Abstract
(E)-Pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one was designed then synthesized using a multi-step pathway starting from commercially available 2-nitroaniline. Structure characterization of this original substituted pyrrolo[1,2-a]quinoxaline compound was achieved by using FT-IR, 1H-NMR, 13C-NMR, X-Ray and HRMS spectral analysis. This new pyrroloquinoxaline shows interesting cytotoxic potential against different human leukemia cell lines (MV4-11, K562, MOLM14 and Jurkat cells).
1. Introduction
Among heterocyclic nitrogen derivatives that have attracted attention due to their broad profile of biological activities, the pyrrolo[1,2-a]quinoxaline moiety has shown wide interest for its various biological properties. [1,2]. Thus, many pyrrolo[1,2-a]quinoxaline compounds have been designed, synthesized and described as antipsychotic agents [3], antiviral agents [4], adenosine receptor modulators [5], antituberculosis agents [6,7], antiparasitic [8,9,10,11,12,13,14,15] and anticancer compounds [16,17,18,19,20,21,22]. However, despite the recent and promising development of targeted therapies, there is still a need to discover new cytotoxic molecules for the treatment of human cancers.
We previously published a series of new pyrrolo[1,2-a]quinoxaline derivatives endowed with promising pharmacological activity towards human leukemia cells [16,17,18,19,20,21]. In this context, and as an extension of our research work on the development and assessment of new antiproliferative heterocyclic derivatives, we decided to further modulate and substitute our heterocyclic pyrrolo[1,2-a]quinoxaline pharmacophore. DYT-1, a substituted quinoline, was recently screened from a chemical library and identified as a hit compound active against several leukemia [23,24]. Consequently, taking into account the experience of our lab in the domain of the synthesis of new bioactive nitrogen heterocyclic compounds based on this pyrrolo[1,2-a]quinoxaline heterocyclic scaffold, we report herein the design, synthesis and structural identification of (E)-pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (JG1679) that could be considered a new structural bioisostere analogue of the previously described bioactive quinoline DYT-1 compound (Figure 1). This novel substituted pyrrolo[1,2-a]quinoxaline derivative JG1679 is then tested against four leukemia cell lines, namely, MV4-11, K562, Jurkat and MOLM14.
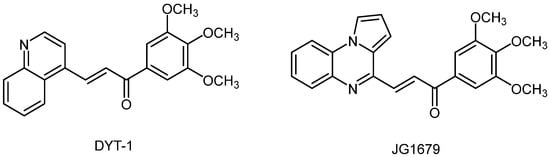
Figure 1.
Chemical structures of compounds DYT-1 and JG1679.
2. Results and Discussion
2.1. Synthesis of (E)-Pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (JG1679)
The preparation of (E)-pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (JG1679) has been accomplished in six steps starting from the commercially available 2-nitroaniline 1 according to the sequence depicted in Scheme 1. The Clauson-Kaas reaction of commercially available 2-nitroaniline 1 with 2,5-dimethoxytetrahydrofuran (DMTHF) in refluxing acetic acid under microwave conditions gave phenylpyrrole 2, which was then reduced using a NaBH4-CuSO4 treatment to provide 1-(2-aminophenyl)pyrrole 3. The reaction of acetyl chloride with 3 led to acetamide 4. 4-methylpyrrolo[1,2-a]quinoxaline 5 was prepared via the cyclisation of this amide 4 in refluxing phosphorus oxychloride according to the Bischler–Napieralski reaction. Then, we synthesized pyrrolo[1,2-a]quinoxaline-4-carboxaldehyde 6 via the oxidation of 5 with SeO2 in refluxing dioxane. Finally, aldehyde 6 reacted with 3′,4′,5′-trimethoxyacetophenone under the presence of an aqueous NaOH solution, resulting in the synthesis of compound JG1679 as a single E isomer [23]. Thus, a coupling constant value of 15.15 Hz was in favor of a trans configuration in the double bond. The structure of this original synthesized pyrroloquinoxaline derivative JG1679 was then corroborated by FTIR, 1H/13C-NMR, X-ray and ESI-MS experiments (see Supplementary Materials, Figures S1–S4). The 3D structural determination of this new substituted pyrrolo[1,2-a]quinoxaline JG1679 was established by X-ray crystallography (Figure 2) [25], and the confirmation of the structure being in the solid state was anticipated on the basis of NMR analysis.
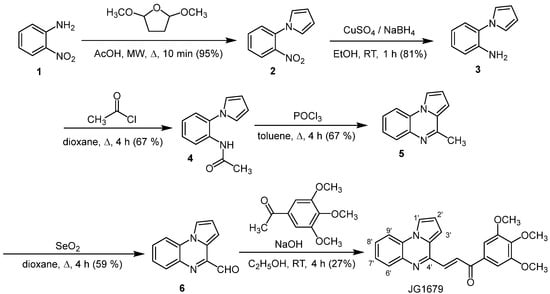
Scheme 1.
Synthesis of (E)-Pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (JG1679).
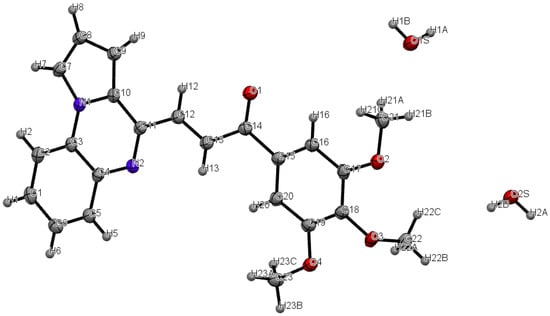
Figure 2.
The ORTEP (Oak Ridge Thermal Ellipsoid Plot) drawing of the 1(E)-Pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (JG1679) with thermal ellipsoids at 30% level.
2.2. Cytotoxic Activity
The cytotoxic activity of this new (E)-pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one JG1679 was evaluated against the following human leukemia K562, MV4-11, Jurkat and MOLM14 cell lines with the MTS assay [16,17] (Table 1). The IC50 values of quinoline DYT-1, used as reference a drug, are reported in Table 1 as previously described [23,24]. Against the human myeloid leukemia cell line K562, our new pyrroloquinoxaline derivative JG1679 displayed none antiproliferative activity (IC50 > 50 μM), while this new compound inhibited the growth of MV4-11 human myeloid leukemia cells with an IC50 value of 1.7 µM. Moreover, the IC50 of JG1679 was noticed to be superior to 2 μM against the human MOLM14 acute myeloid leukemia cell line. Against the T-acute lymphoblastic leukemia Jurkat cell line, the biological result of compound JG1679 exhibited potent cytotoxicity with an IC50 value of 3.0 μM.

Table 1.
In vitro activity of compounds JG1679 and DYT-1 on K562, MV4-11, Jurkat, MOLM14 and HEK293 cells.
In addition, (E)-pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one JG1679 was also tested against human epithelial cell line HEK293 to evaluate its cytotoxicity on normal cells. Thus, compound JG1679 showed a low cytotoxicity against this HEK293 cell line (IC50 = 31.2 μM). Index of selectivity (IS) was defined as the ratio of the IC50 value on normal cell line HEK293 to the IC50 value on the different leukemia cell lines. Derivative JG1679 showed a promising selectivity towards the MV4-11 cell line (IS = 18.35) and also towards the lymphoblastic leukemia Jurkat cell line with a calculated index of selectivity of 10.4.
3. Materials and Methods
Commercial reagents were used as received without additional purification. Melting points were determined with an SM-LUX-POL Leitz hot-stage microscope (Leitz GMBH, Midland, ON, USA) and are uncorrected. IR spectra were recorded on a NICOLET 380FT-IR spectrophotometer (Thermo Electron Scientific Instruments LLC, Madison, WI, USA). NMR spectra were recorded with tetramethylsilane as an internal standard using a BRUKER AVANCE 300 spectrometer (Bruker BioSpin, Wissembourg, France). Splitting patterns were reported as follows: s = singlet; bs = broad singlet; d = doublet; t = triplet; q = quartet; dd = double doublet; ddd = double double doublet; dt = double triplet; m = multiplet. 2D-NMR experiments were used for resonance assignments. Analytical TLC was carried out on 0.25 precoated silica gel plates (POLYGRAM SIL G/UV254) and the visualization of compounds after UV light irradiation. Silica gel 60 (70–230 mesh) was used for column chromatography. High-resolution mass spectra (electrospray in positive mode, ESI+) were recorded on a Waters Q-TOF Ultima apparatus (Bruker Daltonics, Bremen, Germany) [13,14].
3.1. (E)-Pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (JG1679)
To a solution of pyrrolo[1,2-a]quinoxaline-4-carboxaldehyde 5 (1.7 mmol) and 3′,4′,5′-trimethoxyacetophenone (1.7 mmol) in ethanol (7 mL) at 0 °C, a 40% aqueous solution of NaOH (0.8 mL) was added dropwise and stirred at this temperature for 30 min. The reaction mixture was then stirred at room temperature for 4 h, the formed solid was filtered, washed with water and then ethanol and dried to give the crude product. Column chromatography of this precipitate on silica gel using ethyl acetate-cyclohexane (3/7) as an eluent gave the pure product JG1679 (27%). Yellow crystals, m.p. 196–198 °C; IR (KBr) 1656 (CO), 1607 (C=N). 1H-NMR (δ, ppm, CDCl3, 300 MHz): 8.38 (d, 1H, J = 15.15 Hz, HC=), 8.18 (d, 1H, J = 15.15 Hz, =CH), 8.07 (dd, 1H, J = 8.05 and 1.30 Hz, H-9′), 8.03 (dd, 1H, J = 2.80 and 1.10 Hz, H-1′), 7.92 (dd, 1H, J = 8.05 and 1.30 Hz, H-6′), 7.59 (ddd, 1H, J = 8.05, 7.60 and 1.30 Hz, H-8′), 7.47 (ddd, 1H, J = 8.05, 7.60 and 1.30 Hz, H-7′), 7.44 (s, 2H, 2 CHphenyl), 7.20 (dd, 1H, J = 4.05 and 1.10 Hz, H-3′), 6.99 (dd, 1H, J = 4.05 and 2.80 Hz, H-2′), 4.00 (s, 6H, 2OCH3), 3.98 (s, 3H, OCH3). 13C-NMR (δ, ppm, CDCl3, 75 MHz): 188.7 (C=O), 153.2 (C-3phenyl and C-5phenyl), 147.8 (C-4′), 143.1 (C-4phenyl), 137.9 (HC=), 135.9 (C-5a), 133.0 (C-7′), 130.4 (C-8′), 128.3 (C-6′), 128.0 (C-9′), 127.6 (C-3a), 126.4 (C-1phenyl), 125.5 (C-9a), 114.8 (=CH), 114.3 (C-1′), 113.8 (C-2′), 106.5 (C-2phenyl and C-6phenyl), 106.2 (C-3′), 61.0 (OCH3), 56.5 (2OCH3). HR-MS m/z [M+H]+ Calcd for C23H21N2O4: 389.1501, Found: 389.1498.
3.2. X-ray Data
The structure of compound JG1679 was established using X-ray crystallography (Figure 2). The yellow single crystal of JG1679 was obtained by slow evaporation from a methanol/chloroform solution (v/v: 15/85): monoclinic, space group C c, a = 30.4488(6) Å, b = 4.8817(1) Å, c = 14.3011(3) Å, α = 90°, β = 109.613(1)°, γ = 90°, V = 2002.41(7)Å3, Z = 4, δ(calcd) = 1.408 Mg.m−3, FW = 424.44 for C23H20N2O4.2H2O, F(000) = 896.0. Full crystallographic results were deposited at the Cambridge Crystallographic Data Centre (CCDC-2237246), UK, as shown in the Supplementary X-ray Crystallographic Data [25]. The data were corrected for Lorentz and polarization effects and for empirical absorption correction [26]. The structure was solved by direct methods Shelx 2013 [26] and refined using Shelx 2013 [27] suite of programs.
3.3. Cell Culture
All leukemic cell lines (MV4-11, Jurkat, MOLM-14, K562) were cultured in Roswell Park Memorial Institute (RPMI) 1640 1X Glutamax (Gibco™, Thermo Fisher Scientific, Villebon-sur-yvette, France), supplemented with 10% fetal calf serum (FCS), 50 U/mL penicillin and 50 µg/mL streptomycin (Gibco™, Thermo Fisher Scientific, Villebon-sur-yvette, France). Cells were cultured with vehicle or the different compounds dissolved in dimethyl sulfoxide (0.1%) at 37 °C in a fully humidified atmosphere (37 °C, 5% CO2) in a CO2-regulated incubator (Jouan, Saint-Nazaire, France).
3.4. Cytotoxic Activity
Cell proliferation assays were performed using MTS tetrazolium (Cell Titer96 Aqueous; Promega, Charbonnières-les-Bains, France) on the human leukemic cell lines K562, MV4-11, Jurkat and MOLM14 as previously described by our team [16,17,21]. Cells (105) were plated in quadruplicate into microtiter-plate wells in 100 µL of culture medium with various doses of compounds (1; 5; 10; 20; 50 µM). After 3 h of incubation of 20 µL of MTS, plates were read in a microplate autoreader (Imark Biorad, Marnes-la-Coquette, France) at a wavelength of 490 nm. The MTT proliferation test on the epithelial cell line HEK293 was performed as previously described [28]. The 50% inhibiting concentrations (IC50) were determined by linear regression analysis.
4. Conclusions
By taking into account our previous research using the valuable and biological active pyrrolo[1,2-a]quinoxaline scaffold, we synthesized here a new (E)-pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one JG1679 and then evaluated its antileukemic activity on different human leukemic cell lines, such as MV4-11, K562, Jurkat and MOLM14. This novel pyrrolo[1,2-a]quinoxaline JG1679 exhibited promising antileukemia properties on the MV4-11 and Jurkat human leukemia cell lines, meaning it could become an interesting candidate for further pharmacomodulations and biological investigations.
Supplementary Materials
FTIR, 1H-NMR, 13C-NMR and HRMS spectra of title compound 9 are available online. Figure S1: 1H-NMR spectrum of compound JG1679. Figure S2: 13C-NMR spectrum of compound JG1679. Figure S3: FT-IR spectrum of compound JG1679. Figure S4: HRMS data for compound JG1679.
Author Contributions
J.G. and S.M. conducted the synthesis and prepared and revised the manuscript; S.S. and N.F. carried out the experiments; S.A.-R. helped in the analysis of the compounds; V.B. and V.D. conducted the in vitro tests; N.P. carried out the crystallographic experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M. New achievements in the synthesis of pyrrolo[1,2-a]quinoxaline. Chem. Heterocycl. Compd. 2019, 55, 584–597. [Google Scholar] [CrossRef]
- Huang, A.; Ma, C. Recent progress in biological activities and synthetic methodologies of pyrroloquinoxalines. Mini-Rev. Med. Chem. 2013, 13, 607–616. [Google Scholar] [CrossRef]
- Campiani, G.; Butini, S.; Fattorusso, C.; Trotta, F.; Franceschina, S.; De Angelis, M.; Nielsen, K.S. Novel Aryl Piperazine Derivatives with Medical Utility. WO2006072608, 13 July 2006. [Google Scholar]
- Campiani, G.; Aiello, F.; Fabbrini, M.; Morelli, E.; Ramunno, A.; Armaroli, S.; Nacci, V.; Garofalo, A.; Greco, G.; Novellino, E.; et al. Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J. Med. Chem. 2001, 44, 305–315. [Google Scholar] [CrossRef]
- Schann, S.; Mayer, S.; Gardan, S. Pyrrolo[1,2-a]quinoxaline Derivatives as Adenosine A3 Receptor Modulators and Uses Thereof. EP1798233, 20 June 2007. [Google Scholar]
- Wang, T.; Tang, Y.; Yang, Y.; An, Q.; Sang, Z.; Yang, T.; Liu, P.; Zhang, T.; Deng, Y.; Luo, Y. Discovery of novel anti-tuberculosis agents with pyrrolo[1,2-a]quinoxaline-base scaffold. Bioorg. Med. Chem. Lett. 2018, 28, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Makane, V.B.; Vamshi Krishna, E.; Karale, U.B.; Babar, D.A.; Kalari, S.; Rekha, E.M.; Shukla, M.; Kaul, G.; Sriram, D.; Chopra, S.; et al. Synthesis of novel 4,5-dihydropyrrolo[1,2-a]quinoxalines, pyrrolo[1,2-a]quinoxalin-2-ones and their antituberculosis and anticancer activity. Arch. Pharma. 2020, 353, e2000192. [Google Scholar] [CrossRef]
- Guillon, J.; Forfar, I.; Mamani-Matsuda, M.; Desplat, V.; Saliège, M.; Thiolat, D.; Massip, S.; Tabourier, A.; Léger, J.-M.; Dufaure, B.; et al. Synthesis, analytical behaviour and biological evaluation of new 4-substituted pyrrolo[1,2-a]quinoxalines as antileishmanial agents. Bioorg. Med. Chem. 2007, 15, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.-M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; et al. Synthesis, antimalarial activity and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef]
- Guillon, J.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Thiolat, D.; Massip, S.; Carrie, H.; Mossalayi, D.; Jarry, C. Synthesis of New 4-(E)-Alkenylpyrrolo[1,2-a]quinoxalines as Antileishmanial Agents by Suzuki-Miyaura Cross-coupling Reactions. J. Enzyme Inhib. Med. Chem. 2007, 22, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Moreau, S.; Ronga, L.; Basmaciyan, L.; Cohen, A.; Rubio, S.; Bentzinger, G.; Azas, N.; Mullié, C.; Sonnet, P. Design, Synthesis and Antimalarial Activity of Some New Aminoalcohol-pyrrolo[1,2-a]quinoxaline Derivatives. Lett. Drug Des. Discov. 2016, 13, 932–942. [Google Scholar] [CrossRef]
- Guillon, J.; Moreau, S.; Mouray, E.; Sinou, V.; Forfar, I.; Belisle-Fabre, S.; Desplat, V.; Millet, P.; Parzy, D.; Jarry, C.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and In Vitro Antimalarial Activity. Bioorg. Med. Chem. 2008, 16, 9133–9144. [Google Scholar] [CrossRef]
- Guillon, J.; Mouray, E.; Moreau, S.; Mullié, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Ravanello, F.; Le-Naour, A.; Pinaud, N.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and in Vitro Antimalarial Activity—Part II. Eur. J. Med. Chem. 2011, 46, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le Pape, P.; Savrimoutou, S.; Pinaud, N.; Mullié, C.; Daulouede, S.; Vincendeau, P.; et al. Design, Synthesis and Biological Evaluation of Novel 4-Alkapolyenylpyrrolo[1,2-a]quinoxalines as Antileishmanial Agents—Part III. Eur. J. Med. Chem. 2014, 81, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Cohen, A.; Gueddouda, N.M.; Das, R.N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; et al. Design, synthesis and antimalarial activity of novel bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J. Enzym. Inhib. Med. Chem. 2017, 32, 547–563. [Google Scholar] [CrossRef]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; et al. Synthesis and evaluation of the cytotoxic activity of novel ethyl 4-[4-(4-substitutedpiperidin-1-yl)]benzyl-phenylpyrrolo[1,2-a]quinoxaline-carboxylate derivatives in myeloid and lymphoid leukemia cell lines. Eur. J. Med. Chem. 2016, 113, 214–227. [Google Scholar] [CrossRef]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; et al. Synthesis and Antiproliferative Effect of Ethyl 4-[4-(4-Substituted Piperidin-1-yl)]benzylpyrrolo[1,2-a]quinoxalinecarboxylate Derivatives on Human Leukemia Cells. ChemMedChem 2017, 12, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Desplat, V.; Geneste, A.; Begorre, M.-A.; Belisle-Fabre, S.; Brajot, S.; Massip, S.; Thiolat, D.; Mossalayi, D.; Jarry, C.; Guillon, J. Synthesis of New Pyrrolo[1,2-a]quinoxaline Derivatives as Potential Inhibitors of Akt Kinase. J. Enzym. Inhib. Med. Chem. 2008, 23, 648–658. [Google Scholar] [CrossRef]
- Desplat, V.; Moreau, S.; Gay, A.; Belisle-Fabre, S.; Thiolat, D.; Massip, S.; Macky, G.; Godde, F.; Mossalayi, D.; Jarry, C.; et al. Synthesis and evaluation of the antiproliferative ctivity of novel pyrrolo[1,2-a]quinoxaline derivatives, potential inhibitors of Akt Kinase. Part II. J. Enzym. Inhib. Med. Chem. 2010, 25, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Moreau, S.; Pinaud, N.; Marchivie, M.; Desplat, V. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-leukemic Activity. Molbank 2020, 2020, M1113. [Google Scholar] [CrossRef]
- Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Moreau, S.; Desplat, V. Synthesis, crystal structure and anti-leukemic activity of 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one. Molbank 2022, 2022, M1333. [Google Scholar] [CrossRef]
- Grande, F.; Aiello, F.; Garofalo, A.; Neamati, N. Identification and Preclinical Evaluation of SC144, a Novel Pyrroloquinoxaline Derivative with Broad-Spectrum Anticancer Activity. Mini Rev. Med. Chem. 2016, 16, 644–650. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, T.; Wang, Y.; Wang, L.; Feng, S.; Cheng, W.; Yang, L.; Duan, Y. Angiogenesis and anti-leukaemia activity of novel indole derivatives as potent colchicine binding site inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Shunichi, Y.; Uichiro, K.; Tadashi, A.; Katsunari, G.; Hiromitsu, S. Preparation of Heterocyclylpropenones as Antitumor Agents. JP08277242, 22 October 1996. [Google Scholar]
- Supplementary X-ray Crystallographic Data; Guillon, J.; Cambridge Crystallographic Data Centre, University Chemical Lab: Cambridge, UK; Available online: https://www.ccdc.cam.ac.uk/ (accessed on 20 January 2023).
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tang, Z.; Fan, S.; Li, C.; Li, Y.; Liu, W.; Long, X.; Zhang, W.; Zhang, Y.; Li, Z.; et al. Synthesis and biological assessment of indole derivatives containing penta-heterocycles scaffold as novel anticancer agents towards A549 and K562 cells. J. Enzym. Inhib. Med. Chem. 2023, 38, 2163393. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).