Abstract
Urazolyl radicals are a class of persistent nitrogen-centered radicals. In a previous work, we successfully formed self-assembled monolayers of substituted urazolyl radicals on gold surfaces. To extend the scope of these investigations, we sought to form a self-assembled monolayer using a urazolyl radical species that we knew existed predominantly in the dimerized N-N form instead of existing predominantly as free N-centered radical species, as had previously been investigated. We successfully synthesized the precursor urazole compound needed to generate the desired urazolyl radical, and completely characterized its structure. Most importantly, it was determined that the alkyne functional group that is needed to adhere to the gold surface remained intact. Unfortunately, however, we only obtained ambiguous results from attempts at forming self-assembled monolayers of this species on gold.
1. Introduction
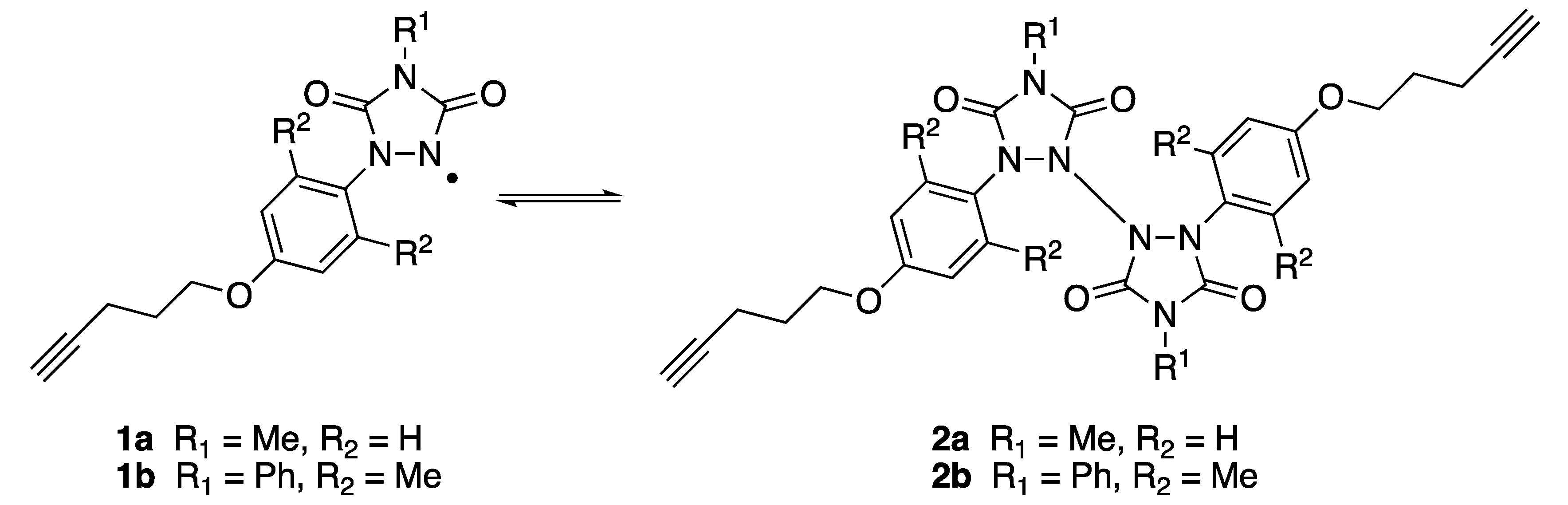
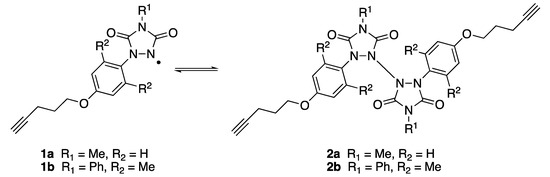
N-aryl substituted urazolyl radicals are a class of persistent nitrogen-centered radicals [1,2,3,4]. We recently reported that self-assembled monolayers (SAMS) of appropriately substituted urazolyl radicals (1a, Scheme 1) could be formed on gold surfaces [1]. The radicals were anchored to the surface via a terminal alkyne group. The resulting SAMs exhibited interesting EPR and electrochemical (cyclic voltammetry [CV]) properties as a result of the unpaired electron centered on the urazole nitrogen atom [1]. The results observed on the surface-anchored species mimicked the same properties of radical 1a when it was unbound in the solution, thus suggesting that the urazolyl radical character remained active even when the molecule was tethered to the surface. Solutions of radical 1a are deep purple in color, despite the fact that they exist in equilibrium with the corresponding colorless N-N dimer (2a, Scheme 1); this suggests that appreciable concentrations of the free radical remain available [2,3]. Phenyl-substituted urazole radicals that bear the bis-ortho substitution on the aromatic ring (e.g., 1b, Scheme 1), on the other hand, are known to favor the N-N dimer form (2b, Scheme 1), even in the solution [3,4]; therefore, we synthesized compound 3 (see Scheme 2), the precursor to urazolyl radical 1b, to determine whether 1b would exhibit radical behavior after it adheres to the gold surface.
Scheme 1.
Urazolyl radicals 1a and 1b in equilibrium, in a solution with N-N dimers 2a and 2b.
Scheme 2.
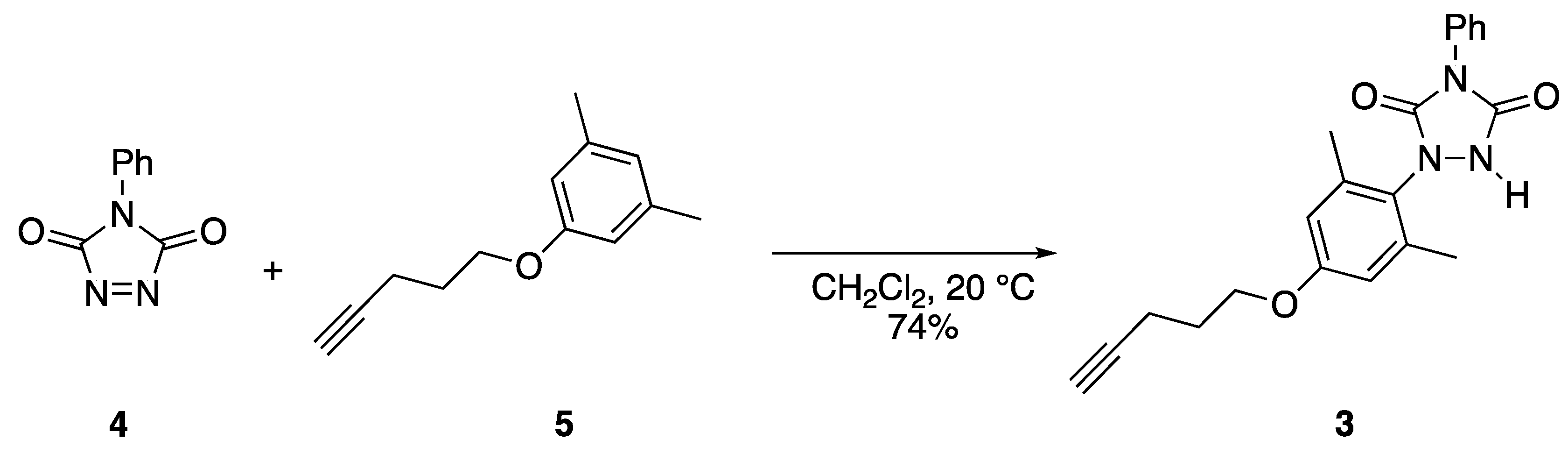
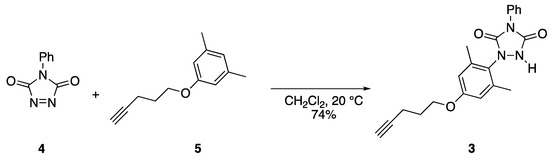
Reaction of ether (5) with PhTAD (4) to yield urazole (3).
2. Results
Urazolyl radicals are generated via the oxidation of the corresponding NH urazole species [2,3,4]. The synthesis of urazole compound 3, the precursor to 1b, was accomplished, the process of which is shown in Scheme 2. The thermal reaction of N-phenyl-1,2,4-triazoline-3,5-dione (4, PhTAD), with the known phenyl ether 5 [5], in CH2Cl2, at room temperature, produced a 74% yield of urazole 3, in the form of a crystalline white solid. The structural assignment was accomplished via 1H and 13C NMR spectroscopy (including 2D HETCOR), IR analysis, and high-resolution mass spectrometry (HRMS). As triazolinedione compounds such as PhTAD are known to react with alkynyl compounds in certain circumstances [6], it was important to ensure that the alkyne function remained unaltered during the reaction. The 1H NMR spectrum clearly showed the alkynyl CH as a triplet, with a small coupling constant of only 2.5 Hz, and with a signal integration of only one proton. The 13C NMR displayed the two alkynyl carbons at 83.4 and 69.2 ppm, which were good matches for the same two carbons in compound 5 (at 83.6 and 68.8 ppm) [5]. IR analysis clearly showed the presence of the NH of the urazole ring at 3248 cm−1, along with carbonyl groups (1765 and 1680 cm−1), and C–O stretching (1140 cm−1). Finally, the methyl groups on the aromatic ring were chemically equivalent, as determined by both 1H (a single signal at 2.22 ppm) and 13C NMR spectroscopy (a single signal at 18.2 ppm that was confirmed to be the methyl groups via HETCOR analysis). This observation confirmed that the PhTAD was added at position para to the strongly electron donating –OR group on the benzene ring. If PhTAD had been added to the position ortho relative to the –OR group site, the two methyls would have been chemically inequivalent. Finally, high-resolution mass spectrometry confirmed the molecular formula for urazole 3; thus, all spectral data were consistent with the assigned structure.
3. Discussion
The reaction between N1-substituted urazoles and the heterogenous oxidant Ni2O3 is known to provide high yields of corresponding urazolyl radicals [2,3,4]. Although solutions of urazolyl radical 1a were deep blue in color, thus indicating the presence of appreciable amounts of N-centered radicals in the solution [1], the oxidation of urazole 3 did not yield a deeply colored solution. The lack of color suggests that urazolyl radical 1b exists predominantly in the form of the N-N dimer 2b when in the solution.
Attempts at tethering solutions of freshly generated 1b/2b onto gold surfaces (as had been successfully accomplished with 1a) yielded ambiguous experimental results. We could not definitively ascertain as to how (or if) the compound had successfully adhered onto the gold surface as we had been able to do with SAMs that were generated with 1a; therefore, unfortunately, we had to abandon further studies with this compound. Future work will continue with other urazolyl radical species that are known to exist in a solution, and which retain their radical character.
4. Materials and Methods
4.1. General Methods
The 1H and 13C NMR spectra were obtained using a 400 MHz NMR spectrometer. The chemical shifts were reported in units of parts per million downfield from TMS. High-resolution mass spectra (HRMS) were acquired via electron spray ionization on an LTQ-FTMS hybrid mass spectrometer. Ether 5 was synthesized, in accordance with the procedure given in the literature [5]. All of the other compounds were commercially available and used as received.
4.2. Synthesis of 1-[2,6-Dimethyl-4-(pent-4-yn-1-yloxy)phenyl]-4-phenyl-1,2,4-triazolidine-3,5-dione (3)
To a solution of 0.5 g (2.66 mmol) of ether 5 [5] in 20 mL of CH2Cl2, 0.44 g (0.95 equivalents) of PhTAD was added as a solid, and it was stirred at room temperature. The resultant deep red solution was stirred for 48 h, after which, the red color gave way to a pale orange colored solution. The reaction mixture was concentrated, and the resultant residue was chromatographed (SiO2; 1:1 hexanes: ethyl acetate) so that 0.71 g (74% yield) of 3 as a crystalline white solid was produced (m.p., 136–138 °C: IR (ATR) cm−1 3248, 2925, 2887, 1766, 1689, 1429, 1140, 1061). 1H NMR (400 MHz, DMSO-d6) δ 11.30 (br s, 1H), 7.55–7.49 (m, 4H), 7.47–7.38 (m, 1H), 6.80 (s, 2H), 4.06 (t, J = 6.2 Hz, 2H), 2.84 (t, J = 2.5 Hz, 1H), 2.33 (dt, J = 2.5, 6.8 Hz, 2H), 2.22 (s, 6H), 1.89 (p, J = 6.8 Hz, 2H); 13C{1H} NMR (100 MHz, CDCl3); δ 159.8, 153.4, 150.4, 139.9, 131.4, 129.3, 128.4, 125.8, 124.8, 114.6, 83.4, 69.2, 66.3, 28.1, 18.2, 15.2. HRMS (electron spray ionization) m/z [M + H]+ Calculated for C21H22N3O3 364.16557; Found 364.16541.
Supplementary Materials
The following supporting information can be downloaded online. For compound 3: 1H NMR spectrum, 13C NMR spectrum, HETCOR spectrum, IR spectrum, HRMS spectra.
Funding
This research was funded by Berry College.
Data Availability Statement
Copies of spectral data for novel compound 3 are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Campos-Lendinez, A.; Crivillers, N.; Bromley, S.T.; Rovira, C.; Breton, G.W.; Mas-Torrent, M. Efficient Routes for the Preparation of Urazole Radical Self-Assembled Monolayers on Gold Surfaces. J. Phys. Chem. 2022, 126, 133358–133365. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Gravel, P.L. Persistent Cyclic Diacylhydrazyl Radicals from Urazoles and Pyrazolidine-3,5-diones. J. Org. Chem. 1978, 43, 808–815. [Google Scholar] [CrossRef]
- Breton, G.W.; Martin, K.L. Probing the Dynamic Covalent Chemistry Behavior of Nitrogen-Centered Di- and Triurazole Radicals. J. Org. Chem. 2020, 85, 10865–10871. [Google Scholar] [CrossRef] [PubMed]
- Breton, G.W.; Bowron, J.A. 1-(4-{[3,5-bis({[3,5-Dimethyl-4-(4-methyl-3,5-dioxo-1,2,4-triazolindin-1-yl)-phenoxy]methyl})phenyl]methoxy}-2,6-dimethylphenyl)-4-methyl-1,2,4-triazolidine-3,5-dione. Molbank 2023, 2323, M1535. [Google Scholar]
- Meng, Z.; Xiang, J.F.; Chen, C.F. Directional Molecular Transportation Based on a Catalytic Stopper-Leaving Rotaxane System. J. Am. Chem. Soc. 2016, 138, 5652–5658. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Greene, F.D.; Blount, J.F. Reaction of Triazolinediones with Acetylenes. Electrophilic Addition. J. Org. Chem. 1984, 49, 2917–2922. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).