2-[Difluoro(phenylselenyl)methyl]benzo-1,3-thiazole
Abstract
:1. Introduction
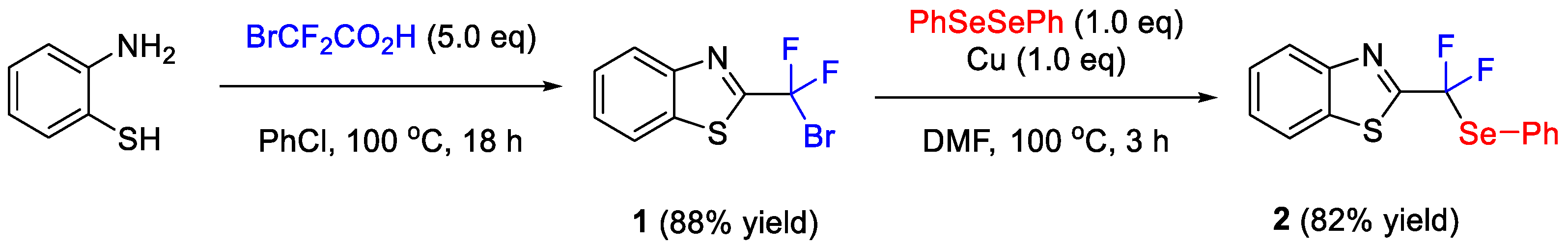
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Welch, J.T.; Eswarakrishnan, S. Fluorine in Bioorganic Chemistry; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Knust, H.; Achermann, G.; Ballard, T.; Buettelmann, B.; Gasser, R.; Fischer, H.; Hernandez, M.; Knoflach, F.; Koblet, A.; Stadler, H.; et al. The discovery and unique pharmacological profile of RO4938581 and RO4882224 as potent and selective GABAA α5 inverse agonists for the treatment of cognitive dysfunction. Bioorg. Med. Chem. Lett. 2009, 19, 5940–5944. [Google Scholar] [CrossRef] [PubMed]
- O′Hagan, D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xiao, Y.-L.; Zhang, X. Transition-metal (Cu, Pd, Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides. Acc. Chem. Res. 2018, 51, 2264–2278. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zhu, L.; Hu, J. Advances in transition-metal-mediated di-and monofluoroalkylations. Huaxue Xuebao 2015, 73, 90–115. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef]
- Mihorianu, M.; Franz, M.H.; Jones, P.G.; Freytag, M.; Kelter, G.; Fiebig, H.H.; Tamm, M.; Neda, I. N-Heterocyclic carbenes derived from imidazo-[1,5-a]pyridines related to natural products: Synthesis, structure and potential biological activity of some corresponding gold(I) and silver(I) complexes. Appl. Organomet. Chem. 2016, 30, 581–589. [Google Scholar] [CrossRef]
- Vollbrecht, A.; Neda, I.; Thonnessen, H.; Jones, P.G.; Harris, R.K.; Crowe, L.A.; Schmutzler, R. Synthesis, structure, and reactivity of tetrakis(o,o-phosphorus)-bridged calix[4]resorcinols and their derivatives. Chem. Ber. 1997, 130, 1715–1720. [Google Scholar] [CrossRef]
- Reddy, P.V.G.; Lin, Y.-W.; Chang, H.-T. Synthesis of novel benzothiazole compounds with an extended conjugated system. Arkivoc 2007, 16, 113–122. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, M.; Lu, W.; Tian, W.; Wan, W.; Chen, Y.; Deng, H.; Wu, S.; Hao, J. Direct gem-difluoromethylenation of sp3-hybridized carbon center through copper-mediated radical/radical cross-coupling for the construction of a CH2-CF2 linkage. Chem. Commun. 2015, 51, 15756–15759. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lu, W.; Yang, K.; Ma, G.; Xu, M.; Li, J.; Yao, J.; Wan, W.; Deng, H.; Wu, S.; et al. Enhancement of neighbouring-group participation in Cu0-promoted cross-coupling gem-difluoromethylenation of aryl/alkenyl halides with 1,3-azolic difluoromethyl bromides. Chem.-A Eur. J. 2014, 20, 10084–10092. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-L.; Zhang, B.; Feng, Z.; Zhang, X. Heteroaryldifluoromethylation of organoborons catalyzed by palladium: Facile access to aryl(heteroaryl)difluoromethanes. Org. Lett. 2014, 16, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, Z.-F.; An, L.; Zhang, X. Nickel-catalyzed difluoroalkylation-alkylation of enamides. ACS Catal. 2019, 9, 8224–8229. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Wang, P.; Deng, H.; Jiang, H. Copper-catalyzed aryldifluoromethylenation of N-arylacrylamides to synthesis of the diheterocyclic compounds linked by gem-difluoromethylene moiety. Tetrahedron 2019, 75, 130778. [Google Scholar] [CrossRef]
- Jiang, H.; Yuan, S.; Cai, Y.; Wan, W.; Zhu, S.; Hao, J. A facile preparation of 2-bromodifluoromethyl benzo-1,3-diazoles and its application in the synthesis of gem-difluoromethylene linked aryl ether compounds. J. Fluorine Chem. 2012, 133, 167–170. [Google Scholar] [CrossRef]
- Xu, R.; Liao, F.; Cai, Y.; Liu, J. A convenient synthesis of 2-bromodifluoromethyl benzo-1,3-thiazoles. Chin. J. Syn. Chem. 2022, 30, 588–592. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Cai, Y.; Liao, F.; Liu, J. 2-[Difluoro(phenylselenyl)methyl]benzo-1,3-thiazole. Molbank 2022, 2022, M1450. https://doi.org/10.3390/M1450
Xu R, Cai Y, Liao F, Liu J. 2-[Difluoro(phenylselenyl)methyl]benzo-1,3-thiazole. Molbank. 2022; 2022(4):M1450. https://doi.org/10.3390/M1450
Chicago/Turabian StyleXu, Rongfu, Ying Cai, Fumin Liao, and Jinbiao Liu. 2022. "2-[Difluoro(phenylselenyl)methyl]benzo-1,3-thiazole" Molbank 2022, no. 4: M1450. https://doi.org/10.3390/M1450
APA StyleXu, R., Cai, Y., Liao, F., & Liu, J. (2022). 2-[Difluoro(phenylselenyl)methyl]benzo-1,3-thiazole. Molbank, 2022(4), M1450. https://doi.org/10.3390/M1450





