Abstract
A new alkyne functionalized pterin derivative was synthesized through a reaction of 7-chloropterin with propargyl alcohol in the presence of sodium hydride. The purity and chemical structure of the compound was validated by NMR (1H, 13C) spectroscopy, Mass (APCI source) spectrometry, elemental analysis, and X-ray crystallography. The title compound may be further functionalized by exploiting the yne moiety, for instance, using click chemistry. The novel pterin derivative, most notably, in contrast to typical pterin behavior, is now soluble or even well soluble in almost any solvent except water.
1. Introduction
Pteridines are heterocyclic compounds containing fused pyrimidine and pyrazine rings (Figure 1). Naturally occurring pteridines can be divided into three main classes: lumazines, isoalloxazines, and pterins. Among these, pterins are the most common naturally occurring pteridines bearing an amino group at position 2 and an oxo group at position 4 [1]. The name pterin originates from the Greek word pteron meaning wing since they were first isolated from pierid butterflies where the yellow, orange, and white coloration of the butterfly wings goes back to the presence of pterins. Though two naturally occurring pterins were already isolated in 1889 from the wings of Brimstone butterflies, followed by further isolation of a third pteridine in 1933, their chemical structures and compositions remained ambiguous until 1940 due to difficulties in elemental analysis. Later, these pterins were found to be xanthopterin, leucopterin, and isoxanthopterin respectively and Robert Purrmann completed the comprehensive synthesis of these pteridines shortly after [2,3]. Since then, more than 50 naturally occurring pterins have been found, identified, and in some cases completely synthesized.
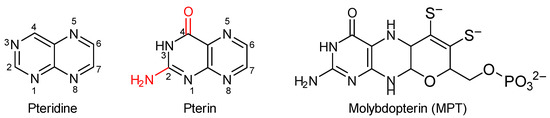
Figure 1.
Pteridine and pterin scaffolds including their numbering schemes and the natural active site ligand in molybdenum-dependent oxidoreductase enzymes, molybdopterin (MPT). In red the two functional groups which define the pterin class of compounds.
Pterins are ubiquitous in nature. They play several different roles in biology, including those of pigments [4], one-carbon transfer cofactors [5], and redox cofactors [6]. Some pterin derivatives can cause harmful effects and are classified as toxins [7]. Folic acid, a form of the water-soluble vitamin B9, is another important pterin-bearing molecule [8].
Not surprisingly, some pterins are medicinally important molecules with several derivatives, especially those substituted at position 7, being used as inhibitors for targets such as ricin [9], methionine synthase, leishmaniasis, nitric oxide synthase or being employed as the anti-folate drug methotrexate [10]. Due to their inherent fluorescence properties pterins have in addition found applications as fluorophores [11,12], in photodynamic [13] and photothermal [14,15] therapies and generally exhibit remarkable photochemical behavior [16,17,18].
The molybdenum cofactor of oxidoreductase enzymes is another significant instance of the pterin system in nature [19]. Here a tetrahydropterin moiety is part of the organic ligand system which is commonly called molybdopterin (acronym: MPT; Figure 1), while some alternative names are also used. In order to better understand the activity of natural active sites at the chemical level, bioinorganic model chemistry can attempt to facilitate respective investigations by addressing certain chemical motifs of the biological cofactors or even the cofactor as a whole. The role of MPT in molybdenum enzymes has been under research since it was discovered and several, mostly relatively smaller models of molybdopterin have been synthesized. However, despite some very serious efforts, the synthesis of a complete mimic of MPT, bearing all the essential groups is still elusive for the scaffold’s difficult chemistry further complicated by issues associated with the acidity, basicity, and tautomerism of pterins [20]. A major disadvantage of pterins is their near or complete insolubility in almost any commonly used solvent. This property can be attributed to the ability of amine and keto groups of the pterin nucleus to form very strong intermolecular H-bonds [21].
It is therefore generally useful to investigate and report the properties of any pterin-bearing compounds in order to develop a better chemical understanding which may guide the way toward suitable synthetic strategies—eventually facilitating the preparation of an artificial MPT. It is particularly valuable in this context if compounds can be made which have an improved solubility as is the case with the title compound, since this will dramatically improve the efficiency of follow-up transformations in solution.
2. Results and Discussion
Title compound 1 was synthetically addressed in order to use it as a synthon for a subsequent ring-closing reaction as depicted in Scheme 1. The synthesis was inspired by a known procedure for chromone synthesis [22]. The respective protocol involves the use of γ-chloropropargyl aryl ethers to access chroman-4-ones via refluxing in ethylene glycol. While this procedure was reported for aryl ethers, for heteroaryl backbones it remains unknown. The approach was supposed to be a synthetic route towards a pterin-based ligand system imitating most of the features of molybdopterin.
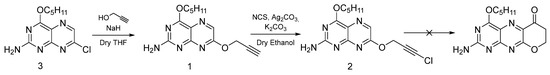
Scheme 1.
Etherification of 7-chloropterin and subsequent successful and unsuccessful transformations.
The synthetic sequence shown in Scheme 1 was successfully carried out up to and including compound 2. However, all attempts to achieve ring closure turned eventually out to be unsuccessful. The starting material, 7-chloropterin (compound 3) was synthesized as described in the literature [23,24]. The etherification reaction between 7-chlorpterin, 3, and propargyl alcohol in the presence of NaH was carried out at room temperature. During this reaction, a single product was formed. It proceeded through the generation of a nucleophile, viz. the sodium salt of propargyl alcohol, followed by nucleophilic substitution of the chloride at position 7 of the pterin substrate by the propargylate group.
The product was purified by column chromatography to yield a colorless solid which exhibits a notably strong fluorescence on the TLC plate under high wavelength UV light (365 nm). The fluorescent emission is also noticeable when dissolved in common polar organic solvents such as alcohols and to a lesser extent also in halogenated solvents (for exemplary UV-vis and fluorescence spectra see Figures S9 and S10 in the Supplementary Materials). Compound 1 is sparingly soluble in very non-polar solvents like hexane; however, it is easily and completely soluble in almost any other organic solvent.
The structure and composition of the resulting product were determined using APCI-mass spectrometry, 1H, 13C, and 13C-dept NMR spectroscopy. The 1H NMR spectrum of compound 1 displayed a doublet at δ = 5.14 ppm with a J-coupling of 2.4 Hz for the CH2 of the propargyl group while the terminal –CH gave a triplet at 2.56 ppm with J = 2.3 Hz. This unique splitting pattern for the –CH2 and terminal –CH of the propargyl fragment is due to long-range coupling and the J value near 3 Hz aligns with reported values for long-range coupling involving intervening π-bonds. The 13C NMR exhibits peaks at δ = 75.6 ppm for the alkynyl carbons and at 54.3 ppm corresponding to the –CH2 carbon of the propargyl group. The aromatic carbons of the pterin backbone are clearly visible in the 13C NMR with signals between 118.41 and 167.30 ppm.
Single crystals of compound 1 were obtained through the slow evaporation from a solution in chloroform which became part of the crystal structure as a lattice solvent. The analyzed crystal system is monoclinic with a P21/c space group.
The co-crystallized solvent chloroform is disordered in the crystal over two positions and the diffraction of the crystal was relatively weak and only moderately satisfying. However, the molecule of interest, compound 1 (Figure 2), behaves well and the important hydrogen atoms (pterin bound, including those on N5, and propargyl bound) could be located on the electron density map and were refined freely. The short C8≡C9 bond 1.197(7) Å and the moderately long C7-C8 bond 1.464(7) Å imply that the π-systems of alkyne and pterin are electronically well separated. The C6–O1–C7–C8 torsion angle of 75.03° emphasizes that the propargyl moiety points away from the pterin plane. The metrical parameters observed (see Table S3 in the Supplementary Materials) are all in line with the expected chemical structure. In-plane bidirectional hydrogen bonding between N2 (acceptor) and N5 (donor) with D⋯A 2.973(5) Å forms pairs of pterin moieties, which again reside in layers roughly along the a/c diagonal of the cell. The distances between the layers are only slightly longer than the lengths of the propargyl moieties which point to the adjacent layers alternately up and down.
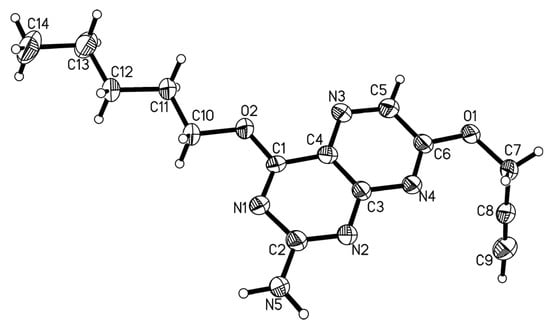
Figure 2.
Molecular structure of compound 1. Ellipsoids are shown at the 50% probability level. Disordered chloroform lattice solvent is not shown for clarity reasons.
Having unambiguously established the chemical structure and purity of compound 1, compound 2 was synthesized by adapting and optimizing a reported procedure for the purpose [25]. The respective chlorination with N-chlorosuccinimide was carried out in the presence of K2CO3 base and catalyst Ag2CO3. Compound 2 was characterized by mass spectrometry and 1H and 13C NMR spectroscopy. The formation of the chlorinated product was confirmed by mass analysis with the presence of the typical chlorine isotope pattern. The former doublet for the CH2 protons of the propargyl fragment of compound 1 appeared now as a singlet in the 1H NMR of compound 2 which further confirms the transformation of compound 1 to compound 2 by chlorination.
Attempts to employ compound 2 in ring-closing reactions by refluxing in ethylene glycol did not yield any reasonable results. In further ring-closing studies acids were employed at room temperature wherein using sulfuric acid led to the decomposition of the pterin. Several additional attempts using low concentrations of sulfuric acids as well as other acids including perchloric acid, and triflic acid proved to be similarly unsuccessful. The predominant reasons for this failure are likely the electron-deficient nature of the pterin scaffold as well as the absence of electron-donating groups (such as a methyl substituent) at the α-position of the propargyl chain [26].
3. Materials and Methods
The starting material 7-chloropterin was synthesized as described in the literature [23,24]. 1H NMR (300 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Avance II-300 spectrometer. Chemical shifts δ are given in ppm and the solvent residual peak (CDCl3: 1H, δ = 7.27; 13C, δ = 77.0) was used as an internal standard. Peak multiplicities are specified as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. Mass spectra (m/z) were recorded on a compact ESI-mass spectrometer Advion expression CMS. An Elementar Vario MICRO cube was used for the experimental determination of elemental compositions of the final pure product. TLC analyses were carried out on aluminum plates coated with silica gel 60 F254, 0.2 mm thickness. The plates were visualized using a 365 nm UV lamp.
3.1. Synthesis
3.1.1. 4-(Pentyloxy)-7-(prop-2-yn-1-yloxy)pteridin-2-amine (1)
Propargyl alcohol (0.41 mL, 7.1 mmol, 1 equiv.) was added to a suspension of NaH (0.341 g, 24.2 mmol, 2 equiv.) in THF (12 mL) at 0 C. After stirring for 20 min, a solution of 7-chloropterin (1.9 g, 7.1 mmol) in 15 mL of THF was added dropwise and the reaction was warmed to room temperature and left to stir for 45 min. The reaction was monitored by thin-layer chromatography. After completion of the reaction, the reaction mixture was poured into a saturated solution of ammonium chloride and the product was extracted with chloroform. The organic fractions were combined, washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was subjected to neutral alumina column chromatography using chloroform as the eluent to give 4-(pentyloxy)-7-(prop-2-yn-1-yloxy) pteridin-2-amine (compound 1) as a colorless (white) powder (Yield: 1.89 g, 90%). (+ve) APCI-MS m/z = 287.32 m/z calcd. for C14H17N5O2 [M]; found: 288.2 [M + H]. NMR (CDCl3, 300 MHz): δ = 8.20 (s, 1H), 5.43 (br s, 2H), 5.14 (d, J = 2.4 Hz, 2H), 4.54 (t, J = 7.0 Hz, 2H), 2.56 (t, J = 2.3 Hz, 1H), 1.92 (quin, J = 7.1 Hz, 2H, –OCH2CH2–), 1.43 (m, 4H), 0.93 ppm (t, J = 1.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): _ [ppm] = 167.3, 162.0, 161.1, 156.5, 133.5, 118.4, 77.2, 75.7, 68.2, 54.6, 28.3, 28.0, 22.4, 13.9. CHNS calcd for C14H17N5O2: C, 58.52; H, 5.96; N, 24.38; found C, 58.86; H, 5.92; N, 23.10.
3.1.2. 7-((3-Chloroprop-2-yn-1-yl)oxy)-4-(pentyloxy)pteridin-2-amine (2)
An oven-dried Schlenk tube was charged with potassium(I) carbonate (0.035 g, 0.25 mmol, 0.5 equiv.), N-chlorosuccinimide (0.2 g, 1.5 mmol, 3 equiv.), silver(I) carbonate (0.069 g, 0.25 mmol, 0.5 equiv.) and compound 1 (0.144 g, 0.5 mmol). The Schlenk tube was evacuated and refilled with nitrogen, and 2 mL of dry ethanol was added. The Schlenk tube was sealed and the reaction was left to stir at 50 °C for 5 h. the reaction was monitored by thin-layer chromatography. After the complete disappearance of the starting material the reaction was cooled to 0 °C and 50 mL of saturated NaCl solution was added. The product was extracted in chloroform. The organic fractions were combined, dried over anhydrous Na2SO4 and the solvent was evaporated in vacuo. The residue was purified by column chromatography over neutral alumina using chloroform as the eluent to give 7-((3-chloroprop-2-yn-1-yl)oxy)-4-(pentyloxy)pteridin-2-amine as an off-white (or beige) powder (Yield: 0.06 g, 38%). (+ve) APCI-MS m/z = 321.1 m/z calcd. for C14H16N5O2Cl [M]; found: 322.5 [M + H], 324.5 [M + 3] 1H NMR (CHLOROFORM-d, 300 MHz): δ = 8.18 (s, 1H), 5.61 (br s, 2H), 5.13 (s, 2H), 4.53 (t, J = 7.0 Hz, 2H), 1.92 (quin, J = 7.2 Hz, 2H), 1.44 (m, 4H), 0.93 ppm (t, J = 7.1 Hz, 3H); 13C NMR (CHLOROFORM-d, 75 MHz): δ = 167.2, 162.0, 161.1, 156.5, 133.2, 118.4, 77.2, 68.1, 63.3, 54.7, 28.2, 27.9, 22.3, 13.9 ppm.
3.2. Single Crystal X-ray Diffraction Analysis
Suitable single crystals of compound 1 were mounted on a thin glass fiber coated with paraffin oil. X-ray single-crystal structural data were collected at low temperature (170 k) with a STOE IPDSII diffractometer (STOE, Darmstadt, Germany) equipped with a normal-focus, 2.4 kW, sealed-tube X-ray source with graphite-monochromatic MoKα radiation (λ = 0.71073 Å). The program X-Area was used for the integration of diffraction profiles; a numerical absorption correction was carried out with the programs X-Shape and X-Red—all by STOE. The structures were solved with SHELXT-2018 [27] and refined by full-matrix least-squares methods using SHELXL-2018 [28] and the WinGX GUI, Ver2021.3 [29]. The methylene and methyl hydrogen atoms of the pentyl substituent, as well as the methine hydrogen atom of the solvent molecule chloroform, were added using the riding model with the Uiso values constrained to the Ueq of the parent carbon atoms (1.5 times for –CH3 hydrogen atoms and 1.2 times for all others). All other hydrogen atoms on nitrogen or carbon were refined entirely freely. The chloroform solvent is disordered over two positions. The disorder was modeled using SADI constraints for all C–Cl bonds and all Cl–Cl distances. Additionally, DELU and SIMU constraints were applied. Occupancies are 62% for the major component and 38% for the minor one. Crystallographic data were deposited at the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB21EZ, UK. These data can be obtained free of charge on quoting the depository number CCDC 2264640 by FAX (+44-1223-336-033), email (deposit@ccdc.cam.ac.uk) or their web interface (at http://www.ccdc.cam.ac.uk).
4. Conclusions
Although the principle objective for the synthesis of compound 1 could not be accomplished, a new pterin derivative exhibiting improved solubility in organic solvents and strong fluorescence upon UV excitation was synthesized. A procedure for the selective functionalization of position C-7 of the pterin moiety was established. This synthetic method may be applied to the synthesis of analogous 7-substituted pterin derivatives, taking into account their importance for medicinal applications.
The triple bond may be further taken forward into click reactions in order to synthesize triazoles; respective studies are being presently carried out by our group. The inherent strong fluorescence of pterins as further tuned by the application of click chemistry will potentially open new synthetic doors towards bio-conjugation applications of respective pterin derivatives.
Supplementary Materials
1. Table S1: Crystal data and structure refinement for 1. 2. Table S2. Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 × 103) for 1. 3. Table S3: Bond lengths [Å] and angles [°] for 1. 4. Table S4: Anisotropic displacement parameters (Å2 × 103) for 1. 5. Table S5: Hydrogen coordinates (×104) and isotropic displacement parameters (Å2 × 103) for 1. 6. Table S6: Hydrogen bonds for 1 [Å and °]. 7. Figure S1: Crystal structure of compound 1 showing the disordered co-crystalized solvent molecule. 8. Figure S2: Crystal structure of compound 1 with the major occupancy component of the co-crystallized solvent molecule. 9. Figure S3: 1H NMR spectrum of compound 1. 10. Figure S4: 13C NMR spectrum of compound 1. 11. Figure S5: Mass (APCI) spectrum of compound 1. 12. Figure S6: 1H NMR spectrum of compound 2. 13. Figure S7: 13C NMR spectrum of compound 2. 14. Figure S8: Mass (APCI) spectrum of compound 2. 15. Figure S9: UV Absorption spectra of compound 1 in protic and aprotic solvents. 16. Figure S10: Fluorescence emission spectra of compound 1 in protic and aprotic solvents. 17. Figure S11: A photo showing fluorescence of 10 µM solutions of compound 1 in chloroform, methanol, and acetonitrile, respectively.
Author Contributions
Methodology, investigation, data curation, writing—original draft preparation, J.V.C.; formal synthesis, reaction condition optimization, characterization, B.W.; visualization, supervision, project administration, funding acquisition, writing the final draft, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the DFG SPP 1927 grant number SCHU 1480/4-2.
Data Availability Statement
The data presented in this study are available in this article and supporting information. Crystallographic data may be downloaded from the CCDC web interface. Primary data, e.g., in simple txt-format, may be requested via email to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ishikawa, T. Product Class 21: Pteridines and Related Structures. In Category 2, Hetarenes and Related Ring Systems; Yamamoto, Y., Shinkai, I., Eds.; Georg Thieme Verlag KG: Stuttgart, Germany, 2004; Volume 16. [Google Scholar]
- Purrmann, R. Die Synthese des Xanthopterins. Über die Flügelpigmente der Schmetterlinge. X. Justus Liebigs Ann. Chem. 1941, 546, 98–102. [Google Scholar] [CrossRef]
- Purrmann, R. Über die Flügelpigmente der Schmetterlinge. VII. Synthese des Leukopterins und Natur des Guanopterins. Justus Liebigs Ann. Chem. 1940, 544, 182–190. [Google Scholar] [CrossRef]
- Andrade, P.; Carneiro, M. Pterin-Based Pigmentation in Animals. Biol. Lett. 2021, 17, 20210221. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Burgmayer, S.J.N. Pterin Chemistry and Its Relationship to the Molybdenum Cofactor. Coord. Chem. Rev. 2011, 255, 1016–1038. [Google Scholar] [CrossRef]
- Colston, K.J.; Basu, P. Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors. Molecules 2022, 27, 3324. [Google Scholar] [CrossRef]
- Kosuge, T.; Tsuji, K.; Hirai, K.; Yamaguchi, K.; Okamoto, T.; Iitaka, Y. Isolation and Structure Determination of a New Marine toxin, Neosurugatoxin, from the Japanese Ivory Shell, Babylonia japonica. Tetrahedron Lett. 1981, 22, 3417–3420. [Google Scholar] [CrossRef]
- Choi, S.-W.; Mason, J.B. Folate and Carcinogenesis: An Integrated Scheme. J. Nutr. 2000, 130, 129–132. [Google Scholar] [CrossRef]
- Saito, R.; Goto, M.; Katakura, S.; Ohba, T.; Kawata, R.; Nagatsu, K.; Higashi, S.; Kurisu, K.; Matsumoto, K.; Ohtsuka, K. Pterin-based small molecule inhibitor capable of binding to the secondary pocket in the active site of ricin-toxin A chain. PLoS ONE 2022, 17, e0277770. [Google Scholar] [CrossRef]
- Bennett, Z.; Grumbles, K.; Pruet, J. Comparative routes to 7-carboxymethyl-pterin: A useful medicinal chemistry building block. Pteridines 2022, 33, 1–8. [Google Scholar] [CrossRef]
- Kawada, T.; Kino, K.; Matsuzawa, Y.; Morikawa, M.; Okamoto, Y.; Kobayashi, T.; Tanaka, Y. N′1,N′4-bis(2-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)ethylidene)succinohydrazide. Molbank 2022, 2022, M1436. [Google Scholar] [CrossRef]
- Walalawela, N.; Urrutia, M.N.; Thomas, A.H.; Greer, A.; Vignoni, M. Alkane Chain-extended Pterin Through a Pendent Carboxylic Acid Acts as Triple Functioning Fluorophore, 1O2 Sensitizer and Membrane Binder. Photochem. Photobiol. 2019, 95, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, M.N.; Sosa, M.J.; Pissinis, D.E.; Cánneva, A.; Miñán, A.G.; Vignoni, M.; Calvo, A.; Thomas, A.H.; Schilardi, P.L. Immobilization of Alkyl-Pterin Photosensitizer on Silicon Surfaces through In Situ SN2 Reaction as Suitable Approach for Photodynamic Inactivation of Staphylococcus aureus. Colloid Surf. B-Biointerfaces 2021, 198, 111456. [Google Scholar] [CrossRef] [PubMed]
- Blach, D.; Alves De Souza, C.E.; Méndez, S.C.; Martínez, F.O. Conjugated anisotropic gold nanoparticles through pterin derivatives for a selective plasmonic photothermal therapy: In vitro studies in HeLa and normal human endocervical cells. Gold Bull. 2021, 54, 9–23. [Google Scholar] [CrossRef]
- Bertel, L.; Mendez-Sanchez, S.C.; Martínez-Ortega, F. Laser photo-thermal therapy of epithelial carcinoma using pterin-6-carboxylic acid conjugated gold nanoparticles. Photochem. Photobiol. Sci. 2021, 20, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Buglak, A.A.; Kononov, A.I. Silver Cluster Interactions with Pterin: Complex Structure, Binding Energies and Spectroscopy. Spectrochim. Acta Mol. Biomol. Spectros. 2022, 279, 121467. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Serrano, M.P.; Lorente, C.; Borsarelli, C.D.; Thomas, A.H. Quenching of the Singlet and Triplet Excited States of Pterin by Amino Acids. Photochem. Photobiol. 2019, 95, 220–226. [Google Scholar] [CrossRef]
- Malcomson, T.; Paterson, M.J. Theoretical determination of two-photon absorption in biologically relevant pterin derivatives. Photochem. Photobiol. Sci. 2020, 19, 1538–1547. [Google Scholar] [CrossRef]
- Johnson, J.L.; Hainline, B.E.; Rajagopalan, K.V. Characterization of the Molybdenum Cofactor of Sulfite Oxidase, Xanthine, Oxidase, and Nitrate Reductase. Identification of a Pteridine as a Structural Component. J. Biol. Chem. 1980, 255, 1783–1786. [Google Scholar] [CrossRef]
- Pfleiderer, W.; Zondler, H.; Mengel, R. Pteridine, XXXIX. Synthese und Struktur von Pterin-carbonsäure-(6) und -(7). Justus Liebigs Ann. Chem. 1970, 741, 64–78. [Google Scholar] [CrossRef]
- Nonogawa, M.; Arai, T.; Endo, N.; Pack, S.P.; Kodaki, T.; Makino, K. Hydrogen bond removal of pterin derivative whose structure is similar to nucleic acid bases. Nucleic Acids Symp. Ser. 2005, 49, 311–312. [Google Scholar] [CrossRef]
- Ariamala, G.; Balasubramanian, K.K. A Simple Route for the Synthesis of 4-Chlorochromenes and Chroman-4-Ones. Tetrahedron Lett. 1988, 29, 3487–3488. [Google Scholar] [CrossRef]
- Chrysochos, N.; Ahmadi, M.; Trentin, I.; Lõkov, M.; Tshepelevitsh, S.; Ullmann, G.M.; Leito, I.; Schulzke, C. Aiding a Better Understanding of Molybdopterin: Syntheses, Structures, and pKa Value Determinations of Varied Pterin-Derived Organic Scaffolds Including Oxygen, Sulfur and Phosphorus Bearing Substituents. J. Mol. Struct. 2021, 1230, 129867. [Google Scholar] [CrossRef]
- Baur, R.; Sugimoto, T.; Pfleiderer, W. Pteridines. Part LXXXV. Chemical Synthesis of Deoxysepiapterin and 6-Acylpteridines by acyl Radical Substitution Reactions. Helv. Chim. Acta 1988, 71, 531–543. [Google Scholar] [CrossRef]
- Shi, D.; Liu, Z.; Zhang, Z.; Shi, W.; Chen, H. Silver-Catalyzed Synthesis of 1-Chloroalkynes Directly from Terminal Alkynes. ChemCatChem 2015, 7, 1424–1426. [Google Scholar] [CrossRef]
- Ariamala, G.; Balasubramanian, K.K. Thermal Behaviour of Aryl γ-Haloprcpargyl Ethers. Tetrahedron 1989, 45, 309–318. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).