Abstract
(S)-N1,N3-dibenzyl-1-cyclohexyl-N1,N3-bis((R)-1-phenylethyl)propane-1,3-diamine was prepared in good yield by the reduction of the corresponding amide, which was obtained by the addition of a chiral lithium amide to an α,β-unsaturated ester. The target compound was fully characterized by NMR (1H and 13C), high-resolution mass spectrometry and polarimetry.
1. Introduction
1,3-Diamines represent a chemical space with attractive bioactivity, such as sparteine and its derivatives (Figure 1). These compounds showed diverse therapeutic effects [1,2], including antiarrhythmic [3], anticonvulsant [4], sodium channel blockers [5], and neuroprotective activity [6]. In addition to its clinical use, chiral 1,3-diamines present interesting applications in organic chemistry used as organocatalysts or chiral auxiliars [7,8,9,10]. The synthesis of 1,3-diamine scaffold has been reported using a wide variety of methodologies and starting materials [11,12,13], commonly using some types of catalyst. Therefore, a cheap and quick methodology for the asymmetric synthesis of this type of compounds would be interesting and useful.
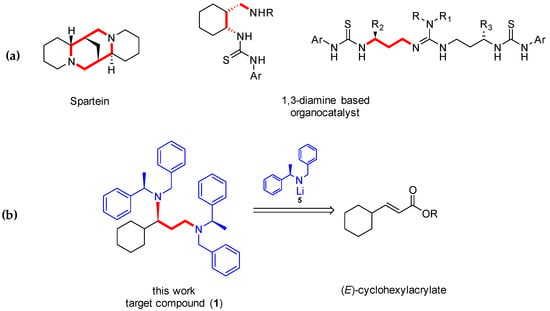
Figure 1.
(a) 1,3-diamine-based compounds [14]. (b) Retrosynthetic analysis of 1.
In this study, we present the synthesis of a chiral 1,3-diamine using chiral lithium amides. Davies et al. had widely studied the asymmetric addition of this chiral lithium amides to different α,β-unsaturated esters, with a total control of the stereochemistry [15,16,17]. In our laboratory, this methodology has been used several times to afford the synthesis of a series of compounds with interesting biological activity [18,19,20], and recently using Ezetimibe analogs, through a domino protocol by the addition of the chiral lithium amide to Baylis–Hillman adducts.
The newly formed stereocenter is determined by the chirality of the amine which formed the lithium amide [21,22,23], giving us a quick method to the asymmetric synthesis of promising compounds obtaining the 1,3-diamine scaffold (Figure 2).
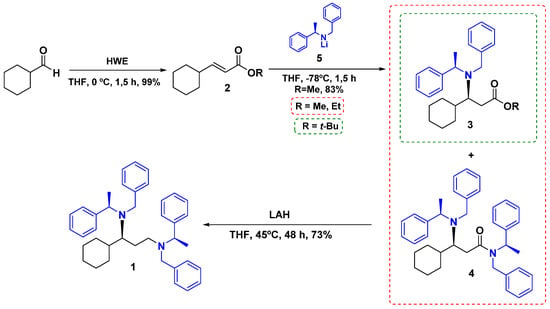
Figure 2.
Synthetic pathway of 1.
2. Results and Discussion
The addition of n-BuLi (1,6 M in hexane) to a THF solution of (R)-(+)-N-benzyl-N-methylbenzylamine at −78 °C furnished the corresponding lithium amide (5) [24]. Different (E)-3-cyclohexyl acrylates were used as substrates, particularly, methyl, ethyl, and tert-butyl acrylates. Table 1 shows the results obtained for each case.

Table 1.
Different R groups and their yields.
When tert-butyl acrylate is used as a substrate, the only product obtained is the corresponding amino-ester as a result of the attack of the lithium amide in the β-position (98% yield) [Entry 1]. The product is obtained in high yield because of the high steric hindrance of the tert-butyl group, preventing the attack of another equivalent of lithium amide to the carbonyl moiety. If the ester’s protecting group is replaced with an ethyl group, the yield of the corresponding amino-ester 3 (spectroscopic information in the Supplementary Materials) drops to 37% and the main product is amide 4, resulting from the attack of two equivalents of lithium amide (5) (54% yield) [Entry 2]. Yield is increased to 72% if methyl acrylate is used [Entry 3], and when doubling the equivalents of lithium amide used, the yield increases up to 83% [Entry 4], due the low steric hindrance of this group.
The 13C NMR of amide 4 shows the duplicity of several signals, due the existence of rotamers. The C–N bond of the amide group has no free rotation; thus, the amide N-substituents show duplicated signals in both 1C NMR and 1H NMR. Thus, the spectra of compound 4 are presented, but the signals are not assigned. Spectra at different temperatures will be derived in order to fully characterize this compound. The results of the 1H and 13C heteronuclear correlation experiments (HSQC and HMBC/Supplementary Materials) corroborate their structure and the full assignment of 1H and 13C data of 1.
Chiral 1,3-diamine 1 is obtained in 73% yield by amide 4 treatment with strong reductive conditions. Amine substituents are suitable for modification by debenzylations and further functionalization.
3. Materials and Methods
All reagents were purchased from Sigma-Aldrich (Merck Group, Darmstadt, Germany) and used without purification, except for the solvents, purified by distillation. Specific rotation was measured with a digital polarimeter Perkin-Elmer 241 (PerkinElmer, Waltham, MA, USA) using chloroform as solvent and 1 dm optical pitch cuvettes. IR spectra were taken with a spectrometer Shimazdu FT IR-Affinity 1 (Shimadzu Europa GmbH, Duisburg, Germany) Mass spectra were referred to the Mass Spectrometry service of University of Salamanca. NMR spectra were recorded on a Bruker Avance Neo 400 MHz spectrometer (Bruker, Zagreb, Croatia) using the chloroform signal as a reference (7.26 ppm in 1H and 77.00 ppm in 13C). TLC was run with Merck 60 F254 0.2 mm thickness TLC cards (Merck KGaA, Darmstadt, Germany).
Synthesis of N-benzyl-3-(benzyl((R)-1-phenylethyl)amino)-3-cyclohexyl-N-((R)-1-phenylethyl)propanamide (4): A solution of 780 mg (5.14 mmol; 2.4 Eq) of N-benzyl-N-(α)-(methyl)benzyl amine in dry THF (5 mL) and inert atmosphere was cooled at −78 °C, and 2.95 mL (4.73 mmol; 2.2 Eq) of n-BuLi 1.6 M in hexane was added dropwise while stirring in order to elaborate 5. After 15 min, the solution was immersed in an ice bath for a further 15 min. Subsequently, the solution of 5 was returned to −78 °C and transferred via cannula to the solution of 360 mg (2.15 mmol; 1 Eq) of methyl (E)-cyclohexylacrylate in dry THF (5 mL) at −78 °C and inert atmosphere. The mixture was gently stirred at −78 °C for 1.5 h. NH4Cl sat. (10 mL) was added before heating to room temperature. THF was removed in vacuum and the aqueous phase was extracted with AcOEt (5 mL) 3 times. The organic phase was washed 3 times with 10% p/v citric acid, 3 times with H2O, 3 times with brine, and the solvent was removed under vacuum. The concentrated extract was flash-chromatographed (hexane/ethyl ether 95/5 to 9/1) on silica gel obtaining 3 and 4 in 20% and 70% yields, respectively.
The same procedure was carried out but doubling lithium amide equivalents (N-benzyl-N-(α)-(methyl)benzyl amine: 1.54 g; 10.28 mmol; 4.8 Eq; n-BuLi: 5.9 mL; 9.45 mmol; 4.4 Eq) and only 4 was isolated, exhibited as a pale yellow oil in 83% yield. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.52–6.88 (m), 6.12 (q, J = 7.2 Hz), 4.92 (d, J = 15.5 Hz), 4.85 (q, J = 7.0 Hz), 4.03 (d, J = 17.8 Hz), 3.92 (d, J = 17.8 Hz), 3.89 (J = 17.8 Hz), 3.81 (m), 3.73 (m), 3.62 (J = 15.9, 7.0 Hz), 3.51 (d, J = 15.1 Hz), 3.48 (d, J = 15.0), 3.29 (d, J = 15.1 Hz), 2.29 (dd, J = 16.6, 9.4 Hz), 2.13 (d, J = 11.8 Hz), 1.98 (dd, J = 16.7, 9.7 Hz), 1.84 (d, J = 12.5 Hz). 1.43 (d, J = 7.2 Hz), 1.22 (d, J = 7.0 Hz), 1.80–0.85 (m). 13C NMR (101 MHz, CDCl3) δ 172.80, 172.13, 141.66, 141.47, 141.38, 141.35, 141.30, 128.64, 128.46, 128.37, 128.32, 128.30, 128.27, 128.25, 128.18, 128.08, 128.03, 127.98, 127.88, 127.49, 127.38, 127.35, 127.22, 126.89, 126.77, 126.66, 126.61, 126.54, 126.51, 126.46, 125.87, 57.22, 56.93, 55.08, 54,79, 51.84, 51.75, 51.39, 47.04, 46.32, 33.71, 33.16, 31.16, 30.74, 30.45, 26.92, 26.73, 19.81, 19.75, 19.16, 17.06. HR-MS: calculated for [C39H46N2O + H]+ = 559.3688, found = 559.3680 (Δ (ppm) = 1.43). IR: 3061.03 cm−1, 3026.31 cm−1, 2926.01 cm−1, 2850.79 cm−1, 1645.28 cm−1, 1492.90 cm−1, 1452.40 cm−1, 1415.75 cm−1, 1409.96 cm−1, 1028.06 cm−1, 748.38 cm−1, 743.88 cm−1, 698.23 cm−1.
Synthesis of1, reduction of4with LAH: 77 mg (1. Eq, 0.14 mmol) of 4 was dissolved in 10 mL of dry THF and immersed on an ice bath at 0 ºC while stirring. Then, 92 mg (5 Eq, 2.41 mmol) of LAH was slowly added. The solution was slowly heated up to 65 ºC while stirring under an inert atmosphere for 48 h. The crude was cooled to 0 ºC and 5 mL of saturated NH4Cl was slowly added. The THF was removed under vacuum, and the crude was extracted 3 times with AcOEt. The organic phase was washed 3 times with 5 mL of water and 3 times more with 5 mL of saturated NaCl. The organic phase was concentrated under vacuum and the concentrated extract was flash-chromatographed (hexane/ethyl ether 95/5 to 9/1), obtaining 54 mg (72% yield) of 1,5 1 as a very pale yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.36–7.09 (m, 20H), 3.73 (d, J = 15.4 Hz, 1H), 3.68 (q, J = 6.7 Hz, 1H), 3.63 (q, J = 7.0 Hz, 1H), 3.51 (d, J = 15.4 Hz, 1H), 3.42 (d, J = 14.0 Hz, 1H), 3.31 (d, J = 14.0 Hz, 1H),2.12 (m, 2H), 2,07 (td, J = 12.3, 4.9 Hz, 1H), 1.90 (d, J = 12.1 Hz, 1H), 1.63–1.46 (m, 4H), 1.25 (d, J = 6.7 Hz, 3H), 1.15 (d, J = 7.0, 3H), 1.11 (m, 1H), 1.08 (m, 1H), 1.05–0.83 (m, 5H).13C NMR (101 MHz, CDCl3) δ 144.29, 144.18, 142.70, 141.15, 128.56–126.23 (20C, CH Ar), 59.70, 58.97, 57.94, 54.33, 52.06, 49.05, 42.06, 31.58, 30.89, 30.59, 26.83, 26.81, 26.68, 26.46, 22.65, 15.28, 14.11. HR-MS: calculated for [C39H48N2 + H]+ = 545.3896, found = 545.3881 (Δ (ppm) = 2.75). IR: 3085.25 cm−1, 3061.03 cm−1, 3026.31 cm−1, 2968.45 cm−1, 2916.01 cm−1, 2850.79 cm−1, 2804.50 cm−1, 1492.90 cm−1, 1450.47 cm−1, 1371.39 cm−1, 1265.30 cm−1, 1201.65 cm−1, 1147.65 cm−1, 1111.00 cm−1, 1083.99 cm−1, 1074.35 cm−1, 1028.06 cm−1, 759.95 cm−1, 742.59 cm−1, 731.02 cm−1, 451.34 cm−1. [α]D20 = 27.9 (c = 4.16 M, CHCl3).
4. Conclusions
The target chiral diamine (S)-N1,N3-dibenzyl-1-cyclohexyl-N1,N3-bis((R)-1-phenylethyl)propane-1,3-diamine has been synthetized in a 61% overall yield by the aza-Michael addition of lithium (R)-(+)-N-benzyl-α-methylbenzylamide excess (4.4 Eq) to an affordable α,β-unsaturated ester and further reduction with LAH. In the near future, we aim to synthesize a variety of chiral 1,3-diamines using this methodology in order to carry out QSAR studies.
Supplementary Materials
The following supporting information can be downloaded. Horner–Wadsworth–Emmons’ synthetic methodology. NMR spectra (1H, 13C, HMBC, HSQC, and COSY), infrared spectrum, and HR-MS report of diamine 1 and amide 4 [23,25].
Author Contributions
Conceptualization, N.M.G. and C.T.N.; investigation, L.B., Á.G.-G. and A.M.; writing—original draft preparation, L.B.; writing—review and editing, L.B., Á.G.-G., A.M., C.T.N. and N.M.G.; supervision, N.M.G. and C.T.N. All authors have read and agreed to the published version of the manuscript.
Funding
We are indebted to the European Regional Development Fund (FEDER), Spanish Ministerio de Ciencia e Innovación (PID2020-118303GB-I00/MCIN/AEI/10.13039/501100011033) and Junta de Castilla y León (SA076P20).
Data Availability Statement
The data of this study are available in this paper and its Supplementary Materials.
Acknowledgments
The authors are also grateful for the support from Servicios de la Universidad de Salamanca (Nucleus): A.M. Lthgow for the NMR and César Raposo for the mass spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hidalgo, M.; Asmat Marrufo, P.; Lezama Asencio, P.; Ramos, C.; Chimoy Tuñoque, C.A.; Zolla, G. Evaluation of in vitro susceptibility to sparteine in four strains of Mycobacterium tuberculosis. Rev. Peru. Med. Exp. Salud Pública 2022, 39, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kassenbrock, A.; Li, B.X.; Xiao, X. Discovery of a potent anti-tumor agent through regioselective mono-N-acylation of 7 H-pyrrolo [3, 2-f] quinazoline-1, 3-diamine. MedChemComm 2013, 4, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Pugsley, M.K.; Saint, D.A.; Hayes, E.; Berlin, K.D.; Walker, M.J. The cardiac electrophysiological effects of sparteine and its analogue BRB-I-28 in the rat. Eur. J. Pharmacol. 1995, 294, 319–327. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Lee, C.S.; Wang, J. Enantio-and Regioselective Construction of 1, 4-Diamines via Cascade Hydroamination of Methylene Cyclopropanes. Angew. Chem. Int. Ed. 2022, 61, e202202160. [Google Scholar] [CrossRef]
- Gawali, V.S.; Simeonov, S.; Drescher, M.; Knott, T.; Scheel, O.; Kudolo, J.; Kählig, H.; Hochenegg, U.; Roller, A.; Todt, H. C2-Modified Sparteine Derivatives Are a New Class of Potentially Long-Acting Sodium Channel Blockers. ChemMedChem 2017, 12, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Gavilan, J.; Mennickent, D.; Ramirez-Molina, O.; Trivino, S.; Perez, C.; Silva-Grecchi, T.; Godoy, P.A.; Becerra, J.; Aguayo, L.G.; Moraga-Cid, G. 17 Oxo Sparteine and Lupanine, Obtained from Cytisus scoparius, Exert a Neuroprotection against Soluble Oligomers of Amyloid-β Toxicity by Nicotinic Acetylcholine Receptors. J. Alzheimer’s Dis. 2019, 67, 343–356. [Google Scholar] [CrossRef]
- Kodama, K.; Sugawara, K.; Hirose, T. Synthesis of Chiral 1, 3-Diamines Derived from cis-2-Benzamidocyclohexanecarboxylic Acid and Their Application in the Cu-Catalyzed Enantioselective Henry Reaction. Chem. Eur. J. 2011, 17, 13584–13592. [Google Scholar] [CrossRef]
- Kizirian, J. Chiral tertiary diamines in asymmetric synthesis. Chem. Rev. 2008, 108, 140–205. [Google Scholar] [CrossRef]
- Mayans, E.; Gargallo, A.; Álvarez-Larena, Á.; Illa, O.; Ortuño, R.M. Diastereodivergent Synthesis of Chiral vic-Disubstituted-Cyclobutane Scaffolds: 1, 3-Amino Alcohol and 1, 3-Diamine Derivatives–Preliminary Use in Organocatalysis. Eur. J. Org. Chem. 2013, 2013, 1425–1433. [Google Scholar] [CrossRef]
- Csillag, K.; Szakonyi, Z.; Fülöp, F. Stereoselective syntheses of pinane-based 1, 3-diamines and their application as chiral ligands in the enantioselective addition of diethylzinc to benzaldehyde. Tetrahedron Asymmetry 2013, 24, 553–561. [Google Scholar] [CrossRef]
- Ji, X.; Huang, H. Synthetic methods for 1, 3-diamines. Org. Biomol. Chem. 2016, 14, 10557–10566. [Google Scholar] [CrossRef]
- Li, K.; Weber, A.E.; Tseng, L.; Malcolmson, S.J. Diastereoselective and enantiospecific synthesis of 1, 3-diamines via 2-azaallyl anion benzylic ring-opening of aziridines. Org. Lett. 2017, 19, 4239–4242. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, T.; Leitch, J.A.; Grainger, R.; Dixon, D.J. Photocatalytic three-component umpolung synthesis of 1, 3-diamines. Org. Lett. 2018, 20, 6794–6798. [Google Scholar] [CrossRef] [PubMed]
- Ričko, S.; Svete, J.; Štefane, B.; Perdih, A.; Golobič, A.; Meden, A.; Grošelj, U. 1, 3-Diamine-Derived Bifunctional Organocatalyst Prepared from Camphor. Adv. Synth. Catal. 2016, 358, 3786–3796. [Google Scholar] [CrossRef]
- Davies, S.G.; Smith, A.D.; Price, P.D. The conjugate addition of enantiomerically pure lithium amides as homochiral ammonia equivalents: Scope, limitations and synthetic applications. Tetrahedron Asymmetry 2005, 16, 2833–2891. [Google Scholar] [CrossRef]
- Davies, S.G.; Fletcher, A.M.; Roberts, P.M.; Thomson, J.E. The conjugate addition of enantiomerically pure lithium amides as chiral ammonia equivalents part II: 2005–2011. Tetrahedron Asymmetry 2012, 23, 1111–1153. [Google Scholar] [CrossRef]
- Davies, S.G.; Fletcher, A.M.; Roberts, P.M.; Thomson, J.E. The conjugate addition of enantiomerically pure lithium amides as chiral ammonia equivalents part III: 2012–2017. Tetrahedron Asymmetry 2017, 28, 1842–1868. [Google Scholar] [CrossRef]
- Garrido, N.M.; Nieto, C.T.; Diez, D. Enantioselective synthesis of a (1R, 5R, 9R)-2-azabicyclo [3.3.1] nonane-9-carboxylic acid with an embedded morphan motif: A multipurpose product. Synlett 2013, 24, 169–172. [Google Scholar] [CrossRef]
- Nieto, C.T.; Gonzalez-Nunez, V.; Rodríguez, R.E.; Diez, D.; Garrido, N.M. Design, synthesis, pharmacological evaluation and molecular dynamics of β-amino acids morphan-derivatives as novel ligands for opioid receptors. Eur. J. Med. Chem. 2015, 101, 150–162. [Google Scholar] [CrossRef]
- Salgado, M.M.; Manchado, A.; Nieto, C.T.; Díez, D.; Garrido, N.M. Synthesis and Modeling of Ezetimibe Analogues. Molecules 2021, 26, 3107. [Google Scholar] [CrossRef]
- Urones, J.G.; Garrido, N.M.; Díez, D.; Dominguez, S.H.; Davies, S.G. Asymmetric synthesis of (R)-and (S)-methyl (2-methoxy-carbonylcyclopent-2-enyl) acetate and (R)-and (S)-2-(2-hydroxy-methyl-cyclopent-2-enyl) ethanol. Tetrahedron Asymmetry 1997, 8, 2683–2685. [Google Scholar] [CrossRef]
- Davies, S.G.; Walters, I.A. Asymmetric synthesis of anti-α-alkyl-β-amino Acids. J. Chem. Soc. Perkin Trans. 1 1994, 1129–1139. [Google Scholar] [CrossRef]
- Davies, S.G.; Ichihara, O. Asymmetric synthesis of R-β-amino butanoic acid and S-β-tyrosine: Homochiral lithium amide equivalents for Michael additions to α, β-unsaturated esters. Tetrahedron Asymmetry 1991, 2, 183–186. [Google Scholar] [CrossRef]
- Koperniku, A.; Schafer, L.L. Zirconium catalyzed hydroaminoalkylation for the synthesis of α-Arylated amines and N-Heterocycles. Chem. Eur. J. 2021, 27, 6334–6339. [Google Scholar] [CrossRef]
- Das, M.; Manvar, A.; Fox, I.; Roberts, D.J.; O’Shea, D.F. Bu4N-controlled addition and olefination with ethyl 2-(trimethylsilyl) acetate via silicon activation. Synlett 2017, 28, 2401–2406. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).