Abstract
The title compound was synthesized and characterized for the first time by 1H, 13C NMR, high-resolution mass spectra and single-crystal X-ray diffraction.
1. Introduction
The growing interest in the design and synthesis of 1,3-diketones is, in particular, due to the great potential of these ligands as versatile building blocks for the construction of highly emissive metal complexes for application in organic light-emitting diodes (OLEDs) [1,2,3,4,5,6,7,8,9,10,11]. Aromatic β-diketones are also considered to be promising ligands for the creation of strong light-absorbing metal complexes for dye-sensitized solar cells [12,13,14,15]. This application requires the specific structure of diketones which should contain at least one carboxy- (or ester) group and electron-donating fragment(s) to give the resulting metal complexes robust light-harvesting characteristics. Such β-diketones are evidently asymmetric and, hence, they can be scarcely obtained via the common Claisen condensation. In this work, we synthesized an asymmetric aromatic 1,3-diketone (Figure 1) bearing the carboxymethyl group and the piperidine moiety under mild conditions and characterized the prepared ligand by a 1H, 13C {1H} NMR, high-resolution mass spectra, UV-vis spectroscopy and single-crystal X-ray diffraction.
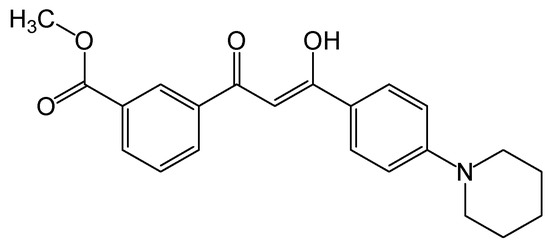
Figure 1.
Chemical structure of the title compound.
2. Results and Discussion
The title diketone was synthesized via the reaction of a benzotriazole amide of isophthalic acid monomethyl ester acting as the mild acylation agent with 1-(4-(piperidin-1-yl)phenyl)ethanone (Scheme 1). The low yield of the product (10%) is likely a result of the side formation of a symmetrical 1,3-di-(3-carboxymethylphenyl)-propane-1,3-dione (see the 1H NMR spectrum of the crude product in Supplementary Materials Figures S3–S5 and chemical structure of the side product) by a previously described intermolecular benzotriazole-mediated acylation/deacetylation process [16].

Scheme 1.
Preparation of methyl-3-(3-hydroxy-3-(4-(piperidin-1-yl)phenyl) prop-2-enoyl)benzoate.
The composition and structure of the β-diketone were identified by various spectroscopic techniques. The 1H NMR spectrum (Figure S1, Supplementary Materials) shows a characteristic singlet of the OCH3 group at 3.98 ppm while two multiples at 1.69 and 3.40 ppm with the relative intensities 6 and 4, respectively, arise from the protons of the piperidine ring; the aromatic protons appear at 6.91–8.60 ppm. The 13C NMR spectrum consists of 18 individual signals consistent with the number of unique carbon atoms in the title compound (Figure S2). In the high-resolution mass spectrum, the dominant molecular ion peak corresponds to the [M + H]+ ion.
The obtained diketone exists in enol form in the solution, as evidenced by its 1H NMR spectrum containing two characteristic singlets at 6.82 and 17.15 ppm corresponding to the α-methine proton and hydroxy-group of the enol, respectively. Single-crystal X-ray diffraction analysis revealed that the title compound exists in enol form in the solid state too.
The molecular structure of the enol is depicted in Figure 2. The conformation is non-planar with interplanar angles between the substituted phenyl rings and the keto-enol plane ranging from 3.77(4)° to 21.31(4)°. The C9–O2 bond (1.2720(13) Å) is significantly shorter than the C11–O1 bond (1.3190(13) Å); that is in good agreement with the data from the Cambridge Structural Database (Version 5.43, March 2022, Cambridge, UK). Though there are a fairly large number of reports on crystal structures of symmetrical and asymmetrical β-diketones (more than 450 structures), analysis of the database shows that the vast majority of these compounds crystallizes in enol form and only 15 are pure diketones.

Figure 2.
Molecular structure of the title enol. Displacement ellipsoids are shown at 50% probability level.
According to the database, in the structures of enols the intramolecular resonance-assisted hydrogen bonds impart short O⋯O distances (2.39–2.55 Å) [17]. The hydrogen atom shared between these oxygen atoms can be ordered, disordered by symmetry, as in dibenzoylmethane [18] and many other symmetrical β-diketones [19], or disordered with unequal occupancies (the most asymmetrical enols). In the case of the title compound, the H2 atom located from the difference Fourier map is closer to the O1 atom with the O1–H2 bond length of 1.07(2) Å, which lies within the expected range 0.76(4)–1.26(7) Å, according to the CSD (273 structures). Although the corresponding C–O–H angle is as small as 103(3)°, it falls within the range from 94 to 112°, which is common for this type of intramolecular hydrogen bonds. In the crystal, enol molecules are assembled by weak van der Waals interactions (Figure S7).
3. Materials and Methods
3.1. General Comment
All commercially available reagents were at least reagent grade and used without further purification. Solvents were distilled and dried according to standard procedures. Benzotriazole amide of isophthalic acid monomethyl ester was previously prepared by the method described in the work [20].
1H and 13C NMR spectra were acquired at 25 °C on a Bruker Avance 400 instrument (Billerica, MA, USA) and chemical shifts were reported in ppm referenced to residual solvent signals. High resolution and accurate mass measurements were carried out using a Brukermicro-TOF-QTM spectrometer (Billerica, MA, USA). The electronic absorption spectrum was measured on an OKB Spectr SF-2000 spectrophotometer (Saint Petersburg, Russia).
3.2. Synthesis
A suspension of MgBr2·Et2O (0.96 g, 3.7 mmol) in dry CH2Cl2 (30 mL) benzotriazole amide (0.5 g, 1.8 mmol) was added and the mixture was sonicated for a minute. 1-(4-(piperidin-1-yl)phenyl)ethenone (0.3 g, 1.48 mmol) was added to the mixture and it was sonicated for a minute. N,N-Diisopropylethylamine (0.775 mL, 4.5 mmol) was added to the resulting suspension and the mixture was stirred at room temperature for 20 h. The reaction mixture was treated by 2 M HCl (30 mL) and vigorously stirred for 0.5 h. The organic layer was separated and the aqueous layer was extracted by CH2Cl2 (3 × 20 mL). The combined organic extracts were filtrated through paper followed by the evaporation of the solvent. The resulting oil was crystallized from CH3OH to give a yellow–brown powder, which was dried in vacuo and purified by column chromatography (SiO2, ethyl acetate/hexane 1:10 → 1:3 vol.). The powder containing ~70% of the symmetrical side product was dissolved in dichloromethane for preparative TLC (SiO2, ethyl acetate/hexane 1:5 vol.). The resulting solid containing ~30% of the side product was purified by column chromatography (SiO2, ethyl acetate/hexane 5:1 vol.). Yield 0.054 g (10%).
1H NMR (400 MHz, CDCl3, δ): 1.69 (m, 6H, l + m + n), 3.40 (m, 4H, k + o), 3.98 (s, 3H, CH3), 6.82 (s, 1H, e), 6.91(d, J = 3.8 Hz, 2H, g + j), 7.57 (t, J = 7.8 Hz, 1H, c), 7.93 (d, J = 4.5 Hz, 2H, h + i), 8.16–8.20 (m, 2H, b + d), 8.60 (s, 1H, a), 17.15 (s, 1H, f).
13C{1H} NMR (101 MHz, CDCl3, δ): 24.0 (m), 25.0 (l), 48.2 (k), 52.0 (OCH3), 91.7 (e), 113.1 (arom.), 123.3 (arom.), 127.5 (arom.), 128.4 (arom.), 129.0 (arom.), 130.1 (arom.), 130.8 (arom.), 132.13 (arom.), 135.9 (arom.), 154.0 (arom.), 166.2 (COOCH3), 181.0 (Cdiketonate), 186.3 (Cdiketonate).
HRMS (ESI) m/z: [M + H]+ calcd for C22H23NO4 366.1705, found 366.1706.
UV-Vis (CH2Cl2): λmax 326 nm (ε = 9900 M−1 cm−1), 338.8 nm (ε = 10,300 M−1 cm−1), 404.4 nm (ε = 6400 M−1 cm−1).
3.3. Crystallography Details
Single crystals of the target enol were obtained by recrystallization from ethyl acetate/hexane mixture. Crystallographic data were collected on a Bruker D8 Venture diffractometer (at T = 100 K) using graphite monochromatized Mo–Kα radiation (λ = 0.71073 Å) using ω-scan mode. Absorption correction based on the measurements of equivalent reflections was applied [21]. The structure was solved by direct methods and refined by full matrix least-squares on F2 with anisotropic thermal parameters for all non-hydrogen atoms [22,23]. All hydrogen atoms were found from the difference Fourier map and refined freely. The crystallographic details are presented in Table S1 and the crystal packing is plotted in Figure S7. CCDC 2204554 contains the supplementary crystallographic data. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Crystal Data for C22H23NO4 (M = 365.41 g/mol): monoclinic, space group P21/c (no. 14), a = 11.7425(6) Å, b = 11.3120(7) Å, c = 13.9594(8) Å, β = 106.159(2)°, V = 1780.99(18) Å3, Z = 4, T = 100 K, μ(MoKα) = 0.094 mm−1, Dcalc = 1.363 g/cm3, 34187 reflections measured (2.36° ≤ 2Θ ≤ 28.27°), 4403 unique (Rint = 0.0396, Rsigma = 0.0239) which were used in all calculations. The final R1 was 0.0379 (I > 2σ(I)) and wR2 was 0.1069 (all data).
Supplementary Materials
The following are available online. Part 1. NMR spectroscopy and high-resolution mass-spectrometry data: Figures S1–S6; Part 2. X-ray crystallography: Table S1, Figure S7; Part 3. Optical data: Figure S8.
Author Contributions
Conceptualization, S.I.B.; methodology, S.V.T.; validation, M.A.K. and S.V.T.; formal analysis, M.A.K.; investigation, S.I.B.; resources, A.V.C. and S.I.B.; data curation, M.A.K., S.V.T. and S.I.B.; writing—original draft preparation, M.A.K. and S.I.B.; writing—review and editing, A.V.C. and S.I.B.; visualization, M.A.K.; supervision, S.I.B. and A.V.C.; project administration, S.I.B. and A.V.C.; funding acquisition, S.I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 22-23-01171.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplemental Materials.
Acknowledgments
X-ray diffraction studies were performed at the Centre of Shared Equipment of the N.S. Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buil, M.L.; Esteruelas, M.A.; López, A.M. Recent Advances in Synthesis of Molecular Heteroleptic Osmium and Iridium Phosphorescent Emitters. Eur. J. Inorg. Chem. 2021, 2021, 4731–4761. [Google Scholar] [CrossRef]
- Podyachev, S.N.; Zairov, R.R.; Mustafina, A.R. 1,3-Diketone Calix[4]Arene Derivatives—A New Type of Versatile Ligands for Metal Complexes and Nanoparticles. Molecules 2021, 26, 1214. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yu, Y.; Yang, X.; Sun, Y.; Zhong, D.; Deng, X.; Zhou, G.; Wu, Z. Manipulating MLCT Transition Character with Phosphors in Organic Light-Emitting Diodes. J. Mater. Chem. C 2021, 9, 12650–12660. [Google Scholar] [CrossRef]
- Clegg, J.K.; Li, F.; Lindoy, L.F. Oligo-β-Diketones as Versatile Ligands for Use in Metallo-Supramolecular Chemistry: Recent Progress and Perspectives. Coord. Chem. Rev. 2022, 455, 214355. [Google Scholar] [CrossRef]
- Kesarkar, S.; Mróz, W.; Penconi, M.; Pasini, M.; Destri, S.; Cazzaniga, M.; Ceresoli, D.; Mussini, P.R.; Baldoli, C.; Giovanella, U.; et al. Near-IR Emitting Iridium(III) Complexes with Heteroaromatic β-Diketonate Ancillary Ligands for Efficient Solution-Processed OLEDs: Structure-Property Correlations. Angew. Chem. Int. Ed. 2016, 55, 2714–2718. [Google Scholar] [CrossRef]
- Nehra, K.; Dalal, A.; Hooda, A.; Bhagwan, S.; Saini, R.K.; Mari, B.; Kumar, S.; Singh, D. Lanthanides β-Diketonate Complexes as Energy-Efficient Emissive Materials: A Review. J. Mol. Struct. 2022, 1249, 131531. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, M.; Wang, N.; Lu, T.; Zhou, L.; Huang, Y.; Lu, Z.; Luo, D.; Pu, X. A Facile Color-Tuning Strategy for Constructing a Library of Ir(III) Complexes with Fine-Tuned Phosphorescence from Bluish Green to Red Using a Synergetic Substituent Effect of –OCH 3 and –CN at Only the C-Ring of C^N Ligand. J. Mater. Chem. C 2016, 4, 4269–4277. [Google Scholar] [CrossRef]
- Kang, J.; Zaen, R.; Park, K.; Lee, K.H.; Lee, J.Y.; Kang, Y. Cyclometalated Platinum(II) Β-Diketonate Complexes with Extremely High External Quantum Efficiency for White Organic Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2101233. [Google Scholar] [CrossRef]
- Liao, X.-J.; Zhu, J.-J.; Yuan, L.; Yan, Z.-P.; Luo, X.-F.; Zhang, Y.-P.; Lu, J.-J.; Zheng, Y.-X. Efficient Organic Light-Emitting Diodes Based on Iridium(III) Complexes Containing Indolo[3,2,1-jk]Carbazole Derivatives with Narrow Emission Bandwidths and Low Efficiency Roll-Offs. J. Mater. Chem. C 2021, 9, 8226–8232. [Google Scholar] [CrossRef]
- Zysman-Colman, E. (Ed.) Iridium(III) in Optoelectronic and Photonics Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2017; ISBN 9781119007166. [Google Scholar]
- Bilyalova, A.A.; Tatarin, S.V.; Kalle, P.; Smirnov, D.E.; Zharinova, I.S.; Kiselev, Y.M.; Dolzhenko, V.D.; Bezzubov, S.I. Synthesis, Structure, Optical, and Electrochemical Properties of Iridium(III) Complexes with 2-Arylphenantroimidazoles and Dibenzoylmethane. Russ. J. Inorg. Chem. 2019, 64, 207–215. [Google Scholar] [CrossRef]
- Ozawa, H.; Kawaguchi, H.; Okuyama, Y.; Arakawa, H. Characterization of Photovoltaic Performance of the Dye-Sensitized Solar Cell with a Novel Ruthenium Complex Having a Bisdemethoxycurcumin as a Ligand. Ambio 2012, 41, 149–150. [Google Scholar] [CrossRef][Green Version]
- Tatarin, S.V.; Kalle, P.; Taydakov, I.V.; Varaksina, E.A.; Korshunov, V.M.; Bezzubov, S.I. Sterically Hindered Phenanthroimidazole Ligands Drive the Structural Flexibility and Facile Ligand Exchange in Cyclometalated Iridium(III) Complexes. Dalt. Trans. 2021, 50, 6889–6900. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Wang, Z.; Wang, M.; Wilkerson, C.R.; Hall, C.D.; Akhmedov, N.G. Preparation of β -Keto Esters and β -Diketones by C-Acylation/Deacetylation of Acetoacetic Esters and Acetonyl Ketones with 1-Acylbenzotriazoles. J. Org. Chem. 2004, 69, 6617–6622. [Google Scholar] [CrossRef]
- Zharinova, I.S.; Bilyalova, A.A.; Bezzubov, S.I. Synthesis and Crystal Structure of Methyl 3-(3-Hydroxy-3-Phenylprop-2-Enoyl)Benzoate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2018, 74, 816–819. [Google Scholar] [CrossRef]
- Thomas, L.H.; Florence, A.J.; Wilson, C.C. Hydrogen Atom Behaviour Imaged in a Short Intramolecular Hydrogen Bond Using the Combined Approach of X-Ray and Neutron Diffraction. New J. Chem. 2009, 33, 2486. [Google Scholar] [CrossRef]
- Andrews, P.C.; Hennersdorf, F.; Junk, P.C.; Thielemann, D.T. Variable Nuclearity in Lanthanoid Coordination Chemistry. Eur. J. Inorg. Chem. 2014, 2014, 2849–2854. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Zharinova, I.S.; Khusyainova, A.A.; Kiselev, Y.M.; Taydakov, I.V.; Varaksina, E.A.; Metlin, M.T.; Tobohova, A.S.; Korshunov, V.M.; Kozyukhin, S.A.; et al. Aromatic Β-Diketone as a Novel Anchoring Ligand in Iridium(III) Complexes for Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2020, 2020, 3277–3286. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).