Synthesis, Spectroscopic, and Thermal Analyses of 2-Oxo-1,2-dihydroquinolin-8-yl 4-chlorobenzoate
Abstract
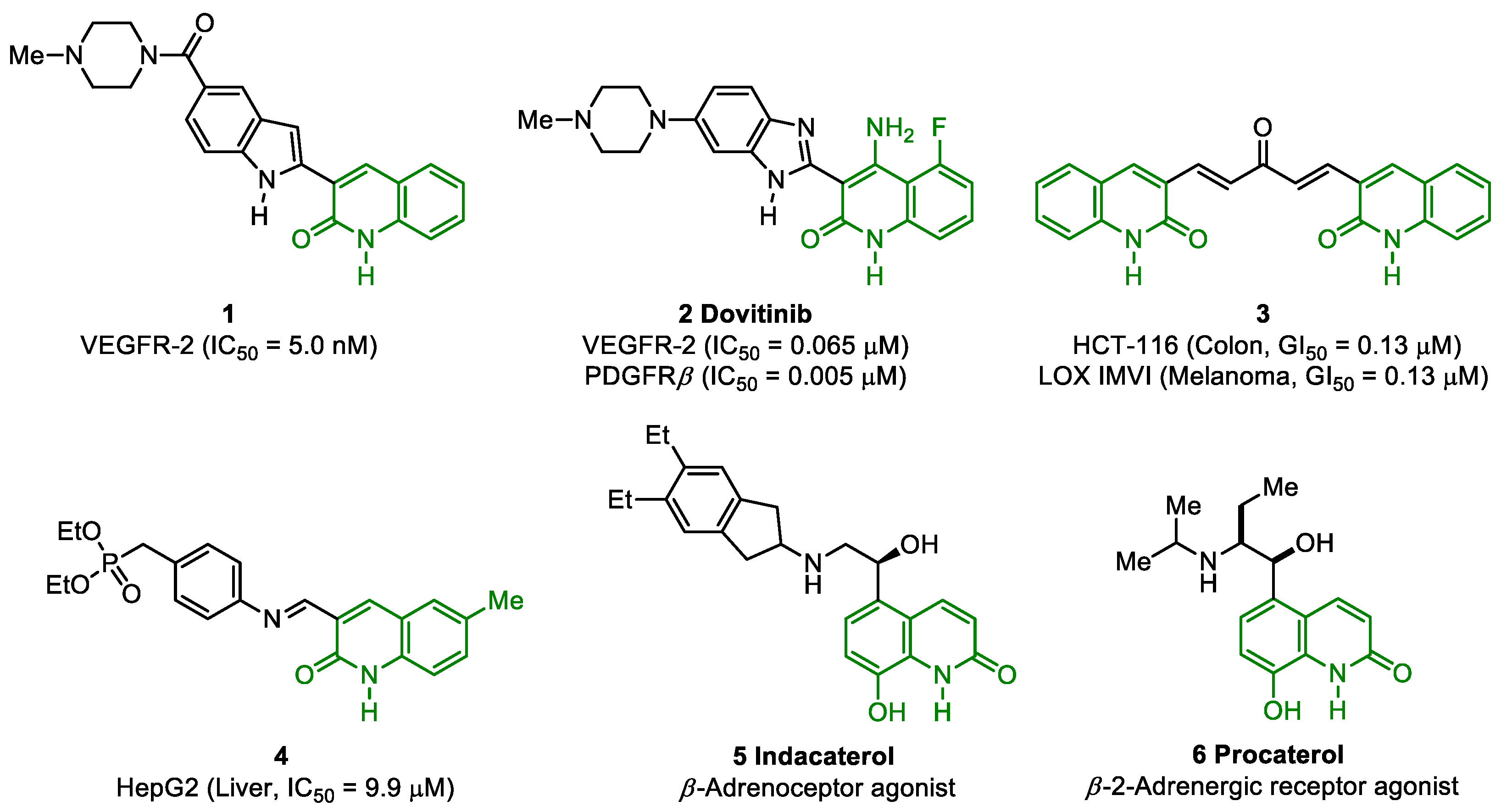
1. Introduction
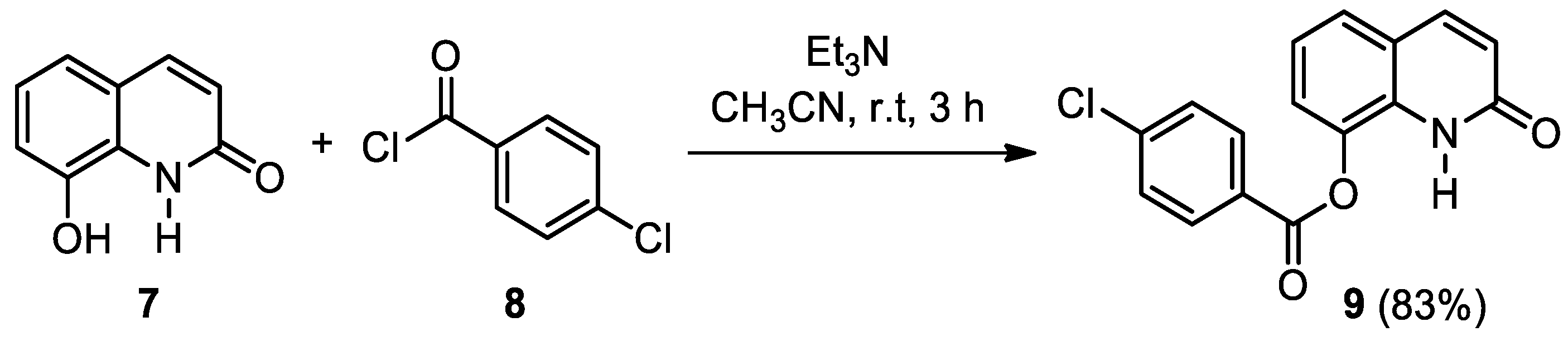
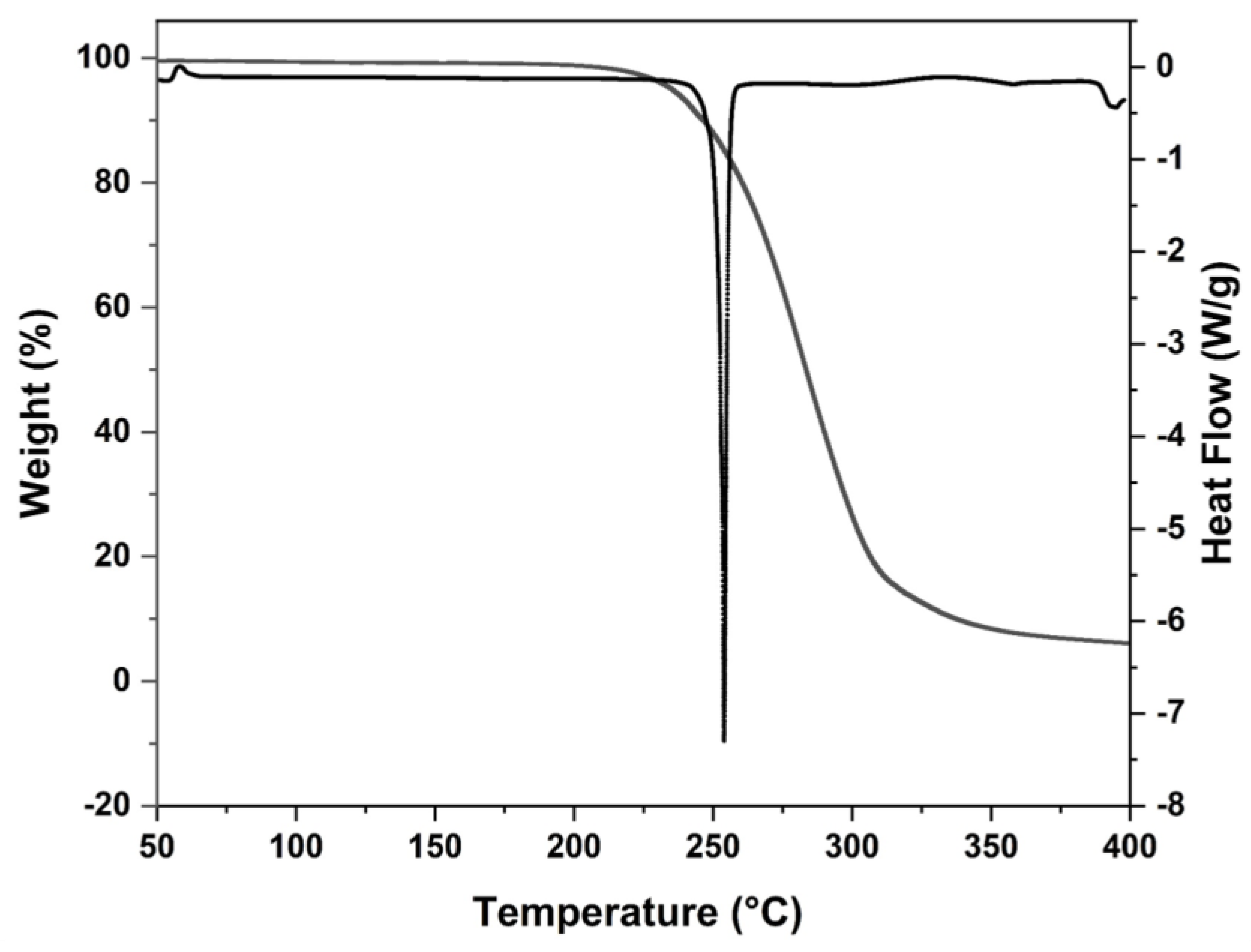
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 8-Hydroxyquinolin-2(1H)-one (7)
3.3. Synthesis of 2-Oxo-1,2-dihydroquinolin-8-yl 4-chlorobenzoate (9)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirek, J.; Sygula, A. Semiempirical MNDO and UV absorption studies on tautomerism of 2-quinolones. Z. Naturforsch. A. 1982, 37, 1276–1283. [Google Scholar] [CrossRef]
- Masoud, M.S.; Mohammed, M.S.; Abdel-Latif, F.F.; Soliman, E.M.A. Spectral studies on some 2-quinolones. Spectrosc. Lett. 1988, 21, 369–383. [Google Scholar] [CrossRef]
- Pan, Y.; Lau, K.-C.; Al-Mogren, M.M.; Mahjoub, A.; Hochlaf, M. Theoretical studies of 2-quinolinol: Geometries, vibrational frequencies, isomerization, tautomerism, and excited states. Chem. Phys. Lett. 2014, 613, 29–33. [Google Scholar] [CrossRef]
- Mohebi, N. Developing new derivatives of 3-X-4-hydroxy-2(1H)-quinolone as quinoline-based chemosensors for detecting fluoride: Theoretical study on nucleophilicity and hydrogen-bonding via various analyses. J. Phys. Org. Chem. 2022, 35, e4422. [Google Scholar] [CrossRef]
- Lewis, F.D.; Reddy, G.D.; Elbert, J.E.; Tillberg, B.E.; Meltzer, J.A.; Kojima, M. Spectroscopy and photochemistry of 2-quinolones and their Lewis acid complexes. J. Org. Chem. 1991, 56, 5311–5318. [Google Scholar] [CrossRef]
- Volle, J.-N.; Mävers, U.; Schlosser, M. The tautomeric persistence of electronically and sterically biased 2-quinolones. Eur. J. Org. Chem. 2008, 2008, 2430–2438. [Google Scholar] [CrossRef]
- Krebs, C.; Förster, W.; Weiss, C.; Hofmann, H.-J. Theoretical description of solvent effects. V. The medium influence on the lactim-lactam tautomerism of hydroxyazines. J. Prakt. Chem. 1982, 324, 369–378. [Google Scholar] [CrossRef]
- Fraley, M.E.; Hoffman, W.F.; Arrington, K.L.; Hungate, R.W.; Hartman, G.D.; McFall, R.C.; Coll, K.E.; Rickert, K.; Thomas, K.A.; McGaughey, G.B. Property-based design of KDR kinase inhibitors. Curr. Med. Chem. 2004, 11, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Renhowe, P.A.; Pecchi, S.; Shafer, C.M.; Machajewski, T.D.; Jazan, E.M.; Taylor, C.; Antonios-McCrea, W.; McBride, C.M.; Frazier, K.; Wiesmann, M.; et al. Design, structure−activity relationships and in vivo characterization of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones: A novel class of receptor tyrosine kinase inhibitors. J. Med. Chem. 2009, 52, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Insuasty, D.; Castillo, J.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J. Synthesis of novel quinoline-2-one based chalcones of potential anti-tumor activity. Eur. J. Med. Chem. 2012, 57, 29–40. [Google Scholar] [CrossRef]
- Yu, Y.-C.; Kuang, W.-B.; Huang, R.-Z.; Fang, Y.-L.; Zhang, Y.; Chen, Z.-F.; Ma, X.-L. Design, synthesis and pharmacological evaluation of new 2-oxo-quinoline derivatives containing α-aminophosphonates as potential antitumor agents. Med. Chem. Comm. 2017, 8, 1158–1172. [Google Scholar] [CrossRef]
- Abonia, R.; Castillo, J.; Cuervo, P.; Insuasty, B.; Quiroga, J.; Ortíz, A.; Nogueras, M.; Cobo, J. A simple one-pot synthesis of new imidazol-2-yl-1H-quinolin-2-ones from the direct reaction of 2-chloroquinolin-3-carbaldehyde with aromatic o-diamines. Eur. J. Org. Chem. 2010, 2010, 317–325. [Google Scholar] [CrossRef]
- Valencia, J.; Sánchez-Velasco, O.A.; Saavedra-Olavarría, J.; Hermosilla-Ibáñez, P.; Pérez, E.G.; Insuasty, D. N-Arylation of 3-formylquinolin-2(1H)-ones using copper(II)-catalyzed Chan–Lam coupling. Molecules 2022, 27, 8345. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Duan, Y.; Lu, L.-H.; Li, Y.-L.; Peng, S.; Wu, C.; Liu, K.-J.; Wang, Z.; He, W.-M. Fast, base-free and aqueous synthesis of quinolin-2(1H)-ones under ambient conditions. ACS Sustain. Chem. Eng. 2017, 5, 10407–10412. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Wu, X.-F. Palladium-catalyzed reductive aminocarbonylation of benzylammonium triflates with o-nitrobenzaldehydes for the synthesis of 3-arylquinolin-2(1H)-ones. J. Org. Chem. 2021, 86, 13824–13832. [Google Scholar] [CrossRef]
- Dao, P.D.Q.; Lim, H.-J.; Cho, C.S. Weak base-promoted lactamization under microwave irradiation: Synthesis of quinolin-2(1H)-ones and phenanthridin-6(5H)-ones. ACS Omega 2018, 3, 1211412121. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.P.; Shin, I.; Lim, H.N. Recent advances in one-pot modular synthesis of 2-quinolones. Molecules 2020, 25, 5450. [Google Scholar] [CrossRef]
- Cheng, P.; Gu, Q.; Liu, W.; Zou, J.-F.; Ou, Y.-Y.; Luo, Z.-Y.; Zeng, J.-G. Synthesis of quinolin-2-one alkaloid derivatives and their inhibitory activities against HIV-1 reverse transcriptase. Molecules 2011, 16, 7649–7661. [Google Scholar] [CrossRef]
- Nieto, C.I.; García, M.Á.; Farrán, Á.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Alkorta, I.; Elguero, J. Two polymorphs of 8-hydroxycarbostyril: X-ray crystallography, solid-state NMR and DFT calculations. J. Mol. Struct. 2012, 1008, 88–94. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, I.-L.; Chen, J.J.; Wei, D.C.; Hsieh, H.J.; Chang, K.M.; Tzeng, C.-C.; Wang, T.-C. Studies on the alkylation of quinolin-2(1H)-one derivatives. J. Chil. Chem. Soc. 2015, 60, 2812–2816. [Google Scholar] [CrossRef]
- Xing, G.; Zhi, Z.; Yi, C.; Zou, J.; Jing, X.; Woo, A.Y.-H.; Lin, B.; Pan, L.; Zhang, Y.; Cheng, M. 8-Hydroxyquinolin-2(1H)-one analogues as potential β2-agonists: Design, synthesis and activity study. Eur. J. Med. Chem. 2021, 224, 113697. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Pan, L.; Yi, C.; Li, X.; Ge, X.; Zhao, Y.; Liu, Y.; Li, J.; Woo, A.; Lin, B.; et al. Design, synthesis and biological evaluation of 5-(2-amino-1-hydroxyethyl)-8-hydroxyquinolin-2(1H)-one derivatives as potent β2-adrenoceptor agonists. Bioorg. Med. Chem. 2019, 27, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Chen, Y.; Hegde, S.S.; Jasper, J.R.; Jaw-Tsai, S.; Lee, T.-W.; McNamara, A.; Pulido-Rios, M.T.; Steinfeld, T.; Mammen, M. Discovery of (R)-1-(3-((2-chloro-4-(((2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethyl)amino)methyl)-5-methoxyphenyl)-amino)-3-oxopropyl)-piperidin-4-yl[1,1′-biphenyl]-2-ylcarbamate (TD-5959, GSK961081, Batefenterol): First-in-class dual pharmacology multivalent muscarinic antagonist and β2 agonist (MABA) for the treatment of chronic obstructive pulmonary disease (COPD). J. Med. Chem. 2015, 58, 2609–2622. [Google Scholar] [CrossRef] [PubMed]
- Becerra, D.; Portilla, J.; Castillo, J.-C. 2-Oxo-2H-chromen-7-yl 4-chlorobenzoate. Molbank 2021, 2021, M1279. [Google Scholar] [CrossRef]
- Salinas-Torres, A.; Jiménez, E.; Becerra, D.; Martínez, J.J.; Rojas, H.; Castillo, J.-C.; Macías, M.A. Synthesis, anticancer evaluation, thermal and X-ray crystallographic analysis of 2-oxo-2H-chromen-7-yl 4-chlorobenzoate using a conductively heated sealed-vessel reactor. J. Mol. Struct. 2023, 1274, 134414. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Becerra, D.; Macías, M.A. Crystal structure, Hirshfeld surface analysis, and computational study of quinolin-8-yl 4-chlorobenzoate: Insights from spectroscopic, thermal, and antitumor properties. Crystals 2023, 13, 694. [Google Scholar] [CrossRef]
- Jhong, H.-M.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Oxidative cleavage of styrenes catalyzed by a PdII complex of an 8-hydroxyquinolinonate ligand. Eur. J. Inorg. Chem. 2016, 2016, 5449–5455. [Google Scholar] [CrossRef]
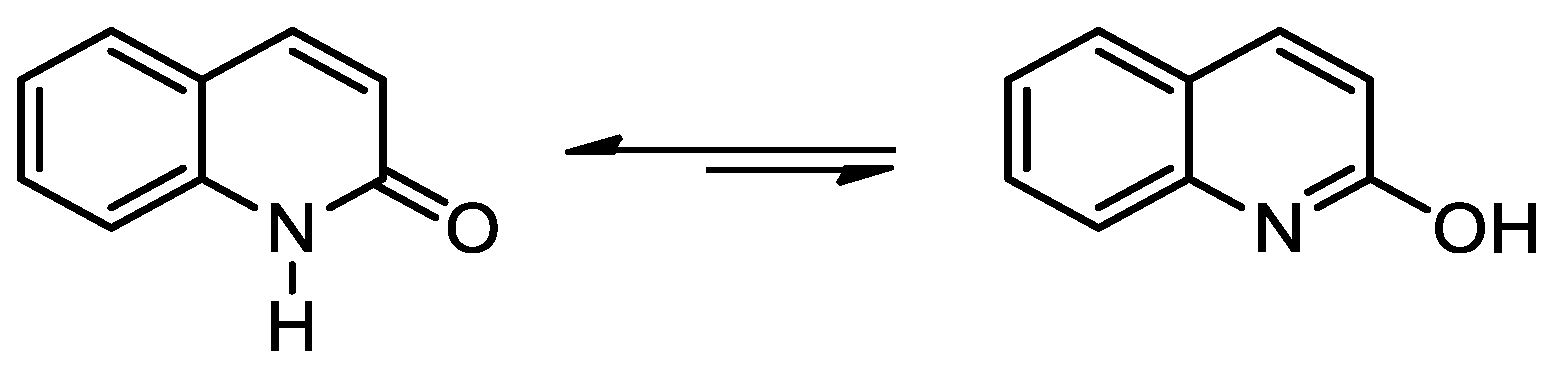

| Number | δH (Mult, J in Hz) | δC (ppm) | COSY | NOESY | HMBC |
|---|---|---|---|---|---|
| 2 | -- | 162.0 | -- | -- | H–3 (2J) |
| H–4 (3J) | |||||
| 3 | 6.55 (d, J = 9.6) | 122.7 | H–4 (3J) | H–4 | -- |
| 4 | 7.98 (d, J = 9.6) | 140.2 | H–3 (3J) | H–3 | H–5 (3J) |
| H–5 | |||||
| 4a | -- | 120.8 | -- | -- | H–3 (3J) |
| H–4 (2J) | |||||
| H–5 (2J) | |||||
| H–6 (3J) | |||||
| 5 | 7.63 (dd, J = 7.8, 1.2) | 126.0 | H–6 (3J) | H–4 | H–4 (3J) |
| H–6 | H–7 (3J) | ||||
| 6 | 7.23 (dd, J = 7.8, 7.8) | 121.6 | H–5 (3J) | H–5 | -- |
| H–7 (3J) | H–7 | ||||
| 7 | 7.44 (dd, J = 8.0, 1.2) | 124.0 | H–6 (3J) | H–6 | H–5 (3J) |
| H–6 (2J) | |||||
| 8 | -- | 136.6 | -- | -- | H–4 (4J) |
| H–6 (3J) | |||||
| H–7 (2J) | |||||
| 8a | -- | 132.0 | -- | -- | H–4 (3J) |
| H–5 (3J) | |||||
| H–7 (3J) | |||||
| i | -- | 128.6 | -- | -- | Hm (3J) |
| o | 8.16 (d, J = 8.6) | 132.2 | Hm (3J) | Hm | -- |
| m | 7.68 (d, J = 8.6) | 128.6 | Ho (3J) | Ho | Ho (2J) |
| p | -- | 138.5 | -- | -- | Ho (3J) |
| Hm (2J) | |||||
| NH | 11.87 (br s) | -- | -- | -- | -- |
| CO2Ar | -- | 164.1 | -- | -- | Ho (3J) |
| Hm (4J) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra, D.; Rojas, H.; Castillo, J.-C. Synthesis, Spectroscopic, and Thermal Analyses of 2-Oxo-1,2-dihydroquinolin-8-yl 4-chlorobenzoate. Molbank 2023, 2023, M1672. https://doi.org/10.3390/M1672
Becerra D, Rojas H, Castillo J-C. Synthesis, Spectroscopic, and Thermal Analyses of 2-Oxo-1,2-dihydroquinolin-8-yl 4-chlorobenzoate. Molbank. 2023; 2023(2):M1672. https://doi.org/10.3390/M1672
Chicago/Turabian StyleBecerra, Diana, Hugo Rojas, and Juan-Carlos Castillo. 2023. "Synthesis, Spectroscopic, and Thermal Analyses of 2-Oxo-1,2-dihydroquinolin-8-yl 4-chlorobenzoate" Molbank 2023, no. 2: M1672. https://doi.org/10.3390/M1672
APA StyleBecerra, D., Rojas, H., & Castillo, J.-C. (2023). Synthesis, Spectroscopic, and Thermal Analyses of 2-Oxo-1,2-dihydroquinolin-8-yl 4-chlorobenzoate. Molbank, 2023(2), M1672. https://doi.org/10.3390/M1672







