A.M.A.; Ravi, H.; Philip, R.C.; Galons, J.-P.; Trouard, T.P.; Parra, N.A.; Von Hoff, D.D.; Read, W.L.; Tibes, R.; Korn, R.L.; et al. Dose–response assessment by quantitative MRI in a phase 1 clinical study of the anti-cancer vascular disrupting agent crolibulin. Sci. Rep. 2020, 10, 14449. [Google Scholar] [CrossRef]
- Choi, Y.; Shimogawa, H.; Murakami, K.; Ramdas, L.; Zhang, W.; Qin, J.; Uesugi, M. Chemical genetic identification of the IGF-linked pathway that is mediated by STAT6 and MFP2. Chem. Biol. 2006, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Patil, R.; Pfeffer, L.M.; Miller, D.D. Chromenes: Potential new chemotherapeutic agents for cancer. Future Med. Chem. 2013, 5, 1647–1660. [Google Scholar] [CrossRef]
- Semba, S.; Itoh, N.; Ito, M.; Harada, M.; Yamakawa, M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer cells. Clin. Cancer Res. 2002, 8, 1957–1963. [Google Scholar]
- Kulshrestha, A.; Katara, G.K.; Ibrahim, S.A.; Patil, R.; Patil, S.A.; Beaman, K.D. Microtubule inhibitor, SP-6-27 inhibits angiogenesis and induces apoptosis in ovarian cancer cells. Oncotarget 2017, 8, 67017–67028. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Singh, J.P.; Wilson, L. Suppression of Microtubule Dynamics by LY290181: A potential mechanism for its antiproliferative action. J. Biol. Chem. 1997, 272, 7681–7687. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.M.; Tian, D.; Xing, C. Structure−Activity Relationship Studies of Ethyl 2-Amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (HA 14-1), an Antagonist for Antiapoptotic Bcl-2 Proteins To Overcome Drug Resistance in Cancer. J. Med. Chem. 2006, 49, 7731–7739. [Google Scholar] [CrossRef]
- Osyanin, V.A.; Lukashenko, A.V.; Osipov, D.V. Cycloaddition reactions of o-quinone methides with polarized olefins. Russ. Chem. Rev. 2021, 90, 324–373. [Google Scholar] [CrossRef]
- Lukashenko, A.V.; Osyanin, V.A.; Osipov, D.V.; Klimochkin, Y.N. Reaction of Push-Pull Enaminoketones and in Situ Generated ortho-Quinone Methides: Synthesis of 3-Acyl-4H-chromenes and 2-Acyl-1H-benzo[f]chromenes as Precursors for Hydroxybenzylated Heterocycles. J. Org. Chem. 2017, 82, 1517–1528. [Google Scholar] [CrossRef]
- Osyanin, V.A.; Osipov, D.V.; Semenova, I.A.; Korzhenko, K.S.; Lukashenko, A.V.; Demidov, O.P.; Klimochkin, Y.N. Eco-friendly synthesis of fused pyrano [2,3-b]pyrans via ammonium acetate-mediated formal oxa-[3 + 3] cycloaddition of 4H-chromene-3-carbaldehydes and cyclic 1,3-dicarbonyl compounds. RSC Adv. 2020, 10, 34344–34354. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.A.; Pergomet, J.L.; Gandon, V.; Riveira, M.J. Iodine-Catalyzed Iso-Nazarov Cyclization of Conjugated Dienals for the Synthesis of 2-Cyclopentenones. Org. Lett. 2018, 20, 7298–7303. [Google Scholar] [CrossRef]
- Riveira, M.J.; Marsili, L.A.; Mischne, M.P. The iso-Nazarov reaction. Org. Biomol. Chem. 2017, 15, 9255–9274. [Google Scholar] [CrossRef]
- Attanonchai, S.; Poonswat, K.; Ruchirawat, S.; Ploypradith, P. Indenoindenes, Indenoindoles, and Indenobenzofurans via the Interrupted iso-Nazarov Reaction. Asian J. Org. Chem. 2025, 14, e202400655. [Google Scholar] [CrossRef]
- La-Venia, A.; Passaglia, L.; Gurgone, L.; Gandon, V.; Riveira, M.J. Acid-Promoted Iso-Nazarov Cyclization of Conjugated trans-Dienones and Dienals for the Synthesis of 2-Cyclopentenones. J. Org. Chem. 2022, 87, 13469–13479. [Google Scholar] [CrossRef]
- Marques, A.-S.; Marrot, J.; Chataigner, I.; Coeffard, V.; Vincent, G.; Moreau, X. In Situ Generation of Cyclopentadienol Intermediates from 2,4-Dienals. Application to the Synthesis of Spirooxindoles via a Domino Polycyclization. Org. Lett. 2018, 20, 792–795. [Google Scholar] [CrossRef]
- Finsen, L.; Becher, J.; Buchardt, O.; Koganty, R.R. Derivatives and Reactions of Glutaconaldehyde. XI. N-Substituted 5-Amino-2,4-Pentadienenals, their Oximes, and 5-Amino-2,4-Pentadienenitriles. Structural Analysis by 1H and 13C NMR Spectroscopy. Acta Chem. Scand. B 1980, 34, 513–518. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.38.41; Rigaku Oxford Diffraction: Abingdon, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).