Abstract
Diffractic acid 1 is a secondary metabolite of depside lichens with antibacterial and insecticidal properties, and anticancer, hepatoprotective and antiviral activities. Novel diffractaic acid derivatives containing a 1,2,4-oxadiazole ring with an aryl substituent have been synthesized by the reaction of diffractaic acid with amidoximes.
1. Introduction
Diffractaic acid 1 (Figure 1) is a secondary metabolite of lichens and belongs to the class of depsides. It is known to have moderate antibacterial and insecticidal properties, and anticancer, hepatoprotective and antiviral activities [1,2,3]. Diffractaic acid 1 is known to exhibit significant activity against the RSV A2 strain at EC50 = 4.8 μM with relatively low cytotoxicity (CC50 = 221.9 μM). The respiratory syncytial virus (RSV) is a common cause of the seasonal onset of acute respiratory illness in the general population. Elderly people and children under two years of age, as well as people with a weakened immune system or with chronic lung and heart diseases, are prone to infections affecting not only the upper but also the lower respiratory tracts, with the development of severe bronchiolitis and pneumonia, potentially leading to fatal outcomes [4,5,6]. Earlier, we found that the benzyl ester of diffractaic acid 2 is more active against respiratory syncytial virus than the original compound 1 (EC50 = 0.3 μM) [7] (Figure 1).
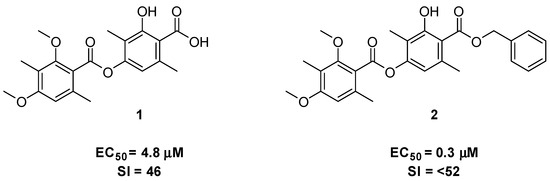
Figure 1.
Diffractaic acid 1 and benzyl diffractate 2.
The aim of the present work was to synthesize new diffractaic acid derivatives containing a bioisostere of the ester group-1,2,4-oxadiazole. Such an alternative may also contribute to the increase in metabolic stability of the compounds [8,9].
2. Results and Discussion
Diffractaic acid 1 was obtained from a mixture of lichens from the genus Usnea (Altai, Russian Federation, 2006) according to the method described in Ref. [7]. The spectrum of the obtained compound was consistent with the literature data [1,7].
The target compounds 5a,b were obtained by the reaction of diffractaic acid 1 with amidoximes under conditions of activation of the carboxyl group by CDI action according to the procedure adapted from Ref. [10]. Amidoximes 4a,b were obtained by reaction of nitriles 3a,b with hydroxylamine hydrochloride and sodium hydrogen carbonate in ethanol under reflux. Compounds 4a,b were isolated in 95% yield. (Scheme 1). The spectral data coincide with the data described in Ref. [11].
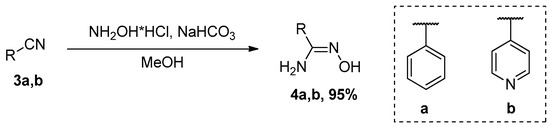
Scheme 1.
Synthesis of amidoximes 4a,b.
For the synthesis of 1,2,4-oxadiazoles 5a,b, diffractaic acid 1 was reacted with carbonyl diimidazole (CDI) for 2 h, and then the corresponding amidoxime 4a,b was added to the reaction mixture (Scheme 2). The resulting mixture was stirred under heating for 1 day, after which potassium hydroxide was added in catalytic amounts. The reaction was carried out for a further 1 day. The target compounds 5a,b were isolated by column chromatography in 43 and 31% yields, respectively.
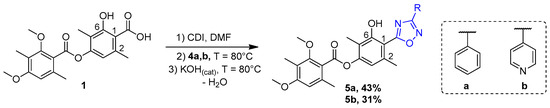
Scheme 2.
Synthesis of 1,2,4-oxadizoles 5a,b.
The structure of the new compounds 5a,b was confirmed using NMR 1H and 13C and HRMS. In the 1H NMR spectra, a change in the chemical shift of the proton signal of the aromatic cycle bonded to the 1,2,4-oxadiazole moiety from 6.60 for diffractaic acid 1 to 6.72–6.73 for compounds 5a,b was observed. There was also a change in the chemical shifts of both methyl groups of the same cycle from 2.17 and 2.58 (compound 1) to 2.25 and 2.76 (compounds 5a,b), respectively. There was no significant change in the chemical shift of the hydroxyl group. In the 13C spectra, a chemical shifts of the entire benzene ring was observed. The most noticeable change was observed in the signals belonging, probably, to one, two and six carbon atoms (numbering is indicated in Scheme 2). The change in chemical shifts occurred from 109, 140, 163 and to 105, 137 and 160, respectively. Signals characteristic of the 1,2,4-oxadiazole cycle were also observed at 166 and 176.
The structure of compound 5a was confirmed by an X-ray diffraction analysis (Figure 2). The diphenyloxadiazole fragment is flat with a range of ±0.065 Å. The geometry of this fragment is close to that of a similar 4-(5-(4-benzoyloxyphenyl)-1,2,4-oxadiazol-3-yl) phenyl benzoate compound [12].
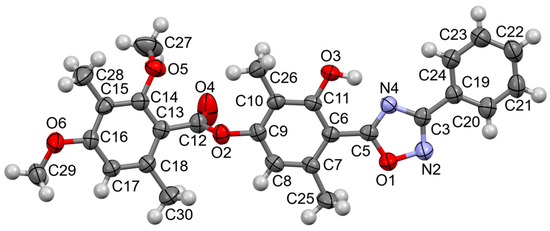
Figure 2.
The molecule of compound 5a in the thermal ellipsoids of the 50% probability level and intramolecular OH…N (O3…N4 2.601(2) Å, H…N 1.72(3) Å, O-H…N 154(3)°) hydrogen bond.
3. Materials and Methods
The analytical and spectral studies were conducted at the Chemical Service Center for the collective use of the Siberian Branch of the Russian Academy of Science.
The 1H and 13C-NMR spectra for solutions of the compound in CDCl3 were recorded on a Bruker AV-400 spectrometer (Bruker Corporation, Germany; operating frequencies 400.13 MHz for 1H and 100.61, for 13C). The residual signals of the solvent were used as references (δH 7.24, δC 76.90 for CDCl3). The mass spectra (ionizing electron energy 70 eV) were recorded on a DFS Thermo Scientific high-resolution mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Electron impact ionization was used in the measurement of mass spectra. Thin-layer chromatography was performed on TLC Silica gel 60F254 (Merck KGaA, Darmstadt, Germany). Synthetic starting materials, reagents and solvents were purchased from Sigma Aldrich, Acros Organics and AlfaAesar (95–99% purity). Reagent grade solvents were redistilled prior to the experiments.
Diffractaic acid 1 was obtained from a mixture of lichens from the genus Usnea (Altai, Russian Federation, 2006) according to the method described in Ref. [7]
Amidoximes 4a,b were prepared according to the procedure described in Ref. [11]
The Synthesis of Compounds 5a,b
Diffractaic acid 1 (1 eq., 2.6 mmol) and CDI (0.9 eq., 2.4 mmol) were dissolved in 5 mL of DMF. The reaction mixture was stirred at room temperature for 2 h. After that, amidoxime 4a,b (0.9 eq., 2.4 mmol) was introduced into the solution. The reaction mixture was stirred at 70 °C for 1 day, followed by addition of KOH in catalytic amounts to the reaction mixture, and stirred again under heat for 1 day. The course of the reaction was controlled using TLC. After the reaction was completed, the mixture was diluted with water, and 1M HCl was added to adjust the pH to 5. The mixture was extracted with a hexane-ethyl acetate (1:1) mixture (3 × 10 mL). The combined extract was dried over Na2SO4. The product was isolated by column chromatography on silica gel (eluent: hexane-ethyl acetate 6:1 for compound 5a and 1:1 for compound 5b).
3-hydroxy-2,5-dimethyl-4-(3-phenyl-1,2,4-oxadiazole-5-yl)phenyl 2,4-dimethoxy-3,6-dimethylbenzoate (5a)
White powder. Yield: 43%. M. p. 155.7–158.1 °C. 1H NMR (CDCl3, δ): 2.15 and 2.26 (6H, s, CH3), 2.47 and 2.76 (6H, s, CH3), 3.85 (6H, s, OCH3), 6.53 (1H, s, Ar-H), 6.71 (1H, s, Ar-H), 7.54 (3H, m, Ar-H), 8.12 (2H, d, Ar-H), 12.08 (1H, s, OH). 13C NMR (CDCl3, δ): 8.7, 9.3, 20.1, 22.9, 55.5, 62.0, 107.9, 116.5, 117.3, 117.4, 119.4, 125.5, 127.4, 128.6, 128.9, 131.5, 135.3, 137.9, 153.2, 157.0, 159.4, 159.9, 165.5, 166.1, 175.8. HRMS: m/z 474.1783 [M]+ (calcd for (C27H26O6N2)+: 474.1785).
3-hydroxy-2,5-dimethyl-4-(3-(pyridine-4-yl)-1,2,4-oxadiazole-5-yl)phenyl 2,4-dimethoxy-3,6-dimethylbenzoate (5b)
White powder. Yield: 31%. M. p. 206.7–208.6 °C. 1H NMR (CDCl3, δ): 2.15 and 2.25 (6H, s, CH3), 2.47 and 2.76 (6H, s, CH3), 3.85 (6H, s, OCH3), 6.53 (1H, s, Ar-H), 6.73 (1H, s, Ar-H), 7.98 (2H, d, Ar-H), 8.82 (2H, d, Ar-H), 11.75 (1H, s, OH). 13C NMR (CDCl3, δ): 8.7, 9.2, 20.0, 22.8, 55.5, 61.9, 107.9, 116.7, 117.3, 117.5, 119.2, 121.1, 133.0, 135.2, 137.9, 150.5, 153.5, 157.0, 159.3, 159.9, 164.0, 166.0, 176.4. HRMS: m/z 475.1736 [M]+ (calcd for (C26H25O6N3)+: 475.1738).
Crystal data for compound 5a: C27H26N2O6, M = 474.50 g·mol−1, crystal size 0.10 × 0.34 × 0.63 mm3, monoclinic, space group C2/c: a = 31.780(2) Å, b = 8.0955(4) Å, c = 22.3532(13) Å, β = 123.1697(18)°, V = 4813.9(5) Å3, Z = 8, Dcalc = 1.309 g/cm3, T = 296 K, 18448 reflections measured (5.2° ≤ 2θ ≤ 52.0°), 4705 unique (Rint = 0.0578). These data were used in all calculations. The final R1 = 0.0555 (3206 I > 2σ(I)) and wR2 = 0.1812 (all data); GOF = 1.006. Largest diff. peak/hole/e Å−3 0.22/−0.20. The data were deposited at the Cambridge Crystallographic Data Centre as CCDC 2386869 (Supplementary Materials). The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/structures (accessed on 26 September 2024).
Supplementary Materials
The following supporting information can be downloaded. Figures S1 and S4: 1H NMR spectra of compounds 5a,b; Figures S2 and S5: 13C NMR spectra of compounds 5a,b. Figures S3 and S6 Mass spectra of compound 5a,b.
Author Contributions
Conceptualization, A.F. and O.L.; data curation, O.L.; investigation, A.D., A.F. and Y.G.; supervision, N.S.; writing—original draft, A.F. and A.D.; writing—review and editing, O.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (grant No. 24-13-00134).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for their assistance with the spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bayir, Y.; Odabasoglu, F.; Cakir, A.; Aslan, A.; Suleyman, H.; Halici, M.; Kazaz, C. The inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine Int. J. Phytother. Phytopharm. 2006, 13, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.B.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q.F. Antimycobacterial activity of lichen substances. Phytomedicine Int. J. Phytother. Phytopharm. 2010, 17, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, I.D.; Ozaslan, M.; Kilic, I.H.; Guler, I.; Uyar, C.; Tuter, D.; Kazanci, U.; Aslan, A.; Cakir, A.; Gezici, S. Hepatoprotective effect of diffractaic acid on carbon tetrachloride-induced liver damage in rats. Biotechnol. Biotechnol. Equip. 2015, 29, 1011–1016. [Google Scholar] [CrossRef]
- Rezaee, F.; Linfield, D.T.; Harford, T.J.; Piedimonte, G. Ongoing developments in RSV prophylaxis: A clinician’s analysis. Curr. Opin. Virol. 2017, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, T.; Sarnari, C.; Gaito, R.; Tabarrini, O.; Manfroni, G. Recent progress toward the discovery of small molecules as novel anti-respiratory syncytial virus agents. J. Med. Chem. 2024, 67, 11543–11579. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, A.S.; Ismangulova, D.U.; Diveikina, A.A.; Komarova, N.I.; Luzina, O.A.; Shtro, A.A.; Galochkina, A.V.; Nikolaeva, Y.V.; Klabukov, A.M.; Krivitskaya, V.Z.; et al. Diffractaic acid and its ethers as anti-respiratory syncytial virus agents. Med. Chem. Res. 2024, 33, 677–686. [Google Scholar] [CrossRef]
- Pitasse-Santos, P.; Sueth-Santiago, V.; Lima, M.E.F. 1,2,4- and 1,3,4-oxadiazoles as scaffolds in the development of antiparasitic agents. J. Braz. Chem. Soc. 2018, 29, 435–456. [Google Scholar] [CrossRef]
- Rosa, M.; Morcelli, A.; Lobo, V. 1,2,4-oxadiazole: A brief review from the literature about the synthesis and pharmacological applications. Visão Acad. 2015, 16, 130–157. [Google Scholar] [CrossRef]
- Reutskaya, E.; Osipyan, A.; Sapegin, A.; Novikov, A.S.; Krasavin, M. Rethinking hydrolytic imidazoline ring expansion: A common approach to the preparation of medium-sized rings via side-chain insertion into [1.4]oxa- and [1.4]thiazepinone scaffolds. J. Org. Chem. 2018, 84, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.V.S.; Nascimento, A.K.P.; Caiana, R.R.A.; Santos, C.S.; Oliveira, W.A.; Menezes, P.H.; Freitas, J.C.R. Synthesis and antifungal activity of new O-alkylamidoximes. Biosci. J. 2021, 37, e37049. [Google Scholar] [CrossRef]
- Manjunath, B.C.; Kumar, S.M.; Kumar, K.S.V.; Prabhuswamy, M.; Sadashiva, M.P.; Lokanath, N.K. 5-(4-Benzoyl-oxyphen-yl)-1,2,4-oxa-diazol-3-yl]phenyl benzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2013, 69, 0543. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).