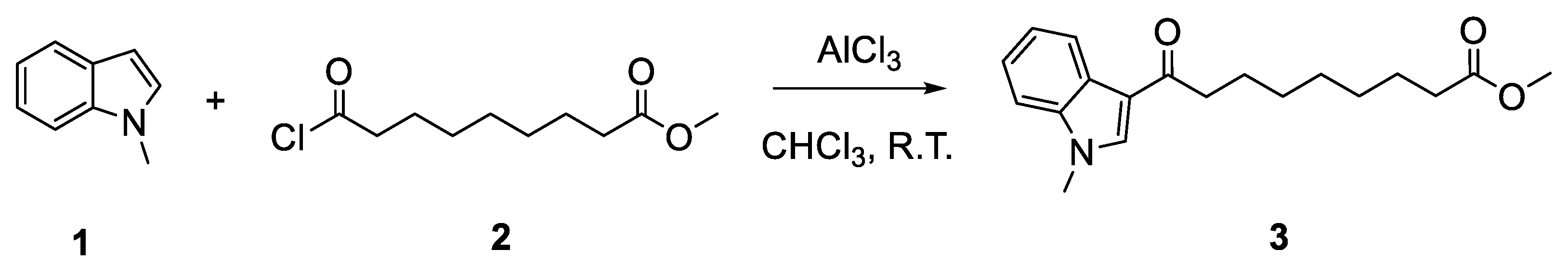
Methyl 9-(1-methyl-1H-indol-3-yl)-9-oxononanoate
Abstract
:1. Introduction
2. Results
3. Materials and Methods
Methyl 9-(1-methyl-1H-indol-3-yl)-9-oxononanoate (3)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedel, C.; Crafts, J.M. Sur une nouvelle méthode générale de synthèse d’hydrocarbures, d’acétones, etc. Compt. Rendus 1877, 84, 1392–1450. [Google Scholar]
- Fairbrother, F. The Mechanism of FriedEl-Crafts reaction. Trans. Faraday Soc. 1941, 37, 763–769. [Google Scholar] [CrossRef]
- Leveson-Gower, R.B.; Roelfes, G. Biocatalytic Friedel-Crafts reactions. ChemCatChem 2022, 14, e202200636. [Google Scholar] [CrossRef] [PubMed]
- Sumita, A.; Ohwada, T. Friedel-Crafts-Type acylation and amidation reactions in strong Bronsted acid: Taming superelectrophiles. Molecules 2022, 27, 5984. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. A brief review of the biological potential of indole derivatives. Future J. Pharm. Sci. 2020, 6, 121. [Google Scholar] [CrossRef]
- Li, L.H.; Niu, Z.J.; Liang, Y.M. New Friedel–Crafts strategy for preparing 3-acylindoles. Org. Biomol. Chem. 2018, 16, 7792–7796. [Google Scholar] [CrossRef] [PubMed]
- Verardi, L.; Fiori, J.; Andrisano, V.; Locatelli, A.; Morigi, R.; Naldi, M.; Bertucci, C.; Strocchi, E.; Boga, C.; Micheletti, G.; et al. Indole Derivative Interacts with Estrogen Receptor Beta and Inhibits Human Ovarian Cancer Cell Growth. Molecules 2020, 25, 4438. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, G.; Calonghi, N.; Farruggia, G.; Strocchi, E.; Palmacci, V.; Telese, D.; Bordoni, S.; Frisco, G.; Boga, C. Synthesis of novel structural hybrids between aza–heterocycles and azelaic acid moiety with a specific activity on osteosarcoma cells. Molecules 2020, 25, 404. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Micheletti, G.; Orlando, I.; Strocchi, E.; Vitali, B.; Verardi, L.; Sartor, G.; Calonghi, N. New hybrids with 2-aminobenzothiazole and azelayl scaffolds: Synthesis, molecular docking and biological evaluation. Curr. Org. Chem. 2018, 22, 1649–1660. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheletti, G.; Boga, C. Methyl 9-(1-methyl-1H-indol-3-yl)-9-oxononanoate. Molbank 2023, 2023, M1579. https://doi.org/10.3390/M1579
Micheletti G, Boga C. Methyl 9-(1-methyl-1H-indol-3-yl)-9-oxononanoate. Molbank. 2023; 2023(1):M1579. https://doi.org/10.3390/M1579
Chicago/Turabian StyleMicheletti, Gabriele, and Carla Boga. 2023. "Methyl 9-(1-methyl-1H-indol-3-yl)-9-oxononanoate" Molbank 2023, no. 1: M1579. https://doi.org/10.3390/M1579
APA StyleMicheletti, G., & Boga, C. (2023). Methyl 9-(1-methyl-1H-indol-3-yl)-9-oxononanoate. Molbank, 2023(1), M1579. https://doi.org/10.3390/M1579






