Abstract
We have successfully synthesized the dichloro-bis[(Z)-1-styryl-benzimidazole]-zinc(II) complex, which was fully characterized by IR, elemental analysis, and mass and NMR spectroscopy. The solid-state structure definitively shows that two benzimidazole moieties are coordinated to the zinc atom, which adopts a tetrahedral geometry.
1. Introduction
Azole heterocycles form complexes with transition metals with remarkable ease [1]. Benzimidazole derivatives are particularly noteworthy, as they easily coordinate with the first row of transition metals to form complexes of the type [MX2L2], in which L represents a N-substituted benzimidazole, X a halogen, and M a metal like manganese [2], iron [3], cobalt [4], nickel [5], copper [2], or zinc [5].
In this context, zinc complexes of the type [ZnCl2L2] are particularly interesting due to their promising optical [6,7], catalytic [8], and biological properties, including antioxidant [9], antidiabetic [10,11], antimicrobial [12], and anticancer [13] capabilities.
Based on the above considerations and our extensive experience in coordinating N-alkyl benzimidazole derivatives to transition metals [12,14], we now report the synthesis of a zinc(II) complex 2 substituted with two styryl-benzimidazole moieties (Figure 1).
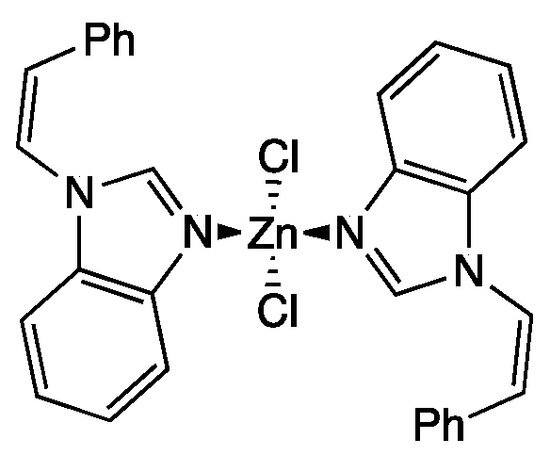
Figure 1.
Targeted zinc(II) complex 2.
2. Results and Discussion
The targeted dichloro-bis[(Z)-1-styryl-benzimidazole]-zinc(II) complex was obtained by mixing two equivalents of (Z)-1-styryl-benzimidazole ligand 1 with one equivalent of ZnCl2 precursor in ethanol. After 4 h at room temperature, the reaction mixture was filtered and the zinc(II) complex was isolated in 92% yield (Scheme 1).
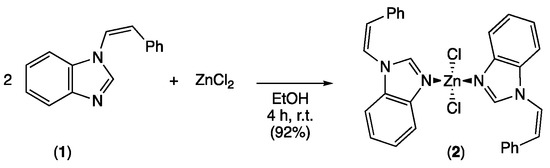
Scheme 1.
Synthesis of zinc complex 2.
The complex was fully characterized by infrared spectroscopy (FT-IR), multi-nuclear magnetic resonance spectroscopy (1H and 13C NMR), mass spectroscopy, and elemental analysis (see Figures S1–S4 in Supplementary Materials).
In comparison with the ligand 1 (ν(CN) 1481 cm−1), the FT-IR spectra of the complex 2 (ν(CN) 1500 cm−1) displayed a redshifted band for the C=N vibration of 19 cm−1, which is characteristic of an azole coordinated to a transition metal [12]. The NMR analysis realized on the zinc(II) complex revealed a downfield singlet (δ = 8.43 ppm) for the NCHN protons with regard to the ligand (δ = 7.74 ppm) (Figure 2). The cis-stereochemistry of the external carbon–carbon double bonds was confirmed by the coupling constants between olefinic protons (3JHH of 9.0 Hz).
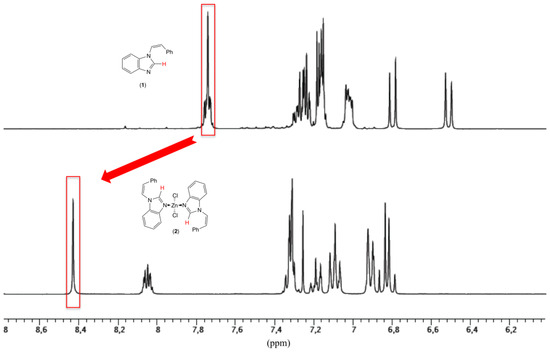
Figure 2.
1H NMR spectra of 1-styryl-benzimidazole (1) (top) and its corresponding dichloro-bis[(Z)-1-styryl-benzimidazole]-zinc(II) (2) complex (bottom) in CDCl3.
The formation of the [ZnCl2(1)2] complex is unambiguously deduced from the mass spectrum, which shows intense peaks at m/z = 539.10 corresponding to the [M − Cl]+ cation with the expected isotopic profiles. Furthermore, the elemental analysis, carried out on dried crystals, is in accordance with the presence of two styryl-benzimidazole moieties per zinc atom.
The formation of the dichloro-bis[(Z)-1-styryl-benzimidazole]-zinc(II) complex (2) was confirmed by a single-crystal X-ray diffraction study. Suitable crystals were obtained by the slow diffusion of diethyl ether into a dichloromethane solution of zinc(II) complex. The complex crystallized in the triclinic space group P-1.
Figure 3 shows that the zinc atom in complex 2 adopts a slightly distorted tetrahedral geometry with L-Zn-L’ angles in the range 103.41(9) to 120.10(6)° (with L and L’ = N or Cl). Two nitrogen atoms of the two benzimidazole moieties (Zn1-N1 2.011(3) and Zn1-N3 2.048(3) Å) and two chloride atoms (Zn1-Cl1 2.2266(16) and Zn1-Cl2 2.224(2) Å) are clearly coordinated to the metal center (Table 1). These distances and angles are in agreement with those reported for the dichloro-{N,N-bis[(1-allyl-1H-benzimidazol-2-yl) methyl]aniline}-zinc(II) or the dichloro-bis{2-(thiophen-2-yl)-1-[(thiophen-2-yl)methyl]- 1H-benzimi-dazole}-zinc(II) complexes [15,16].
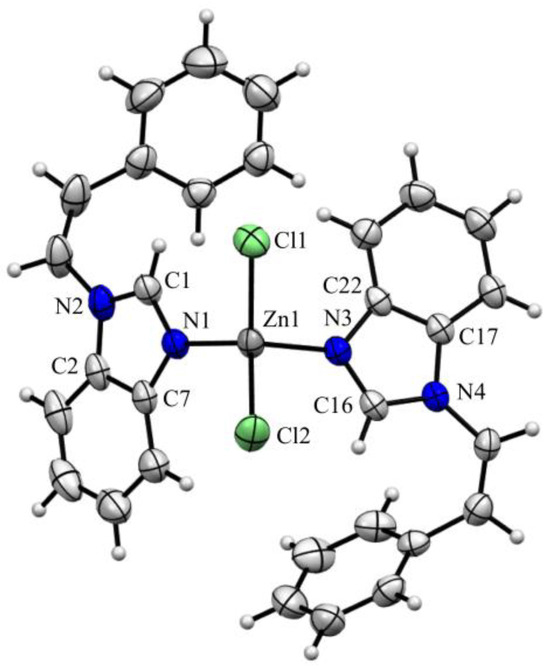
Figure 3.
ORTEP drawing of the zinc(II) complex 2 (50% probability thermal ellipsoids).

Table 1.
Important bond lengths and angles.
It is interesting to note that, in the structure of the zinc complex 2, the point group symmetry is C2 with the two phenyl rings pointing to opposite directions, whereas the point group symmetry of a closely related cobalt complex, the dichloro-bis(1-cinnamyl-benzimidazole)-cobalt(II), recently reported by our group is Cs with a pseudo mirror plane and the two phenyl rings pointing in the same direction [17]. The two benzimidazole rings are inclined at a dihedral angle of 65.61° due to intermolecular interactions with a second molecule of the complex. For ligand 1, the phenyl groups are oblique to the benzimidazoles with dihedral angles of 56.89° and 60.99°, respectively.
3. Materials and Methods
3.1. General
The manipulations were carried out under dry argon with dried solvents. Routine 1H and 13C{1H} spectra were recorded with AC 300 and 500 Bruker FT instruments. Chemical shifts and coupling constants are reported in ppm and Hz, respectively. 1H and 13C NMR spectra were recorded in CDCl3 and they referenced residual protonated solvent (δ = 7.26 and 77.16 ppm, respectively). Mass spectra were recorded on a Bruker MicroTOF spectrometer (ESI-TOF). Infrared spectra were recorded on a Bruker ATR FT-IR Alpha-P spectrometer. Elemental analyses were carried out by the Service de Microanalyse, Institut de Chimie, Université de Strasbourg. (Z)-1-Styryl-benzimidazole (1) was prepared according to an adapted published procedure [18].
3.2. Procedure for the Preparation Dichloro-bis[(Z)-1-styryl-benzimidazole]-zinc(II) (2)
In a Schlenk tube under an inert atmosphere of argon, a solution of ZnCl2 (0.093 g, 0.68 mmol) and (Z)-1-styryl-benzimidazole (0.300 g, 1.36 mmol) in ethanol (10 mL) was stirred at room temperature. After 4 h, the formed precipitate was filtered, washed with diethylether (3 × 10 mL), and dried under vacuum to give complex 2 as white solid in 92% yield (0.362 g). FT-IR: ν(CN) 1500 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.43 (s, 2H, NCHN), 8.09–8.03 (m, 2H, arom CH of C7H5N2), 7.36–7.28 (m, 6H, arom CH of C7H5N2), 7.22–7.17 (m, 2H, arom CH of C6H5), 7.12–7.07 (m, 4H, arom CH of C6H5), 6.94–6.90 (m, 4H, arom CH of C6H5), 6.86 (d, 2H, NCH=CHPh, 3JHH = 9.0 Hz), 6.81 (d, 2H, NCH=CHPh, 3JHH = 9.0 Hz); 13C{1H} NMR (126 MHz, CDCl3): δ = 143.78 (s, NCHN), 139.17, 132.36, 132.33, 129.37, 129.16, 128.51, 125.34, 125.10, 118.97, 111.58 (10 s, arom Cs), 130.22 (s, NCH=CHPh), 119.35 (s, NCH=CHPh) ppm. MS (ESI-TOF): m/z = 539.10 [M − Cl]+ (expected isotopic profile). Elemental analysis (%): calcd for C30H24N4ZnCl2 (576.83): C: 62.47; H: 4.19; N: 9.71; found C: 62.04; H: 4.15 N: 9.64.
3.3. X-Ray Crystal Structure Analysis of Complex 2
Single crystals of zinc(II) complex 2, suitable for X-ray analysis, were obtained by slow diffusion of Et2O into a CH2Cl2 solution of the complex. The samples were studied on a Bruker APEX-II CCD, using Mo-Kα radiation (λ = 0.71073 Å). The structures were solved with SHELXT-2018/2 [19] and the structure was refined with SHELXL-2019/3 [20]. Crystal Data for C30H24Cl2N4Zn (M = 576.80 g/mol): triclinic, space group P-1 (no. 2), a = 10.386(10) Å, b = 10.503(10) Å, c = 14.167(14) Å, α = 97.98(2)°, β = 107.95(2)°, γ = 108.65(3)°, V = 1344(2) Å3, Z = 2, T = 173(2) K, μ(Mo-Kα) = 1.139 mm−1, Dcalc = 1.425 g cm−3, 44,075 reflections collected (1.566 ≤ θ ≤ 25.680°), and 5093 unique (Rint = 0.0676, Rsigma = 0.0404) which were used in all calculations. The final R1 was 0.0354 (I > 2.0 δ(I)) and wR2 was 0.0895 (all data). CCDC 2385094 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
4. Conclusions
In summary, we successfully synthesized and characterized the new dichloro-bis[(Z)-1-styryl-benzimidazole]-zinc(II) complex. Its composition was confirmed by an X-ray study, which clearly showed the presence of two benzimidazole units coordinated to the zinc atom in a slightly distorted tetrahedral conformation.
Supplementary Materials
Characterizing data of dichloro-bis[(Z)-1-styryl-benzimidazole]-zinc(II) (2) with: Figure S1. FT-IR spectrum; Figure S2. Mass spectrum (ESI-TOF); Figure S3. 1H NMR spectrum (CDCl3); Figure S4. 13C{1H} NMR spectrum (CDCl3); Figure S5. Packing pattern in the crystal structure of complex 2; and Figure S6. Self-organized structure of 2 via CH-π interactions (in red).
Author Contributions
Conceptualization, N.Ş. and D.S.; methodology, N.Ş. and D.S.; validation, N.Ş. and D.S.; formal analysis, N.Ş. and D.S.; investigation, N.Ş.; resources, D.S.; data curation, N.Ş. and D.S.; writing—original draft preparation, N.Ş. and D.S.; writing—review and editing, D.S.; supervision, İ.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Contreras, R.; Flores-Parraa, A.; Mijangos, E.; Téllez, F.; López-Sandoval, H.; Barba-Behrens, N. From mono to polydentate azole and benzazole derivatives, versatile ligands for main group and transition metal atoms. Coord. Chem. Rev. 2009, 253, 1979–1999. [Google Scholar] [CrossRef]
- Broughton, V.; Bernardinelli, G.; Williams, A.F. Tetrahedral coordination by a seven-membered chelate ring. Inorganica Chim. Acta 1998, 275–276, 279–288. [Google Scholar] [CrossRef]
- Folkertsma, E.; van der Lit, J.; Di Cicco, F.; Lutz, M.; Gebbink, R.J.M.K.; Swart, I.; Moret, M.-E. Combination of scanning probe microscopy and coordination chemistry: Structural and electronic study of bis(methylbenzimidazolyl)ketone and its iron complex. ACS Omega 2017, 2, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Tarte, N.H.; Woo, S.I.; Cui, L.; Gong, Y.-D.; Hwang, Y.H. Novel non-chelated cobalt(II) benzimidazole complex catalysts: Synthesis, crystal structures and cocatalyst effect in vinyl polymerization of norbornene. J. Organomet. Chem. 2008, 693, 729–736. [Google Scholar] [CrossRef]
- Maldonado-Rogado, M.A.; Vinuelas-Zahínos, E.; Luna-Giles, F.; Bernalte-García, A. Nickel(II) and zinc(II) complexes with N-(5,6-dihydro-4H-1,3-thiazin-2-yl)-2-aminobenzimidazole (BzTz): Synthesis, spectral and structural characterization. Polyhedron 2007, 26, 3112–3120. [Google Scholar] [CrossRef]
- Smirnova, K.S.; Lider, E.V.; Kozlova, S.G.; Sukhikh, T.S.; Kuratieva, N.V.; Pozdniakov, I.P.; Potapova, A.S. Zinc complexes with 1-(1H-benzimidazol-1-ylmethyl)-1H-benzotriazole: The structure, quantum chemical calculations, and luminescence properties. Russ. Chem. Bull. 2020, 69, 1873–1883. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Deng, Y.; Huang, Y.; Chen, Y.F.; Liao, B. Terminal anion induced zinc(II) mononuclear complexes trans-to-cis isomerization regulate photoluminescence properties and its solution behavior. Polyhedron 2019, 174, 114158. [Google Scholar] [CrossRef]
- Milani, J.L.S.; de Almeida Bezerra, W.; Valdo, A.K.S.M.; Martins, F.T.; de Melo Camargo, L.T.F.; Carvalho-Silva, V.H.; dos Santos, S.S.; Cangussu, D.; das Chagas, R.P. Zinc complexes with 1,2-disubstituted benzimidazole ligands: Experimental and theoretical studies in the catalytic cycloaddition of CO2 with epoxides. Polyhedron 2019, 173, 114134. [Google Scholar] [CrossRef]
- Xia, X.; Xia, L.; Zhang, G.; Xu, J.; Wang, C.; Wu, Y.; Zhao, K.; Wu, H. Preparation, structure and antioxidant property of manganese(II) and zinc(II) complexes with bis(N-ethylbenzimidazol-2-ylmethyl)allylamine. J. Coord. Chem. 2020, 73, 3322–3331. [Google Scholar] [CrossRef]
- Wang, X.; Ling, N.; Che, Q.-T.; Zhang, Y.-W.; Yang, H.-X.; Ruan, Y.; Zhao, T.-T. Synthesis, structure and biological properties of benzimidazole-based Cu(II)/Zn(II) complexes. Inorg. Chem. Commun. 2019, 105, 97–101. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Zhou, T.; Fang, X.; Yang, H. Novel benzotriazole-benzimidazole metal complexes: Structure-activity relationship, synthesis, characterization, and antidiabetic activity. J. Mol. Struct. 2023, 1292, 136141. [Google Scholar] [CrossRef]
- Şahina, N.; Üstün, E.; Özdemir, İ.; Günal, S.; Özdemir, N.; Bülbülg, H.; Gürbüz, N.; Özdemir, İ.; Sémeril, D. Antimicrobial activities of bis-(N-alkylbenzimidazole)-cobalt(II) and zinc(II) complexes. Inorg. Chem. Commun. 2023, 157, 111396. [Google Scholar] [CrossRef]
- Su, W.-Y.; Pan, R.-K.; Song, J.-L.; Li, G.-B.; Liu, S.-G. Synthesis, crystal structures and cytotoxic activity of two zinc(II) complexes derived from benzimidazole derivatives. Polyhedron 2019, 161, 268–275. [Google Scholar] [CrossRef]
- Üstün, E.; Şahin, N.; Özdemir, İ.; Günal, S.; Gürbüz, N.; Özdemir, İ.; Sémeril, D. Design, synthesis, antimicrobial activity and molecular docking study of cationic bis-benzimidazole-silver(I) complexes. Arch. Pharm. 2023, 356, e2300302. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, H.; Wang, F.; Peng, H.; Cui, Y.; Li, Y.; Zhang, Y. Zinc(II) and Co(II) complexes based on bis(N-allylbenzimidazol-2-ylmethyl)aniline: Synthesis, crystal structures and antioxidative activity. J. Chem. Res. 2015, 39, 76–81. [Google Scholar] [CrossRef]
- Milani, J.L.S.; Oliveira, I.S.; Dos Santos, P.A.; Valdo, A.K.S.M.; Martins, F.T.; Cangussu, D.; Das Chagas, R.P. Chemical fixation of carbon dioxide to cyclic carbonates catalyzed by zinc(II) complex bearing 1,2-disubstituted benzimidazole ligand. Chin. J. Catal. 2018, 39, 245–249. [Google Scholar] [CrossRef]
- Şahin, N.; Özdemir, İ.; Sémeril, D. Dichloro-bis(1-cinnamyl-benzimidazole)-cobalt(II). Molbank 2024, 2024, M1911. [Google Scholar] [CrossRef]
- Reddy, V.P.; Iwasaki, T.; Kambe, N. Synthesis of imidazo and benzimidazo[2,1-a]-isoquinolines by rhodium-catalyzed intramolecular double C-H bond activation. Org. Biomol. Chem. 2013, 11, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).