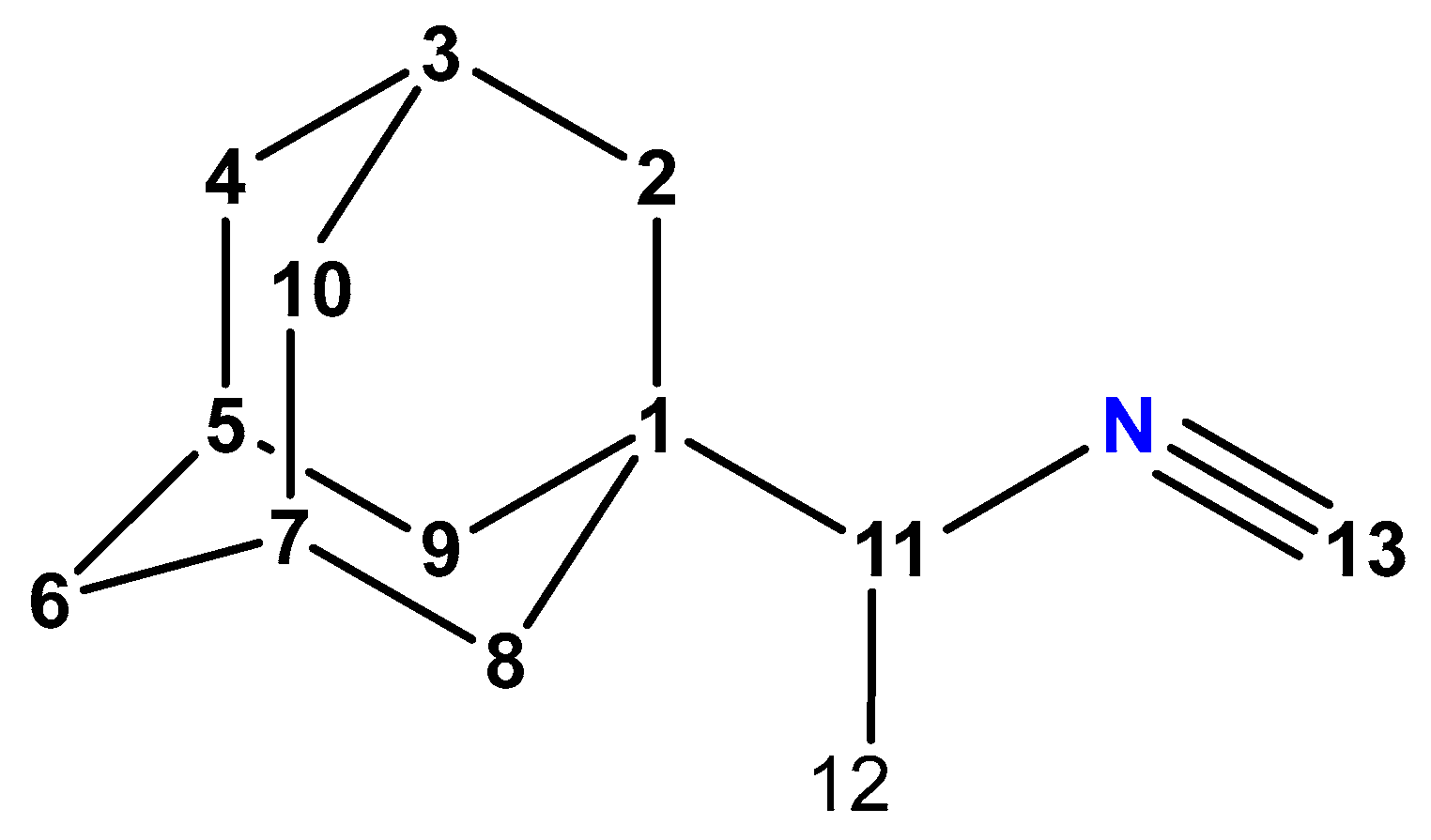
A New Method for the Synthesis of 1-(1-Isocyanoethyl)adamantane
Abstract
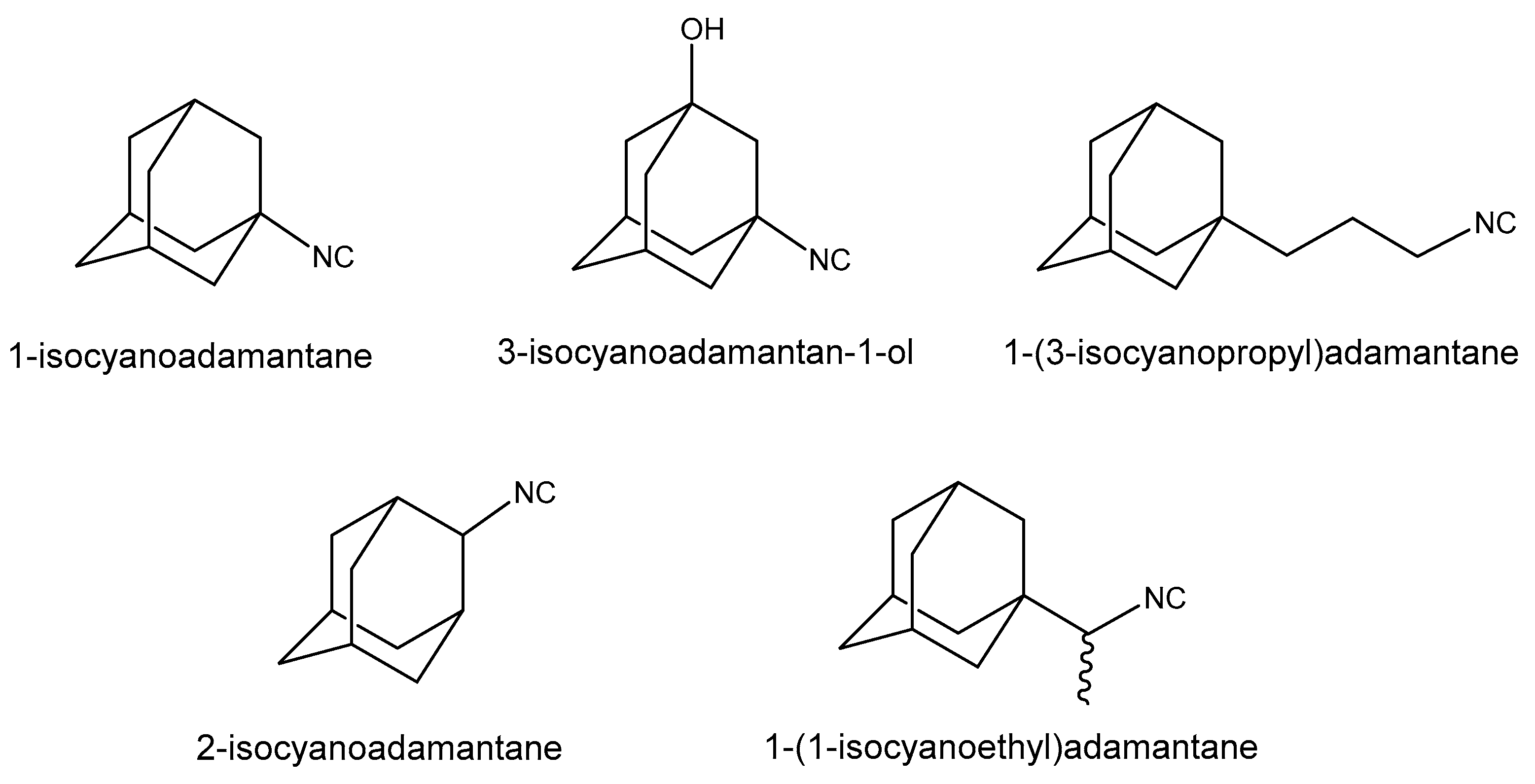
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pirrung, M.C.; Ghorai, S.; Ibarra-Rivera, T.R. Multicomponent Reactions of Convertible Isonitriles. J. Org. Chem. 2009, 74, 4110–4117. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W. Recent Developments in Isonitrile Chemistry. In Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1971; pp. 235–256. [Google Scholar]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168–3210. [Google Scholar] [CrossRef] [PubMed]
- Chandgude, A.L.; Dömling, A. An Efficient Passerini Tetrazole Reaction (PT-3CR). Green Chem. 2016, 18, 3718–3721. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.A.; Siwe-Noundou, X.; Krause, R.W.M. A More Sustainable Isocyanide Synthesis from N-Substituted Formamides Using Phosphorus Oxychloride in the Presence of Triethylamine as Solvent. Molecules 2022, 27, 6850. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Taglialatela-Scafati, O. Marine Antimalarials. Mar. Drugs 2009, 7, 130–152. [Google Scholar] [CrossRef] [PubMed]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Srivastav, N.C.; Puri, S.K. In Vivo Active Antimalarial Isonitriles. Bioorg. Med. Chem. Lett. 2002, 12, 2277–2279. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, J.; Huras, B.; Kiełczewska, A. Synthesis of Isoselenocyanates. Synthesis 2015, 48, 85–96. [Google Scholar] [CrossRef]
- Pitushkin, D.; Burmistrov, V.; Butov, G. 1-(3-Isoselenocyanatopropyl)Adamantane. Molbank 2023, 2023, M1646. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Gazzarrini, S.; He, S.; Chen, L.; Li, J.; Xing, L.; Li, C.; Chen, L.; Neochoritis, C.G.; et al. Isocyanides as Influenza A Virus Subtype H5N1 Wild-type M2 Channel Inhibitors. ChemMedChem 2015, 10, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Ahmadian-Moghaddam, M.; Dömling, A. Isocyanide 2.0. Green Chem. 2020, 22, 6902–6911. [Google Scholar] [CrossRef]
- Popov, K.; Lajunen, L.H.J.; Popov, A.; Rönkkömäki, H.; Hannu-Kuure, M.; Vendilo, A. 7Li, 23Na, 39K and 133Cs NMR Comparative Equilibrium Study of Alkali Metal Cation Hydroxide Complexes in Aqueous Solutions. First Numerical Value for CsOH Formation. Inorg. Chem. Commun. 2002, 5, 223–225. [Google Scholar] [CrossRef]
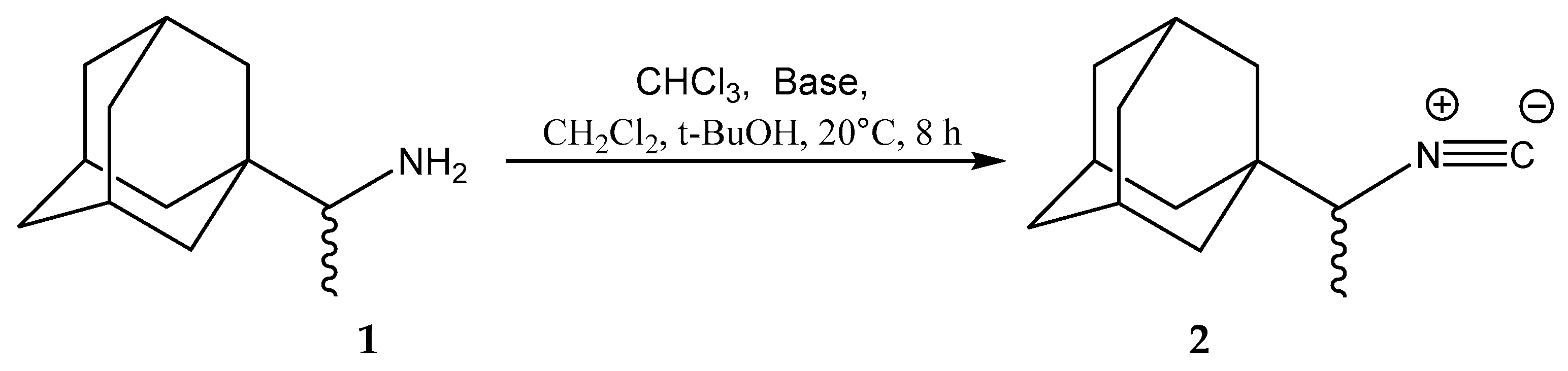
| Reaction Temperature, °C | Reaction Time, h | Base | Isocyanide Content in the Reaction Mass, % | pKb [12] |
|---|---|---|---|---|
| 40 | 8 | LiOH | 0 | 0.36 |
| 40 | 8 | NaOH | 86 | −0.18 |
| 40 | 8 | KOH | 62 | −0.46 |
| 40 | 8 | t-BuOK | 92 | - |
| 20 | 8 | t-BuOK | 91 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitushkin, D.; Butov, G. A New Method for the Synthesis of 1-(1-Isocyanoethyl)adamantane. Molbank 2024, 2024, M1833. https://doi.org/10.3390/M1833
Pitushkin D, Butov G. A New Method for the Synthesis of 1-(1-Isocyanoethyl)adamantane. Molbank. 2024; 2024(2):M1833. https://doi.org/10.3390/M1833
Chicago/Turabian StylePitushkin, Dmitry, and Gennady Butov. 2024. "A New Method for the Synthesis of 1-(1-Isocyanoethyl)adamantane" Molbank 2024, no. 2: M1833. https://doi.org/10.3390/M1833
APA StylePitushkin, D., & Butov, G. (2024). A New Method for the Synthesis of 1-(1-Isocyanoethyl)adamantane. Molbank, 2024(2), M1833. https://doi.org/10.3390/M1833






