1-[4-(4-Chlorophenyl)piperazin-1-yl]-2-[(4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]ethan-1-one
Abstract
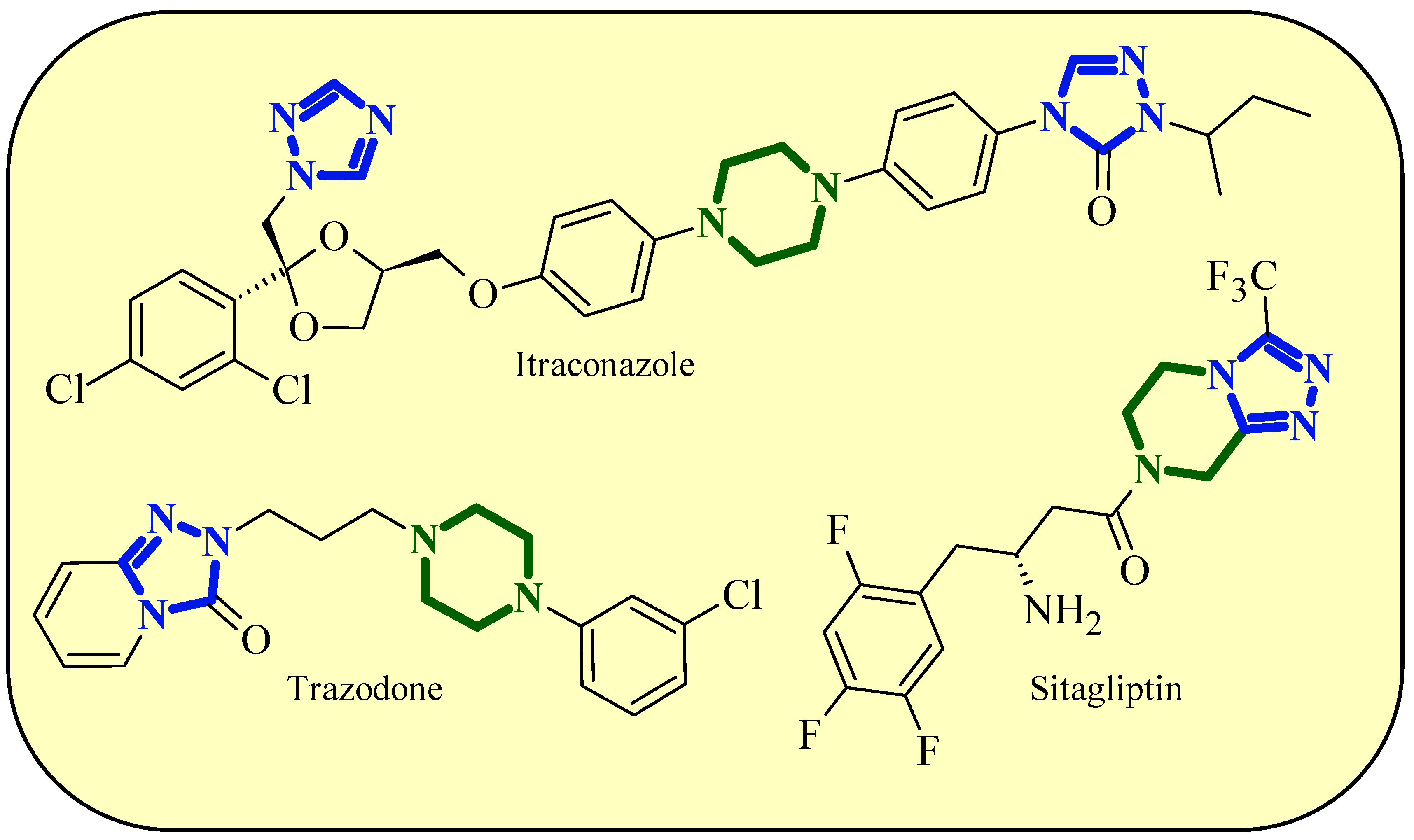
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
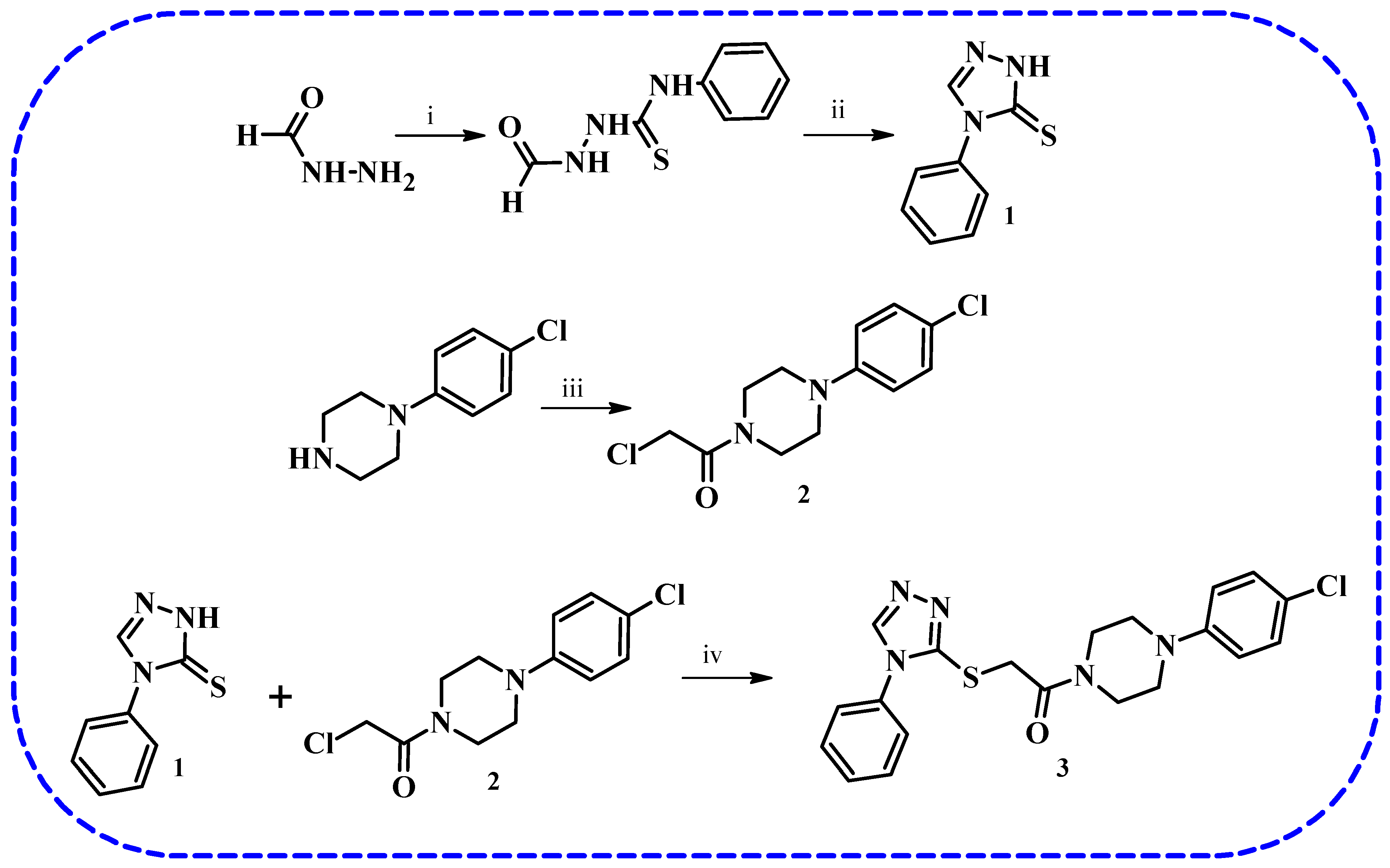
3.2. Synthesis of 4-Phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (1) and 2-Chloro-1-[4-(4-chlorophenyl)piperazin-1-yl]ethan-1-one (2)
3.3. Synthesis of 1-[4-(4-Chlorophenyl)piperazin-1-yl]-2-[(4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]ethan-1-one (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, S.C.; Moratorio de Moraes, R.S.; Tavares de Almeida Pinto, G.; Martins, M.T.M.; Antunes do Nascimento, P.; Alves Soares, D.L.; Mestre Botelho, A.B.; Cardoso Cruz, C.; Cunha, A.C. A review on chemistry and methods of synthesis of 1,2,4-triazole derivatives. Chem. Rec. 2025, 25, e202400190. [Google Scholar] [CrossRef] [PubMed]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1,2,4-triazoles as antifungal agents. BioMed Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef] [PubMed]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as important antibacterial agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- El-Sherief, H.A.M.; Youssif, B.G.M.; Bukhari, S.N.A.; Abdelazeem, A.H.; Abdel-Aziz, M.; Abdel-Rahman, H.M. Synthesis, anticancer activity and molecular modeling studies of 1,2,4-triazole derivatives as EGFR inhibitors. Eur. J. Med. Chem. 2018, 156, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, M.M.S.; Osman, N.A.; Rezq, S.; Abd El-Wahab, H.A.A.; Hassan, A.E.A.; Abdel-Fattah, H.A.; Romero, D.G.; Ghanim, A.M. Design and synthesis of novel 1,3,4-oxadiazole and 1,2,4-triazole derivatives as cyclooxygenase-2 inhibitors with anti-inflammatory and antioxidant activity in LPS-stimulated RAW264.7 Macrophages. Bioorg. Chem. 2022, 124, 105808. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Hassan, A.A.; Makhlouf, M.M.; Bräse, S. Chemistry and biological activities of 1,2,4-triazolethiones—Antiviral and anti-infective drugs. Molecules 2020, 25, 3036. [Google Scholar] [CrossRef] [PubMed]
- Korff, M.; Imberg, L.; Will, J.M.; Bückreiß, N.; Kalinina, S.A.; Wenzel, B.M.; Kastner, G.A.; Daniliuc, C.G.; Barth, M.; Ovsepyan, R.A.; et al. Acylated 1H-1,2,4-triazol-5-amines targeting human coagulation factor XIIa and thrombin: Conventional and microscale synthesis, anticoagulant properties, and mechanism of action. J. Med. Chem. 2020, 63, 13159–13186. [Google Scholar] [CrossRef] [PubMed]
- Karaküçük-Iyidoğan, A.; Başaran, E.; Tatar-Yilmaz, G.; Oruç-Emre, E.E. Development of new chiral 1,2,4-triazole-3-thiones and 1,3,4-thiadiazoles with promising in vivo anticonvulsant activity targeting GABAergic system and voltage-gated sodium channels (VGSCs). Bioorg. Chem. 2024, 151, 107662. [Google Scholar] [CrossRef] [PubMed]
- Plé, C.; Tam, H.-K.; Vieira Da Cruz, A.; Compagne, N.; Jiménez-Castellanos, J.-C.; Müller, R.T.; Pradel, E.; Foong, W.E.; Malloci, G.; Ballée, A.; et al. Pyridylpiperazine-based allosteric inhibitors of RND-type multidrug efflux pumps. Nat. Commun. 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Stępnicki, P.; Targowska-Duda, K.M.; Martínez, A.L.; Zięba, A.; Wronikowska-Denysiuk, O.; Wrobel, M.Z.; Bartyzel, A.; Trzpil, A.; Wrobel, T.M.; Chodkowski, A.; et al. Discovery of novel arylpiperazine-based DA/5-HT modulators as potential antipsychotic agents—Design, synthesis, structural studies and pharmacological profiling. Eur. J. Med. Chem. 2023, 252, 115285. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, K.; Podlewska, S.; Dichiara, M.; Gentile, D.; Patamia, V.; Rosier, N.; Mönnich, D.; Ruiz Cantero, M.C.; Karcz, T.; Łażewska, D.; et al. Structural and molecular insight into piperazine and piperidine derivatives as histamine H3 and sigma-1 receptor antagonists with promising antinociceptive properties. ACS Chem. Neurosci. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, G.M.; Rocamora, F.; Marapana, D.S.; Gnädig, N.F.; Ottilie, S.; Luth, M.R.; Worgall, T.S.; Goldgof, G.M.; Mohunlal, R.; Kumar, T.R.S.; et al. Pan-active imidazolopiperazine antimalarials target the Plasmodium falciparum intracellular secretory pathway. Nat. Commun. 2020, 11, 1780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, M.; Zhou, X.; Tang, L.; Chen, G.; Zhang, Y. Design, synthesis of combretastatin A-4 piperazine derivatives as potential antitumor agents by inhibiting tubulin polymerization and inducing autophagy in HCT116 cells. Eur. J. Med. Chem. 2024, 272, 116497. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, M.; Pitucha, M.; Wujec, M. The reaction of cyclization of thiosemicarbazide derivatives to 1,2,4-triazole or 1,3,4-thiadiazole system. Acta Pol. Pharm. 1996, 53, 31–38. [Google Scholar]
- Sharma, M.; Singh, D.; Gupta, M. Synthesis and evaluation of thiouracil derivatives as dipeptidyl peptidase IV inhibitors. Chem. Biol. Drug Des. 2013, 81, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gupta, M.; Singh, D.; Kumar, M.; Kaur, P. Synthesis, evaluation and molecular docking of thiazolopyrimidine derivatives as dipeptidyl peptidase IV inhibitors. Chem. Biol. Drug Des. 2012, 80, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Król, J.; Siwek, A.; Wujec, M.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial evaluation of new 1-{[4-(4-halogenophenyl)-4H-1,2,4-triazol-3-yl]sulfanyl}acetyl-4-substituted thiosemicarbazides and products of their cyclization. Heteroat. Chem. 2012, 23, 117–121. [Google Scholar] [CrossRef]
- Maliszewska-Guz, A.; Wujec, M.; Pitucha, M.; Dobosz, M.; Chodkowska, A.; Jagiełło-Wójtowicz, E.; Mazur, L.; Kozioł, A.E. Cyclization of 1-{[(4-methyl-4H-1,2,4-triazol-3-yl)sulfanyl]acetyl}thiosemicarbazides to 1,2,4-triazole and 1,3,4-thiadiazole derivatives and their pharmacological properties. Collect. Czech. Chem. Commun. 2005, 70, 51–62. [Google Scholar] [CrossRef]
- Kalhora, M.; Shabania, M.; Nikokarb, I.; Banisaeed, S.R. Synthesis, characterization and antibacterial activity of some novel thiosemicarbazides, 1,2,4-triazol-3-thiols and their S-substituted derivatives. Iran. J. Pharm. Res. 2015, 14, 67–75. [Google Scholar]
- Fizer, M.; Slivka, M.; Korol, N.; Fizer, O. Identifying and explaining the regioselectivity of alkylation of 1,2,4-triazole-3-thiones using NMR, GIAO and DFT methods. J. Mol. Struct. 2021, 1223, 128973. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzał, W.; Sobstyl, J.; Trotsko, N. 1-[4-(4-Chlorophenyl)piperazin-1-yl]-2-[(4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]ethan-1-one. Molbank 2025, 2025, M2097. https://doi.org/10.3390/M2097
Drzał W, Sobstyl J, Trotsko N. 1-[4-(4-Chlorophenyl)piperazin-1-yl]-2-[(4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]ethan-1-one. Molbank. 2025; 2025(4):M2097. https://doi.org/10.3390/M2097
Chicago/Turabian StyleDrzał, Wiktoria, Jarosław Sobstyl, and Nazar Trotsko. 2025. "1-[4-(4-Chlorophenyl)piperazin-1-yl]-2-[(4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]ethan-1-one" Molbank 2025, no. 4: M2097. https://doi.org/10.3390/M2097
APA StyleDrzał, W., Sobstyl, J., & Trotsko, N. (2025). 1-[4-(4-Chlorophenyl)piperazin-1-yl]-2-[(4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]ethan-1-one. Molbank, 2025(4), M2097. https://doi.org/10.3390/M2097





