Abstract
In the present study, (E)-1-(2-(pyridin-2-yl)benzo[d]thiazol-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (4) was designed and synthesized via a three-step reaction sequence. Initially, 6-acetylbenzo[d]thiazol-2(3H)-one (1) was hydrolyzed to the corresponding 5-acetyl-2-aminothiophenol 2 and then cyclized with pyridine-2-carbaldehyde. The final product was synthesized by a base-catalyzed aldol condensation of 1-(2-(pyridin-2-yl)benzo[d]thiazol-6-yl)ethan-1-one (3) and 3,4,5-trimethoxybenzaldehyde and was comprehensively characterized.
1. Introduction
The structure of chalcone is one of the most critical and widely recognized components, and is an integral feature of a range of flavonoids and pharmacological agents [1]. Due to their adaptable structure, chalcones can bind efficiently to various enzymes and receptors, resulting in the diverse biological applications of these compounds [2]. Chalcone is a unique template that is associated with several biological activities such as antioxidant, anticancer, anti-inflammatory, antibacterial, antifungal, antiviral, antitubercular, antimalarial, antileishmanial, antihyperglycemic, tyrosine-inhibitory, and vasorelaxant activity [3].
On the other hand, benzothiazole is a bicyclic heterocycle with N and S as hetero atoms, which possess antitumor, antimicrobial, antimalarial, antitubercular, analgesic, and anti-inflammatory properties. Benzothiazole-derived coordination compounds are of great interest, as they possess a broad spectrum of pharmacological activities [4]. For these reasons, considerable attempts are being made to design molecules integrating diverse pharmacophores. Our research group has experience in the design and synthesis of heterocyclic chalcone, which showed good cytotoxic activity [5,6]. In the present study, we focused on the design and synthesis of a molecule containing a chalcone moiety as a potent cytotoxic pharmacophore, combined with a nitrogen-donor ligand capable of forming coordination complexes with transition metals, analogous to cisplatin, which features amine ligands as non-leaving groups [7].
2. Results and Discussion
To synthesize (E)-1-(2-(pyridin-2-yl)benzo[d]thiazol-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (4), 6-acetylbenzo[d]thiazol-2(3H)-one (1) was used as a starting material in the reaction of hydrolysis with 50% KOH by heating (Scheme 1). The obtained 2-aminothiophenol 2 was cyclized with pyridine-2-carbaldehyde in DMSO at 160 °C [8]. The final product was synthesized by a base-catalyzed aldol condensation of 1-(2-(pyridin-2-yl)benzo[d]thiazol-6-yl)ethan-1-one (3) and 3,4,5-trimethoxybenzaldehyde in good yield. The structures of the new compounds 3 and 4 were confirmed by elemental analysis, FTIR spectra, 1H and 13C NMR, and HRMS. In particular, analysis of the 1H NMR spectra revealed that compound 4 is geometrically pure and adopts the E configuration, as evidenced by the coupling constant (J = 15.5 Hz) for the vinyl protons, see Supplementary Materials.
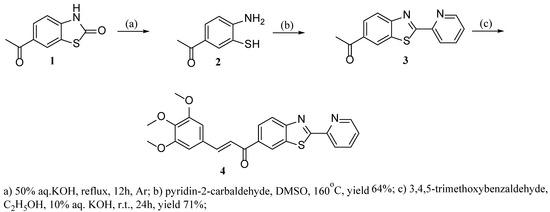
Scheme 1.
Synthesis of (E)-1-(2-(pyridin-2-yl)benzo[d]thiazol-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (4).
3. Materials and Methods
3.1. General
All chemicals were purchased from Acros Organics (Geel, Belgium). Reactions and purity of the final compound were monitored by thin-layer chromatography (TLC) on silica gel plates (Kieselgel 60 F254) using toluene/chloroform/ethylacetate (3:1:1 v/v) as the eluent.
Melting points were determined on a Kruss (Hamburg, Germany) KSN I N melting point meter. NMR spectra were recorded in DMSO-d6 on a Bruker (Billerica, Massachusetts) Avance III HD 500, operating at 500 MHz for 1H and at 125.8 MHz for 13C. Chemical shifts are given in parts per million (δ) relative to the solvent peak. Coupling constants (J) were measured in hertz (Hz). High-resolution mass spectra (HRMS) were obtained with an Orbitrap Exploris 120 Mass Spectrometer, Thermo Fisher Scientific (Waltham, Massachusetts, USA). The elemental analysis was carried on a “VARIO EL III Elemental analyzer (Hesse, Germany)” and the results for C, H, and N were within ±0.4% of the theoretical values. Analytical HPLC was carried out on an Agilent (Santa Clara, California, United States) 1100 HPLC system equipped with a binary pump and diode array detector. The column used was Agilent Eclipse Plus C18 (75 mm × 4.6 mm, 3.5 µm). The mobile phase was AcN:H2O, using a linear gradient of the binary solvent system (AcN:H2O from 30:70 to 90:10 v/v% for 7.5 min, with final time 5 min) and a flow rate of 0.500 mL⁄min.
3.2. Synthesis
3.2.1. 1-(2-(Pyridin-2-yl)benzo[d]thiazol-6-yl)ethan-1-one (3)
6-Acetylbenzo[d]thiazol-2(3H)-one (1, 2.5 g, 13 mmol) was refluxed in 50% aq. KOH (15 mL) for 12 h under argon. The mixture was then diluted with water (50 mL) and filtered. To the filtrate, acetic acid was added with vigorous stirring and cooling until it was just acidic. The obtained product was filtered, washed with water, and used without purification in the next step. A mixture of 2-amino-5-acetylthiophenol (2, 0.50 g, 3 mmol) and pyridine-2-carbaldehyde (0.32 g, 3 mmol) in DMSO (6 mL) was heated to 160 °C for 10 min. After cooling to 100 °C, water (1 mL) was added. The crystalline product was filtered and recrystallized. Yield: 64% (0.48 g).
Light yellow crystals, m.p.: 184–185° C (C2H5OH). IR (nujol): 1669, 1587, 1329, 991, 823, 783 cm−1. 1H NMR (500 MHz, DMSO-d6): δ (ppm) 2.66 (s, 3H, CH3), 7.61 (dd, 1H, pyridine. H, J = 4.9 Hz, J = 7.3 Hz), 8.03–8.08 (m, 2H, pyridine-H,), 8.14 (d, 1H, ArH, J = 8.6 Hz), 8.32 (d, 1H, ArH, J = 7.8 Hz), 8.73 (d, 1H, pyridine-H, J = 4.5 Hz), 8.82 (s, 1H, pyridine-H). 13C NMR (126 MHz, DMSO-d6): δ (ppm) 27.4, 121.1, 123.6, 124.5, 126.6, 127.1, 134.5, 136.1, 138.5, 150.4, 150.6, 156.9, 173.3, 197.6. HRMS (ESI): Found 255.0583. Calcd. for C24H20N2O4S: 255.0592 [M + H]+. Anal. calcd. for C14H10N2OS (254.31): C, 66.12; H, 3.96; N 11.02. Found: C, 66.31; H, 3.84; N, 10.98.
3.2.2. (E)-1-(2-(Pyridin-2-yl)benzo[d]thiazol-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (4)
To a mixture of 1-(2-(pyridin-2-yl)benzo[d]thiazol-6-yl)ethan-1-one (3, 153 mg, 0.6 mmol) and 3,4,5-trimethoxybenzaldehyde (137 mg, 0.7 mmol) in ethanol (6 mL), 10% aq. KOH (2 mL) was added. The obtained yellow mixture was stirred for 24 h at room temperature. The mixture was poured into 20 mL water. The crystalline product was filtered and dried. Yield: 71% (185 mg).
Light yellow crystals, m.p.: 204–206° C (CH3CN). IR (nujol): 1656, 1590, 1124, 1001, 819 cm−1. 1H NMR (500 MHz, DMSO-d6): δ (ppm) 3.73 (s, 3H, OCH3), 3.89 (s, 6H, OCH3), 7.28 (s, 2H, arom. H), 7.63–7.66 (m, 1H, pyridine H), 7.77 (d, 1H, CH=CH, J = 15.5 Hz), 8.04 (d, 1H, CH=CH, J = 15.5 Hz), 8.06–810 (m, 1H, pyridine-H), 8.25 (d, 1H, ArH, J = 8.6 Hz), 8.31 (dd, 1H, ArH, J = 8.6 Hz, J = 1.3 Hz), 8.38 (d, 1H, ArH, J = 7.8 Hz), 8.77 (d, 1H, pyridine-H, J = 4.5 Hz), 9.07 (s, 1H, pyridine-H). 13C NMR (126 MHz, DMSO-d6): δ (ppm) 56.2, 60.2, 106.7, 120.7, 121.1, 123.4, 124.0, 126.7, 126.8, 130.2, 135.0, 135.8, 138.1, 139.9, 144.9, 150.0, 150.2, 153.1, 156.5, 172.8, 188.1. HRMS (ESI): Found 433.1208. Calcd. for C24H20N2O4S: 433.1222 [M + H]+. Anal. calcd. for C24H20N2O4S (432.49): C, 66.65; H, 4.66; N 6.48. Found: C, 66.81; H, 4.37; N, 6.29.
4. Conclusions
The three-step synthetic procedure developed in this study for the preparation of 2-pyridylbenzothiazole derivatives is efficient, reproducible, and affords pure compounds without the need for further purification. The present work provides an experimental foundation for the design of analogous derivatives and the development of novel drug candidates.
Supplementary Materials
FT-IR, 1H- and 13C-NMR, and HRMS results for compounds 3 and 4 are available online; Figure S1: FT-IR spectrum of compound 3; Figure S2: FT-IR spectrum of compound 4; Figure S3: 1H-NMR spectrum of compound 3; Figure S4: 13C-NMR spectrum of compound 3; Figure S5: 1H-NMR spectrum of compound 4; Figure S6: 13C-NMR spectrum of compound 4; Figure S7: HRMS of compound 3; Figure S8: HRMS of compound 4; Figure S9: HPLC of compound 3; Figure S10: HPLC of compound 4.
Author Contributions
Conceptualization, O.I.P. and Y.B.I.; methodology, Y.B.I. and O.I.P.; writing—original draft preparation, Y.B.I. and O.I.P.; writing—review and editing, Y.B.I. and O.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
Financing provided by the University of Forestry, Sofia, Bulgaria, Project NIS-B 1395/08.05.2025, Synthesis of new hybrid molecules and study of their biological potential.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Almutairi, S.M.; Mehmood, R.; Fatima, A.; Ali, D.; Jamal, M.; Ayyaz, M.; Sarfraz, M. Chalcones from the deep: In silico medicinal chemistry and quantum chemical insights into their anticancer and anti-HIV potency. Comput. Theor. Chem. 2025, 1252, 115380. [Google Scholar] [CrossRef]
- Zhang, X.; Rakesh, K.P.; Bukhari, S.N.; Balakrishna, M.; Manukumar, H.M.; Qin, H.L. Multi-targetable chalcone analogs to treat deadly Alzheimer’s disease: Current view and upcoming advice. Bioorg. Chem. 2018, 80, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Suresh, J.; Anbazghagan, S.; Paulraj, J.; Krishnan, G.K. Heteroaryl chalcones: Mini review about their therapeutic voyage. Biomed. Prev. Nutr. 2014, 4, 451–458. [Google Scholar] [CrossRef]
- Radha, V.P.; Bhavani, G.; Vanaraj, R.; Prabakaran, M. Benzothiazole-imidazoline bearing transition metal complexes of Schiff bases: Synthesis, characterization, chemosensing, antimicrobial, anticancer and DNA cleavage activities. Inorg. Chim. Acta 2025, 588, 122860. [Google Scholar] [CrossRef]
- Ivanova, Y.B.; Momekov, G.T.; Petrov, O.I. New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5-or 6-(3-aryl-2-propenoyl)-2 (3H)-benzoxazolones. Heterocycl. Commun. 2013, 19, 23–28. [Google Scholar] [CrossRef]
- Ivanova, Y.B.; Gerova, M.S.; Momekov, G.T.; Petrov, O.I. Synthetic chalcones of 2 (3H)-benzothiazolone with potential cytotoxic activity. Comp. Rend. Acad. Bulg. Sci. 2007, 60, 641–644. [Google Scholar]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A review on metal complexes and its anti-cancer activities: Recent updates from in vivo studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef] [PubMed]
- Deligeorgiev, T.G. An Improved Method for the Preparation of 2-Aryl-,2-Hetaryl- and 2-Styrylbenzothiazoles. Dyes Pigments 1990, 12, 243–248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).