Bis(μ2-2,2′-((2-(hydroxy)propane-1,3-diyl)bis((nitrilo)eth-1-yl-1-ylidene))diphenolato)-dicobalt(III)
Abstract
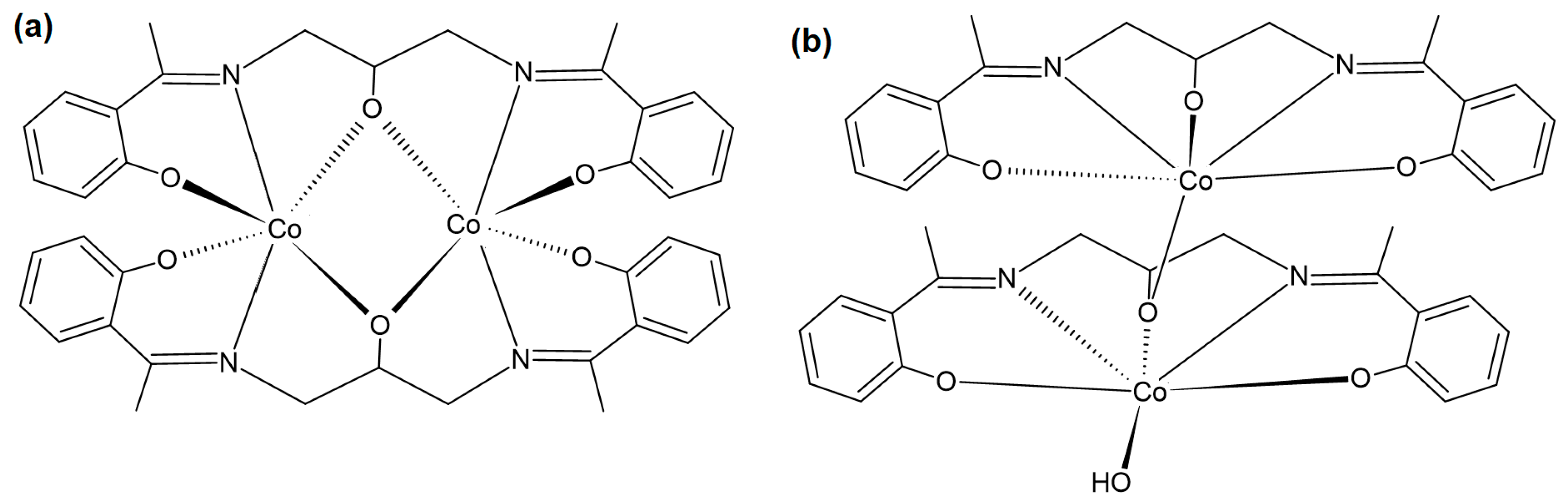
1. Introduction
2. Results and Discussion
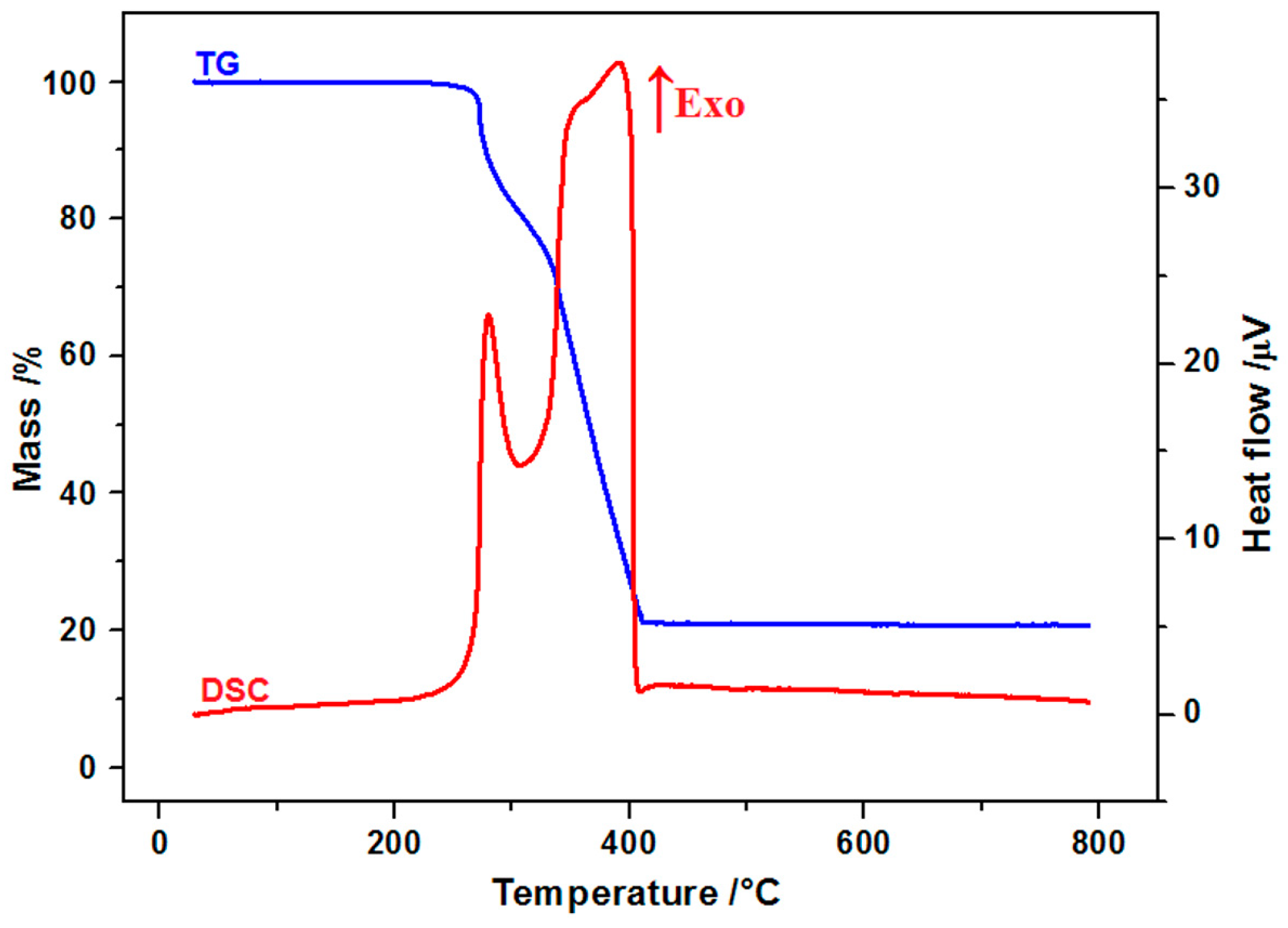
2.1. FT-IR Spectroscopy and Thermal Analysis
2.2. Description of the Molecular Structure
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures
3.3. Methods
3.4. Crystallographic Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maurya, M.R.; Bisht, M.; Chaudhary, N.; Avecilla, F.; Kumar, U.; Hsu, H.-F. Synthesis, structural characterization, encapsulation in zeolite Y and catalytic activity of an oxidovanadium(V) complex with a tribasic pentadentate ligand. Polyhedron 2013, 54, 180–188. [Google Scholar] [CrossRef]
- Biswas, R.; Diaz, C.; Bauzá, A.; Frontera, A.; Ghosh, A. Synthesis, crystal structure, magnetic property and DFT calculations of an unusual dinuclear μ2-alkoxido bridged iron(III) complex. Dalton Trans. 2013, 42, 12274–12283. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Nandy, M.; Sen, S.; Mandal, S.; Rosair, G.M.; Slawin, A.M.Z.; Gómez García, C.J.; Clemente-Juan, J.M.; Zangrando, E.; Guidolin, N.; et al. Isolation of four new CoII/CoIII and NiII complexes with a pentadentate Schiff base ligand: Syntheses, structural descriptions and magnetic studies. Dalton Trans. 2011, 40, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Osypiuk, D.; Cristóvão, B.; Bartyzel, A. New coordination compounds of CuII with Schiff base ligands—Crystal structure, thermal, and spectral investigations. Crystals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Diallo, A.S.; Thiam, I.E.; Gueye-Ndiaye, M.; Dieng, M.; Orton, J.; Simon, C.; Gaye, M. Tetranuclear copper(II) complex of 2-hydroxy-N,N′-bis [1-(2-hydroxyphenyl)ethylidene] propane-1,3-diamine. Acta Crystallogr. Sect. E Cryst. Commun. 2022, 78, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Sen, S.; Rosair, G.; Desplanches, C.; Garribba, E.; Mitra, S. A Novel μ1,1-azido-, μ2-alkoxo-, and μ2-phenoxo-bridged tetranuclear copper(II) complex with a quinquedentate Schiff-base ligand: Magneto-structural and DFT studies. Aust. J. Chem. 2009, 62, 366–375. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Cao, F.; Fu, H.; Li, D.; Dou, J. Crystallization condition-controlled assembly of oxygen-bridged tetranuclear and hexanuclear Ni(II) clusters: Syntheses, structures and properties. RSC Adv. 2012, 2, 1310–1313. [Google Scholar] [CrossRef]
- Kébé, M.; Thiam, I.E.; Sow, M.M.; Diouf, O.; Barry, A.H.; Sall, A.S.; Retailleau, P.; Gaye, M. Hexanuclear copper(II) complex of 2-hydroxy-N,N′-bis [1-(2-hydroxyphenyl)ethylidene]propane-1,3-diamine incorporating an open-cubane core. Acta Crystallogr. Sect. E Cryst. Commun. 2021, 77, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Ignatyev, I.; Kondratenko, Y.; Fundamensky, V.; Kochina, T. Synthesis and characterization of cobalt(II) complexes with triethanolamine and succinate and/or nitrate anions. Transit. Met. Chem. 2017, 43, 127–136. [Google Scholar] [CrossRef]
- Zarei, S.A.; Piltan, M.; Hassanzadeh, K.; Akhtari, K.; Cincic, D. Synthesis, characterization, crystal structure and predicting the second-order optical nonlinearity of a new dicobalt(III) complex with Schiff base ligand. J. Mol. Struct. 2015, 108, 82–87. [Google Scholar] [CrossRef]
- He, X.; Lu, C.Z.; Wu, C.D. Syntheses, structures and properties of di-, trinuclear cobalt compounds. J. Coord. Chem. 2006, 59, 977–984. [Google Scholar] [CrossRef]
- Schenk, K.; Meghdadi, S.; Mehdi, A.; Kashi, A. Co(III) complexes of Me-salpn and Me-salbn and the ring size effect on the coordination modes and electrochemical properties: The crystal structures of trans-[Co III(Me-salpn)(py) 2]PF 6 and cis- α-[Co III(Me-salbn)(4-Mepy) 2]BPh 4 · 4-Mep. Polyhedron 2007, 26, 5448–5457. [Google Scholar] [CrossRef]
- Appleton, T.G. Oxygen uptake by a cobalt(II) complex. An undergraduate experiment. J. Chem. Educ. 1977, 54, 443–444. [Google Scholar] [CrossRef]
- Heffern, M.C.; Reichova, V.; Coomes, J.L.; Harney, A.S.; Bajema, E.A.; Meade, T.J. Tuning Cobalt(III) Schiff Base Complexes as Activated Protein Inhibitors. Inorg. Chem. 2015, 54, 9066–9074. [Google Scholar] [CrossRef] [PubMed]
- Pogány, L.; Moncol, J.; Gál, M.; Šalitroš, I.; Boča, R. Four cobalt(III) Schiff base complexes—Structural, spectroscopic and electrochemical studies. Inorg. Chim. Acta 2017, 462, 23–29. [Google Scholar] [CrossRef]
- CrysAlis PRO Version 1.171.37.35g; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2014.
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. A. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
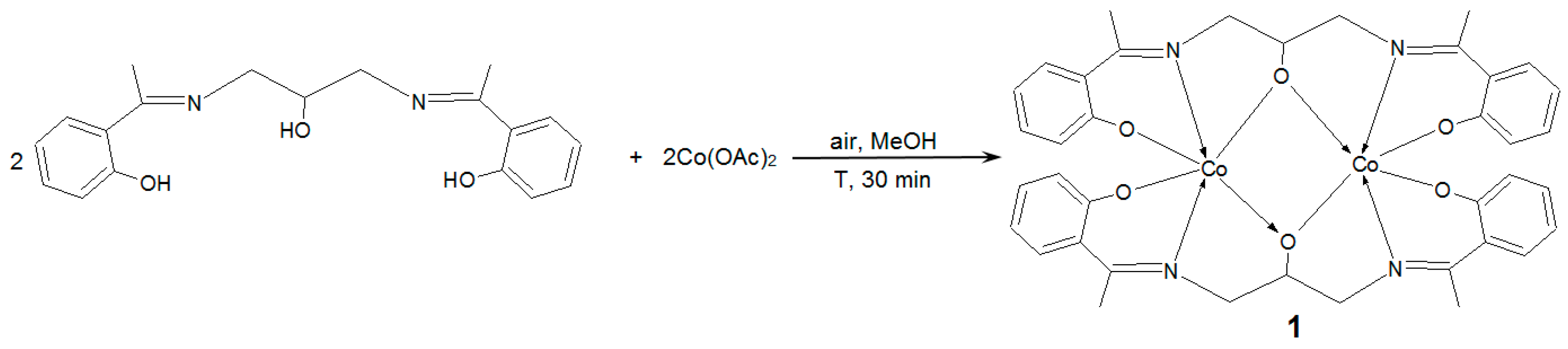
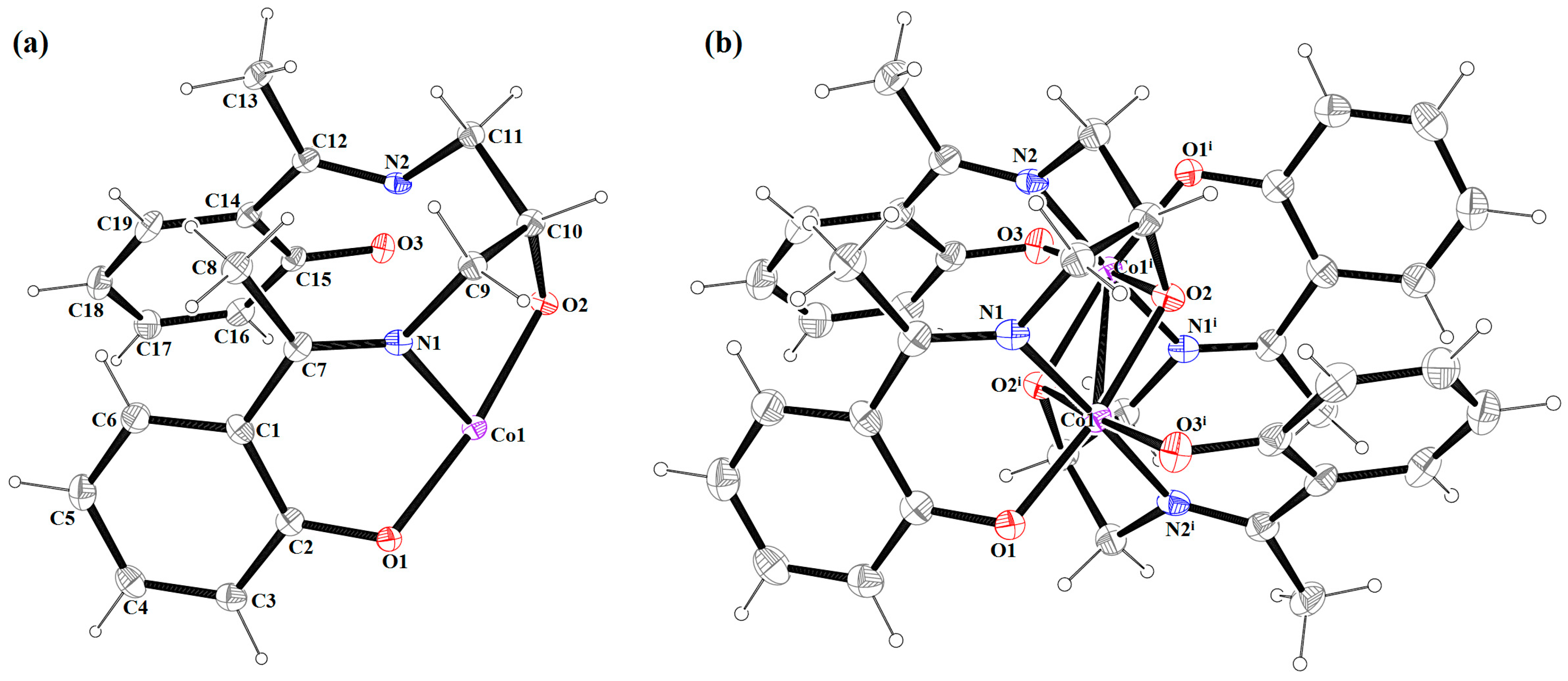
| Bond Lengths (Å) | |||
|---|---|---|---|
| Co(1)-;O(1) | 1.894(2) | Co(1)-;O(3)i | 1.896(2) |
| Co(1)-;O(2) | 1.955(2) | Co(1)-;O(2)i | 1.943(2) |
| Co(1)-;N(1) | 1.896(2) | Co(1)-;N(2)i | 1.908(2) |
| Co(1)-;Co(1)i | 2.930(1) | ||
| Angles (°) | |||
| O(1)-;Co(1)-;N(1) | 88.30(9) | O(3)i-;Co(1)-;O(2)i | 171.63(8) |
| O(1)-;Co(1)-;O(3)i | 90.34(8) | N(2)i-;Co(1)-;O(2)i | 84.78(9) |
| N(1)-;Co(1)-;O(3)i | 90.59(9) | O(1)-;Co(1)-;O(2) | 171.64(8) |
| O(1)-;Co(1)-;N(2)i | 88.65(9) | N(1)-;Co(1)-;O(2) | 84.16(9) |
| N(1)-;Co(1)-;N(2)i | 176.86(10) | O(3)i-;Co(1)-;O(2) | 93.32(8) |
| O(3)i-;Co(1)-;N(2)i | 88.70(9) | N(2)i-;Co(1)-;O(2) | 98.94(9) |
| O(1)-;Co(1)-;O(2)i | 94.74(8) | O(2)i-;Co(1)-;O(2) | 82.54(9) |
| N(1)-;Co(1)-;O(2)i | 96.20(9) |
| CCDC | 2267146 |
| Temperature K | 100(2) |
| Crystal system | monoclinic |
| Space group | P21/c |
| a (Å) | 12.0356(10) |
| b (Å) | 9.7908(7) |
| c (Å) | 13.5890(13) |
| β (°) | 107.086(9) |
| Volume (Å3) | 1530.6(2) |
| Z | 2 |
| Calculated density (g cm−3) | 1.659 |
| μ (mm−1) | 1.144 |
| Absorption correction | multi-scan |
| F(000) | 792 |
| Crystal size (mm) | 0.400 × 0.400 × 0.350 |
| θ range (°) | 2.605-;26.370 |
| Reflections collected/unique | 10,259/3114 |
| Rint | 0.0486 |
| Data/restraints/parameters | 3114/0/228 |
| GooF on F2 | 1.073 |
| Final R indices [I > 2σ(I)] | R1 = 0.0424, wR2 = 0.1015 |
| R indices (all data) | R1 = 0.0543, wR2 = 0.1098 |
| Largest diff. peak/hole, e Å−3 | 0.878/−0.569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartyzel, A.; Cristóvão, B.; Osypiuk, D. Bis(μ2-2,2′-((2-(hydroxy)propane-1,3-diyl)bis((nitrilo)eth-1-yl-1-ylidene))diphenolato)-dicobalt(III). Molbank 2023, 2023, M1690. https://doi.org/10.3390/M1690
Bartyzel A, Cristóvão B, Osypiuk D. Bis(μ2-2,2′-((2-(hydroxy)propane-1,3-diyl)bis((nitrilo)eth-1-yl-1-ylidene))diphenolato)-dicobalt(III). Molbank. 2023; 2023(3):M1690. https://doi.org/10.3390/M1690
Chicago/Turabian StyleBartyzel, Agata, Beata Cristóvão, and Dariusz Osypiuk. 2023. "Bis(μ2-2,2′-((2-(hydroxy)propane-1,3-diyl)bis((nitrilo)eth-1-yl-1-ylidene))diphenolato)-dicobalt(III)" Molbank 2023, no. 3: M1690. https://doi.org/10.3390/M1690
APA StyleBartyzel, A., Cristóvão, B., & Osypiuk, D. (2023). Bis(μ2-2,2′-((2-(hydroxy)propane-1,3-diyl)bis((nitrilo)eth-1-yl-1-ylidene))diphenolato)-dicobalt(III). Molbank, 2023(3), M1690. https://doi.org/10.3390/M1690








