[(N-benzamidomethyl)(N-benzoyl)amino]methyltriphenylphosphonium Tetrafluoroborate
Abstract
1. Introduction
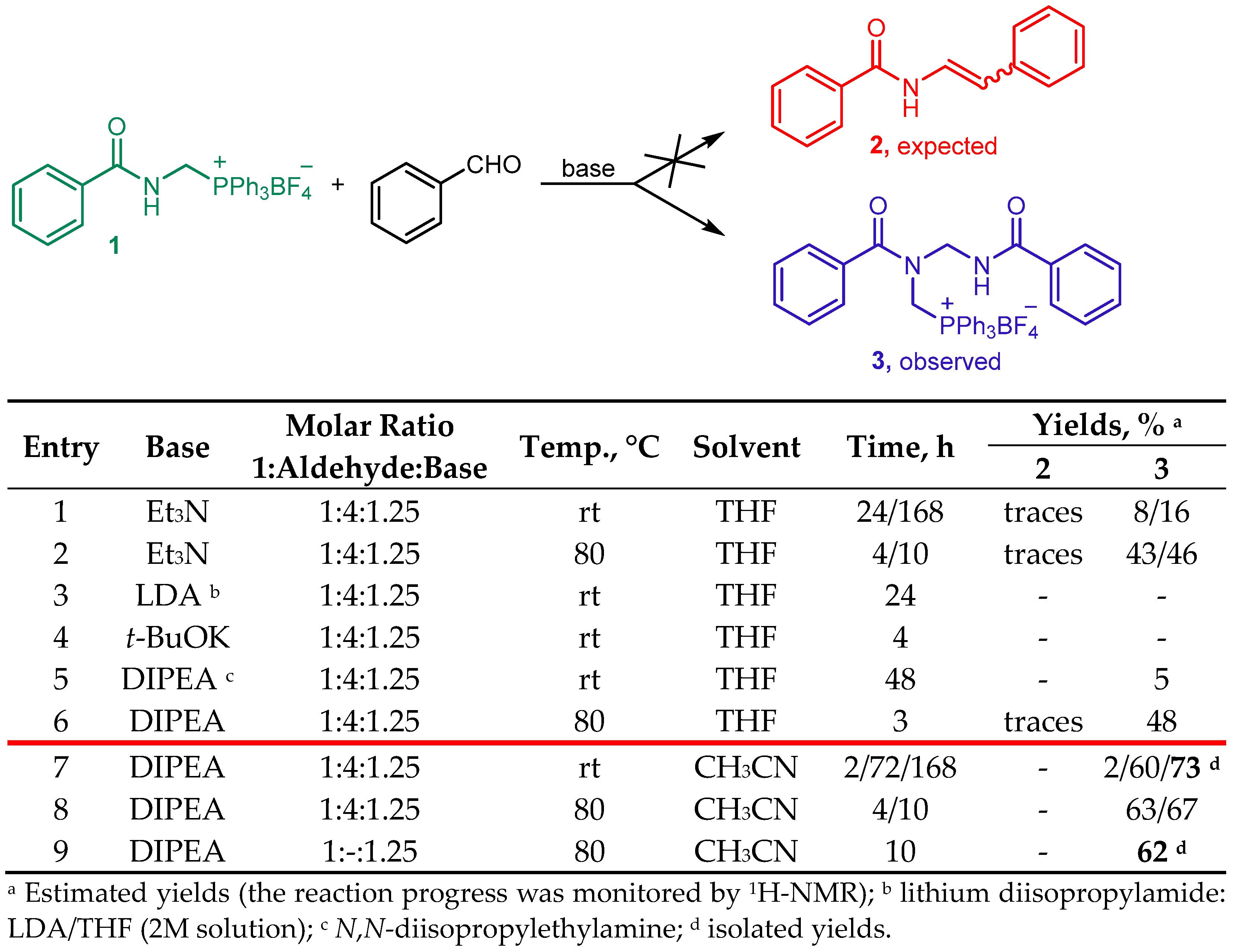
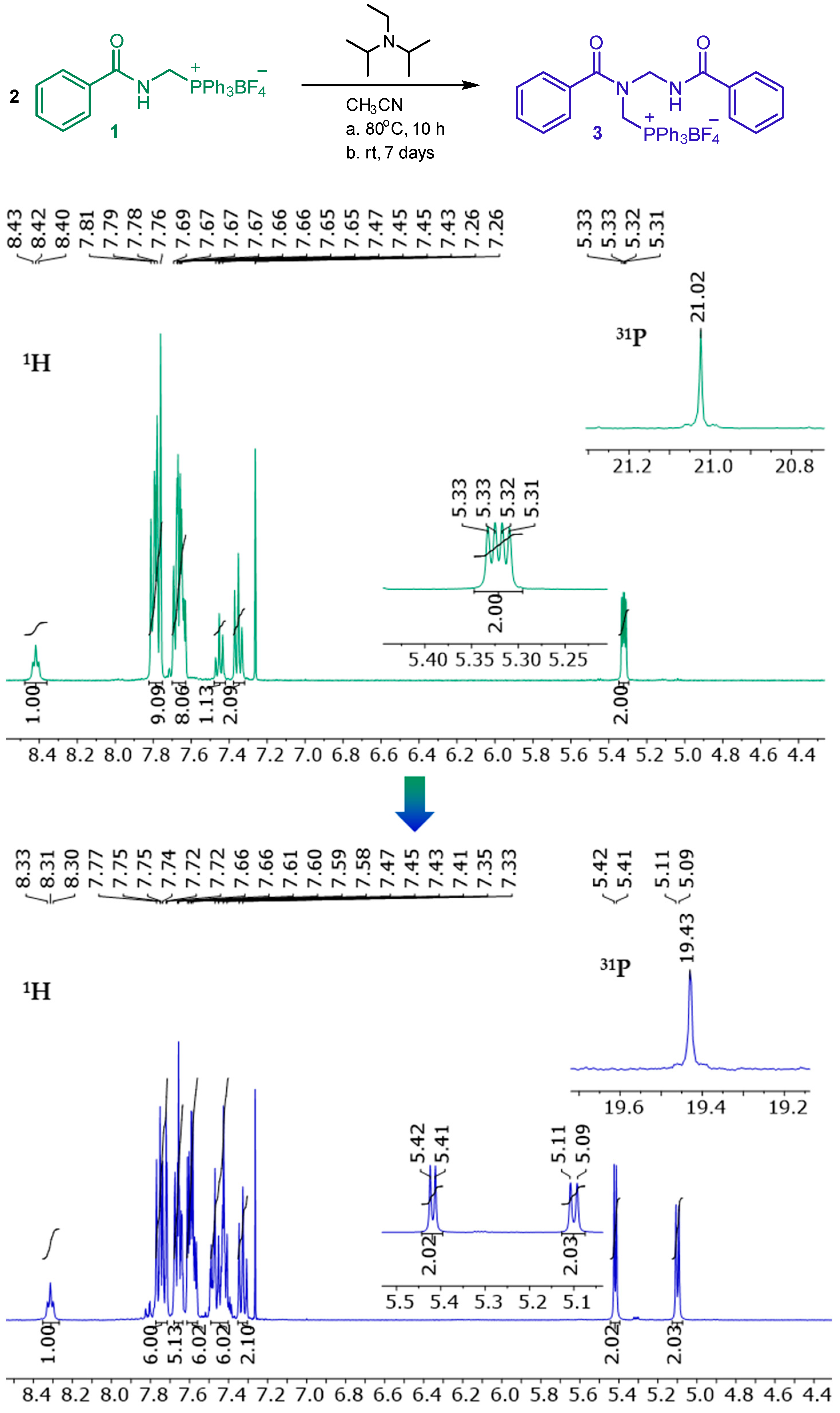
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. [(N-benzamidomethyl)(N-benzoyl)amino]methyltriphenylphosphonium Tetrafluoroborate 3
3.3. Synthesis of [(N-benzamidomethyl)(N-benzoyl)amino]methyltriphenylphosphonium Tetrafluoroborate 3 from N-benzoylaminomethyltriphenylphosphonium Tetrafluoroborate 1 in the Presence of Hünig’s Base
3.4. Reaction of N-benzoylaminomethyltriphenylphosphonium Tetrafluoroborate 1 and Benzaldehyde in the Presence of Base
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neto, B.A.D.; Rocha, R.O.; Rodrigues, M.O. Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis. Molecules 2022, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, E.; Palmieri, A.; Petrini, M. Recent synthetic applications of α-amido sulfones as precursors of N-acylimino derivatives. Org. Chem. Front. 2019, 6, 2142–2182. [Google Scholar] [CrossRef]
- Vinogradov, M.G.; Turova, O.V.; Zlotin, S.G. The progress in the chemistry of N-acyliminium ions and their use in stereoselective organic synthesis. Russ. Chem. Rev. 2017, 86, 1–17. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Cai, C.; Yang, X.; Lv, Z.-C.; Schneider, U. Catalytic Asymmetric Reactions with N,O-Aminals. ACS Catal. 2016, 6, 5747–5763. [Google Scholar] [CrossRef]
- Aranzamendi, E.; Arrasate, S.; Sotomayor, N.; González-Díaz, H.; Lete, E. Chiral Brønsted Acid Catalyzed Enantioselective α-Amidoalkylation Reactions: A Joint Experimental and Predictive Study. ChemistryOpen 2016, 5, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.E.; Manolikakes, G. Bi(OTf)3-Catalyzed Multicomponent α-Amidoalkylation Reactions. J. Org. Chem. 2015, 80, 6193–6212. [Google Scholar] [CrossRef]
- Kataja, A.O.; Masson, G. Imine and iminium precursors as versatile intermediates in enantioselective organocatalysis. Tetrahedron 2014, 70, 8783–8815. [Google Scholar] [CrossRef]
- Mazurkiewicz, R.; Październiok-Holewa, A.; Adamek, J.; Zielińska, K. α-Amidoalkylating agents: Structure, synthesis, reactivity and application. Adv. Heterocycl. Chem. 2014, 111, 43–94. [Google Scholar] [CrossRef]
- Yazici, A.; Pyne, S.G. Intermolecular addition reactions of N-acyliminium ions (Part I). Synthesis 2009, 339–368. [Google Scholar] [CrossRef]
- Yazici, A.; Pyne, S.G. Intermolecular addition reactions of N-acyliminium ions (Part II). Synthesis 2009, 513–541. [Google Scholar] [CrossRef]
- Petrini, M. α-Amido Sulfones as Stable Precursors of Reactive N-Acylimino Derivatives. Chem. Rev. 2005, 105, 3949–3977. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Lan, X.; Yang, J.Z.; Denisko, O.V. Properties and Synthetic Utility of N-Substituted Benzotriazoles. Chem. Rev. 1998, 98, 409–548. [Google Scholar] [CrossRef] [PubMed]
- Zaugh, H.E. α-Amidoalkylation at Carbon: Recent Advances—Part I. Synthesis 1984, 85–110. [Google Scholar] [CrossRef]
- Zaugg, H.E. α-Amidoalkylation at Carbon: Recent Advances—Part II. Synthesis 1984, 181–212. [Google Scholar] [CrossRef]
- Speckamp, W.N.; Hiemstra, H. Intramolecular reactions of N-acyliminium intermediates. Tetrahedron 1985, 41, 4367–4416. [Google Scholar] [CrossRef]
- Hiemstra, H.; Speckamp, W.N. N-Acyliminium Ions as Intermediates in Alkaloid Synthesis. Alkaloids 1988, 32, 271–339. [Google Scholar] [CrossRef]
- Zaugg, H.E. Recent Synthetic Methods Involving Intermolecular alpha-Amidoalkylation at Carbon. Synthesis 1970, 49–73. [Google Scholar] [CrossRef]
- Adamek, J.; Grymel, M.; Kuźnik, A.; Październiok-Holewa, A. 1-Aminoalkylphosphonium Derivatives: Smart Synthetic Equivalents of N-Acyliminium-Type Cations, and Maybe Something More: A Review. Molecules 2022, 27, 1562. [Google Scholar] [CrossRef]
- Adamek, J.; Węgrzyk, A.; Kończewicz, J.; Walczak, K.; Erfurt, K. 1-(N-Acylamino)alkyltriarylphosphonium Salts with Weakened Cα-P+ Bond Strength—Synthetic Applications. Molecules 2018, 23, 2453. [Google Scholar] [CrossRef]
- Mazurkiewicz, R.; Grymel, M. Reaction of N-Acyl-α-triphenylphosphonio-α-amino Acid Esters with Organic Bases: Mechanism of the Base-Catalyzed Nucleophilic Substitution of the Triphenylphosphonium Group. Monatsh. Chem. 2002, 133, 1197–1204. [Google Scholar] [CrossRef]
- Mazurkiewicz, R.; Kuźnik, A.; Grymel, M.; Kuźnik, N. N-Acyl-α-triphenylphosphonioglycinates in the Synthesis of α,β-Dehydro-α-amino Acid Derivatives. Monatsh. Chem. 2004, 135, 807–815. [Google Scholar] [CrossRef]
- Adamek, J.; Zieleźny, P.; Erfurt, K. N-protected 1-aminoalkylphosphonium salts from amides, carbamates, lactams, or imides. J. Org. Chem. 2021, 86, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamek, J.; Kaczmarczyk, W.; Sapia, D. [(N-benzamidomethyl)(N-benzoyl)amino]methyltriphenylphosphonium Tetrafluoroborate. Molbank 2024, 2024, M1834. https://doi.org/10.3390/M1834
Adamek J, Kaczmarczyk W, Sapia D. [(N-benzamidomethyl)(N-benzoyl)amino]methyltriphenylphosphonium Tetrafluoroborate. Molbank. 2024; 2024(2):M1834. https://doi.org/10.3390/M1834
Chicago/Turabian StyleAdamek, Jakub, Wiktoria Kaczmarczyk, and Dawid Sapia. 2024. "[(N-benzamidomethyl)(N-benzoyl)amino]methyltriphenylphosphonium Tetrafluoroborate" Molbank 2024, no. 2: M1834. https://doi.org/10.3390/M1834
APA StyleAdamek, J., Kaczmarczyk, W., & Sapia, D. (2024). [(N-benzamidomethyl)(N-benzoyl)amino]methyltriphenylphosphonium Tetrafluoroborate. Molbank, 2024(2), M1834. https://doi.org/10.3390/M1834






