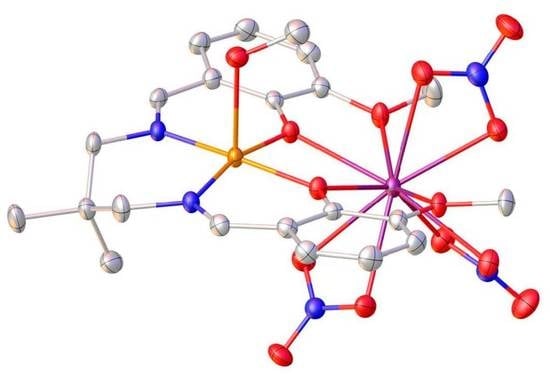
Heteronuclear Bimetallic Complexes with 3d and 4f Elements
Abstract
:1. Introduction
2. Results
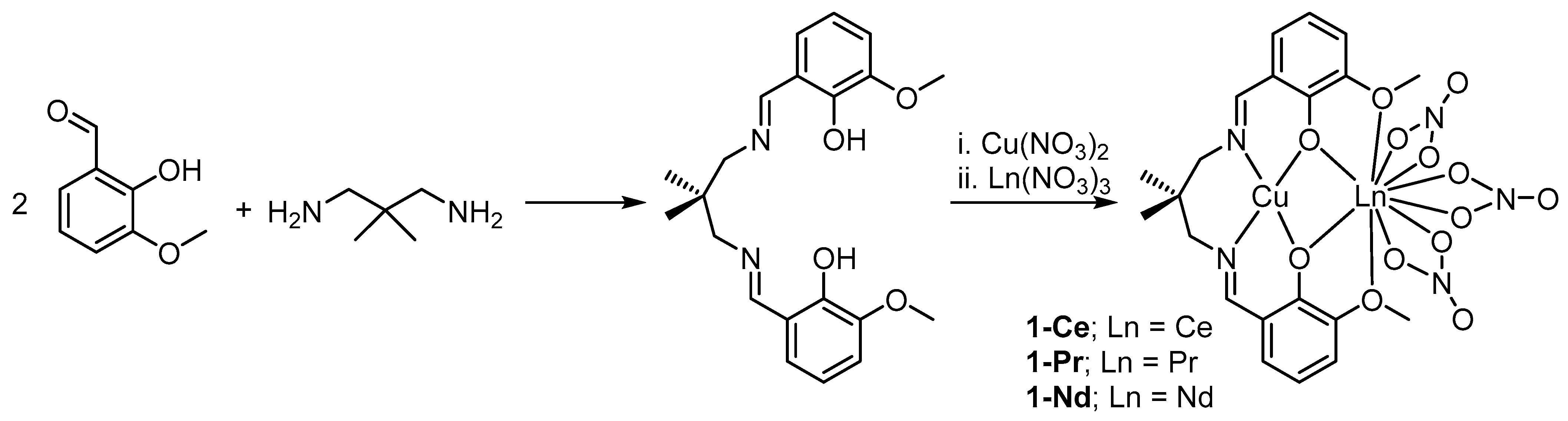
2.1. Synthesis and Characterization
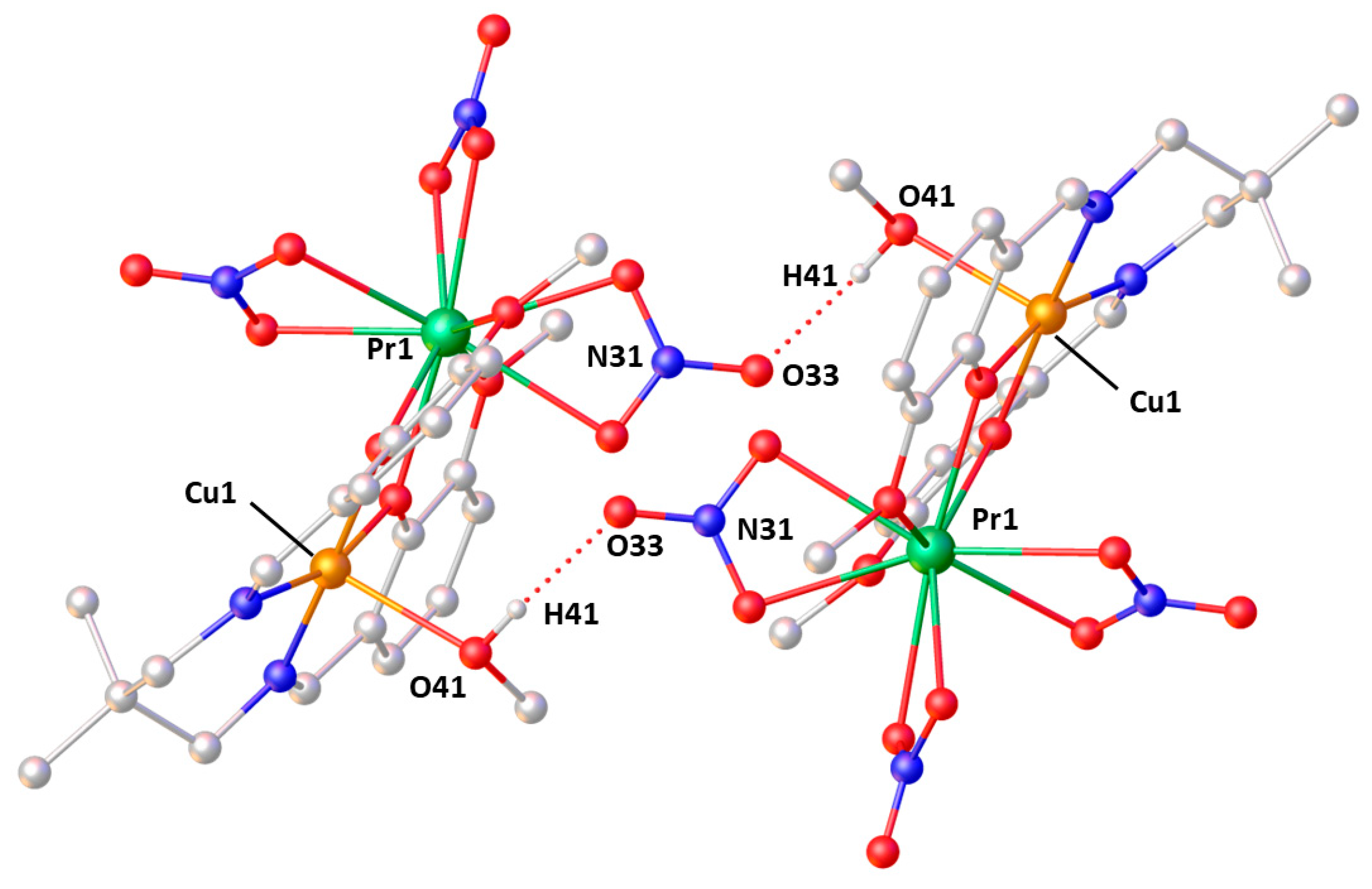
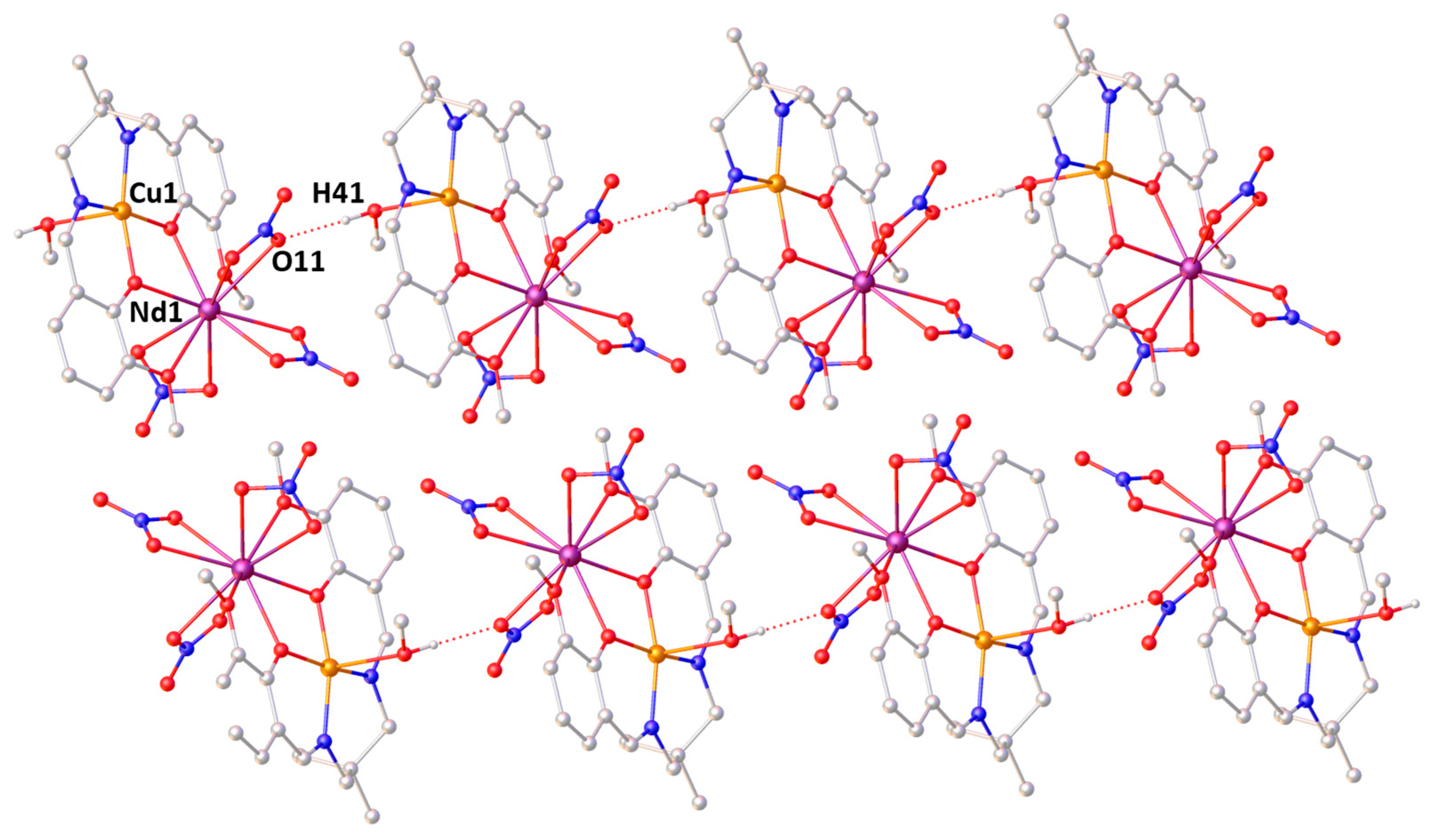
2.2. X-ray Structures
3. Materials and Methods
3.1. General Considerations
3.2. Syntheses
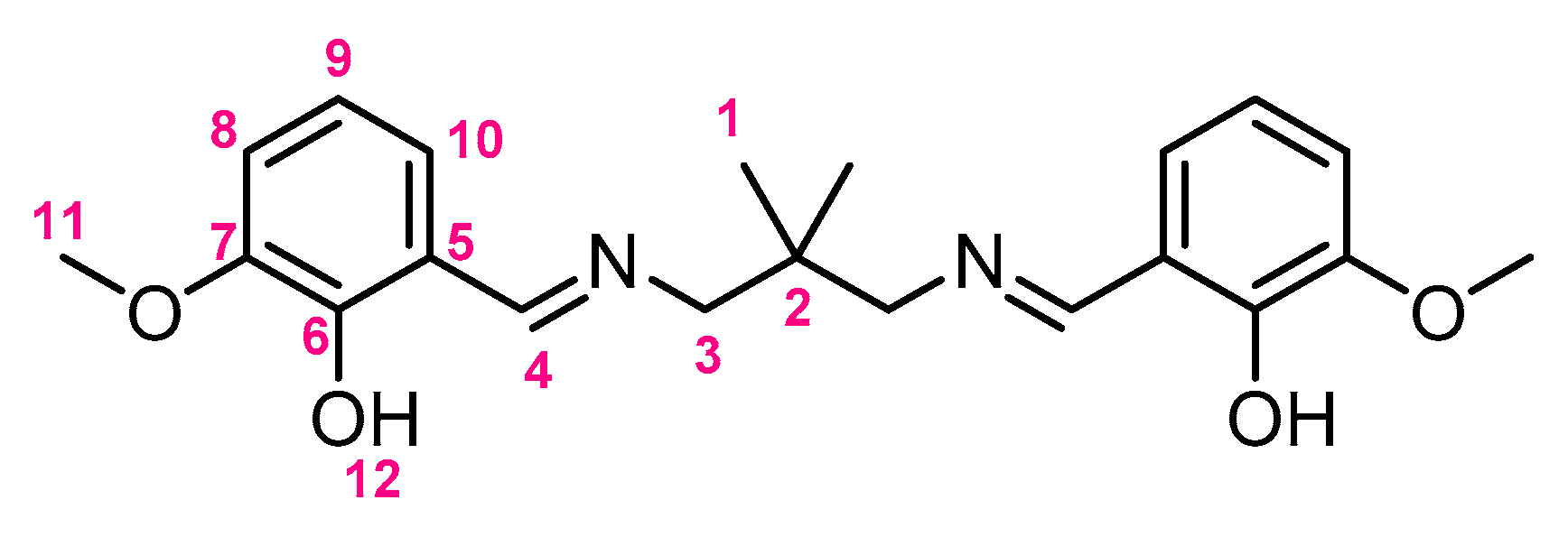
3.2.1. Synthesis of H2L
3.2.2. One-Pot Synthesis of 1-Ce, 1-Pr, and 1-Nd
3.2.3. Analytical Data for 1-Ce
3.2.4. Analytical Data for 1-Pr
3.2.5. Analytical Data for 1-Nd
3.2.6. Crystallographic Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winpenny, R.E.P. The structures and magnetic properties of complexes containing 3d- and 4f-metals. Chem. Soc. Rev. 1998, 27, 447–452. [Google Scholar] [CrossRef]
- Piguet, C.; Bunzli, J.-C.G. Mono- and polymetallic lanthanide-containing functional assemblies: A field between tradition and novelty. Chem. Soc. Rev. 1999, 28, 347–358. [Google Scholar] [CrossRef]
- Sakamoto, M.; Manseki, K.; Okawa, H. d-f Heteronuclear complexes: Synthesis, structures and physicochemical aspects. Coord. Chem. Rev 2001, 219–221, 379–414. [Google Scholar] [CrossRef]
- Abdelbaky, M.S.M.; Amghouz, Z.; Garcia-Granda, S.; Garcia, J.R. Synthesis, Structures and Luminescence Properties of Metal-Organic Frameworks Based on Lithium-Lanthanide and Terephthalate. Polymers 2016, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, M.S.M.; Amghouz, Z.; García-Granda, S.; García, J.R. A metal-organic framework assembled from Y(III), Li(I), and terephthalate: Hydrothermal synthesis, crystal structure, thermal decomposition and topological studies. Dalton Trans. 2014, 43, 5739–5746. [Google Scholar] [CrossRef]
- Aguiari, A.; Bullita, E.; Casellato, U.; Guerriero, P.; Tamburini, S.; Vigato, P.A.; Russo, U. Preparation, properties and coordination behaviour of planar or tridimensional compartmental Schiff bases. Inorg. Chim. Acta. 1994, 1–2, 135–146. [Google Scholar] [CrossRef]
- Thevenon, A.; Garden, J.A.; White, A.J.P.; Williams, C.K. Dinuclear Zinc Salen Catalysts for the Ring Opening Copolymerisation of Epoxides and Carbon Dioxide or Anhydrides. Inorg. Chem. 2015, 54, 11906–11915. [Google Scholar] [CrossRef]
- Rayati, S.; Zakavi, S.; Koliaei, M.; Wojtczak, A.; Kozakiewicz, A. Electron-rich salen-type Schiff based complexes of Cu(II) as catalysts for oxidation of cyclooctene and styrene with tert-butylhydroperoxide: A comparison with electron-deficient ones. Inorg. Chem. Commun. 2010, 13, 203–207. [Google Scholar] [CrossRef]
- Diment, W.T.; Stößer, T.; Kerr, R.W.F.; Phanopoulos, A.; Durr, C.B.; Williams, C.K. Ortho-vanillin derived Al(III) and Co(III) catalyst systems for switchable catalysis using ε-decalactone phthalic anhydride and cyclohexene oxide. Catal. Sci. Technol. 2021, 11, 1737–1745. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. A General Route to Strictly Dinuclear Cu(II)/Ln(III) Complexes. Structural Determination and Magnetic Behavior of Two Cu(II)/Gd(III) Complexes. Inorg. Chem. 1997, 36, 3429–3433. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. Experimental Evidence of a Ferromagnetic Ground State (S = 9/2) for a Dinuclear Gd(III)-Ni(II) Complex. Inorg. Chem. 1997, 36, 4284–4286. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Donnadieu, B.; Garcia-Tojal, J.; Laurent, J.P. Versatility of the Nature of the Magnetic Gadolinium(III)-Vanadium(IV) Interaction—Structure and Magnetic Properties of Two Heterobinuclear [Gd, V(O)] Complexes. Eur. J. Inorg. Chem. 2001, 2001, 363–365. [Google Scholar] [CrossRef]
- Costes, J.-P.; Clemente-Juan, J.M.; Dahan, F.; Dumestre, F.; Tuchagues, J.-P. Dinuclear (FeII, GdIII) Complexes Deriving from Hexadentate Schiff Bases: Synthesis, Structure, and Mössbauer and Magnetic Properties. Inorg. Chem. 2002, 41, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Dahan, F.; Garcia-Tojal, J. Dinuclear CoII/GdIII and CoIII/GdIII Complexes Derived from Hexadentate Schiff Bases: Synthesis, Structure, and Magnetic Properties. Chem. Eur. J. 2002, 8, 5430–5434. [Google Scholar] [CrossRef]
- Cimpoesu, F.; Dahan, F.; Ladeira, S.; Ferbinteanu, M.; Costes, J.-P. Chiral Crystallization of a Heterodinuclear Ni-Ln Series: Comprehensive Analysis of the Magnetic Properties. Inorg. Chem. 2012, 51, 11279–11293. [Google Scholar] [CrossRef]
- Kajiwara, T.; Takahashi, K.; Hiraizumi, T.; Takaishi, S.; Yamashita, M. Coordination enhancement of single-molecule magnet behaviour of Tb(III)–Cu(II) dinuclear systems. Polyhedron 2009, 28, 1860–1863. [Google Scholar] [CrossRef]
- Ishida, T.; Watanabe, R.; Fujiwara, K.; Okazawa, A.; Kojima, N.; Tanaka, G.; Yoshii, S.; Nojiri, H. Exchange coupling in TbCu and DyCu single-molecule magnets and related lanthanide and vanadium analogs. Dalton Trans. 2012, 41, 13609–13619. [Google Scholar] [CrossRef]
- Marius, A.; Costes, J.-P.; Diaz, C.; Gao, S. 3d-4f Combined Chemistry: Synthetic Strategies and Magneitc Properties. Inorg. Chem. 2009, 48, 3342–3359. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. Towards heterometallic single-molecule magnets; Synthetic strategy, structures and properties of 3d-4f discrete complexes. Coord. Chem. Rev. 2015, 289–290, 74–122. [Google Scholar] [CrossRef]
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3d-4f Complexes as Single-Molecule Magnets. Chem. Asian. J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef]
- Pasatoiu, T.D.; Madalan, A.M.; Zamfirescu, M.; Tiseanu, C.; Andruh, M. One- and two-photon induced emission in heterobimetallic ZnII–SmIII and ZnII–TbIII complexes with a side-off compartmental ligand. Phys. Chem. Chem. Phys. 2012, 14, 11448–11456. [Google Scholar] [CrossRef] [PubMed]
- Arnáiz, F.J.; Costes, J.-P.; García-Tojal, J. Inorganic Experiments, 3rd ed; Woollins, J.D., Ed.; Wiley-VCH verlag GmbH & Co, KGaAm: Weinheim, Germany, 2010; pp. 283–286. [Google Scholar]
- Costes, J.-P.; Laussac, J.-P.; Nicodème, F. Complexation of a Schiff baseligand having two coordination sites (N2O2) and O2O2) with lanthanide ions (Ln = La, Pr): An NMR study. J. Chem. Soc. Dalton. Trans 2002, 2731–2736. [Google Scholar] [CrossRef]
- Gaye, M.; Tamboura, F.B.; Sall, A.S. Spectroscopic Studies of Some Lanthanide(III) Nitrate Complexes Synthesised From A New Ligand 2,6-Bis(salicylaldehydehydrazone)-4-chlorophenol. Bull. Chem. Soc. Ethiop. 2003, 17, 27–34. [Google Scholar] [CrossRef]
- Ferraro, J.R. The nitrate symmetry in metallic nitrates. J. Mol. Spectra 1960, 4, 99–105. [Google Scholar] [CrossRef]
- Adiyodi, A.K.; Jyothy, P.V.; Unnikrishnan, N.V. UV-Vis Absorption and NIR-Stimulated Emission of Nd3+: PVA Films. J. Appl. Polym. Sci. 2009, 113, 887–895. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–452. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- CrystalClear-SM Expert v2.1; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2015.
- Beurskens, P.T.; Beurskens, G.; de Gelder, R.; Garcia-Granda, S.; Gould, R.O.; Israel, R.; Smits, J.M.M. DIRDIF-99. Crystallography Laboratory, University of Nijmegen: Nijmegen, The Netherlands, 1999.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- CrystalStructure v4.3.0; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2018.

| 1-Ce | 1-Pr | 1-Nd | |
|---|---|---|---|
| Cu1–N1 | 2.0020(19) | 2.0039(17) | 1.997(2) |
| Cu1–N5 | 1.9579(19) | 1.9560(17) | 1.974(2) |
| Cu1–O8 | 1.9534(15) | 1.9536(14) | 1.9589(17) |
| Cu1–O18 | 1.9689(15) | 1.9634(14) | 1.9639(18) |
| Cu1–O41 | 2.3400(17) | 2.3404(15) | 2.3436(19) |
| Ln1–O8 | 2.4313(15) | 2.4155(14) | 2.4113(18) |
| Ln1–O9 | 2.5912(16) | 2.5789(14) | 2.5391(17) |
| Ln1–O18 | 2.4663(15) | 2.4520(14) | 2.4468(17) |
| Ln1–O19 | 2.6243(16) | 2.6122(14) | 2.5766(19) |
| Cu1⋯Ln1 | 3.5896(4) | 3.5747(4) | 3.5758(6) |
| N1–Cu1–N5 | 96.02(8) | 96.14(7) | 96.68(9) |
| O8–Cu1–O18 | 80.00(6) | 79.82(6) | 78.67(7) |
| O8–Ln1–O18 | 61.96(5) | 62.16(5) | 61.57(6) |
| O9–Ln1–O19 | 148.10(5) | 148.06(5) | 149.70(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmers, B.A.; Cordes, D.B.; Bertram, L.; Harraghy, D.J.; Reid, R.C.; Smellie, I.A.; Tarcza, A.E.; Thomson, B.J. Heteronuclear Bimetallic Complexes with 3d and 4f Elements. Molbank 2023, 2023, M1577. https://doi.org/10.3390/M1577
Chalmers BA, Cordes DB, Bertram L, Harraghy DJ, Reid RC, Smellie IA, Tarcza AE, Thomson BJ. Heteronuclear Bimetallic Complexes with 3d and 4f Elements. Molbank. 2023; 2023(1):M1577. https://doi.org/10.3390/M1577
Chicago/Turabian StyleChalmers, Brian A., David B. Cordes, Lauren Bertram, Daniel J. Harraghy, Rachel C. Reid, Iain A. Smellie, Anna E. Tarcza, and Brodie J. Thomson. 2023. "Heteronuclear Bimetallic Complexes with 3d and 4f Elements" Molbank 2023, no. 1: M1577. https://doi.org/10.3390/M1577
APA StyleChalmers, B. A., Cordes, D. B., Bertram, L., Harraghy, D. J., Reid, R. C., Smellie, I. A., Tarcza, A. E., & Thomson, B. J. (2023). Heteronuclear Bimetallic Complexes with 3d and 4f Elements. Molbank, 2023(1), M1577. https://doi.org/10.3390/M1577