Abstract
Indolo[2,1-b]quinazolin-6,12-dione (tryptanthrin) derivatives present important types of nitrogen-containing heterocyclic compounds which are useful intermediate products in organic synthesis and have potential pharmaceutical applications. The new ethyl 5-oxo-5-(((12-oxoindolo[2,1-b]quinazolin-6(12H)-ylidene)amino)oxy)pentanoate (Compound 2) was synthesized. Compound 2 is the first example of a tryptanthrin derivative containing a dicarboxylic acid residue in the side chain. The Z,E-isomerism of Compound 2 was investigated by DFT calculations. Bioavailability was evaluated in silico using ADME predictions. According to the ADME results, Compound 2 is potentially highly bioavailable and has the prospective to be used as the main component for the development of anti-inflammatory drugs.
1. Introduction
Tetracyclic heteroaromatic compounds with linear or angularly fused indoloquinazoline ring systems constitute an important structural moiety in natural products. Indolo[2,1-b]quinazolin-6,12-dione (tryptanthrin) is a well-known alkaloid and antibiotic isolated from the fungus Candida lipolytica [1,2,3,4,5], higher plants, and several species of different marine micro- and macro-organisms. This alkaloid has various pharmacological effects, such as anti-inflammatory [6], antimicrobial [7,8], antiviral [9], and anti-tumor activities [10,11,12,13,14,15]. It was found that tryptanthrin-6-oxime can serve as a specific small-molecule modulator for mechanistic studies of c-Jun N-terminal kinase (JNK) functioning [16]. The structure of tryptanthrin is attractive for obtaining its derivatives with various functional groups in position 6, as well as those substituted on the indole and quinazoline moieties. At the moment, a huge number of tryptanthrin analogs are known, both natural and chemically synthesized (Scheme 1).
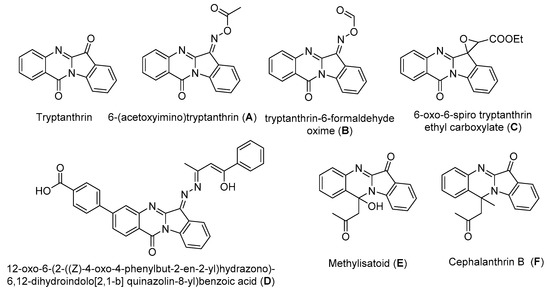
Scheme 1.
Examples of biologically active tryptanthrin analogs with carboxylic groups.
Methylisatoid (E) [17,18,19] and cephalanthrin B (F) [18] are naturally occurring indolo[2,1-b]quinazoline alkaloids which were isolated from various plant sources and different cell cultures. These compounds showed cytotoxicity against MCF-7, NCI-H460, and SF-268 cell lines [18]. The oxime esters A and B were obtained by esterification of tryptanthrin-6-oxime with acetic anhydride or the mixed anhydride of formic and acetic acids. These compounds are antiproliferative and cytotoxic agents [20]. Compound D showed potent anti-oxidant activity. The anti-cancer activity was investigated using MTT assay protocol and the results show that compounds containing the 4-pyridyl or 4-carboxyphenyl substituents at position 8 of the tryptanthrin framework are the most promising cytotoxic agent against A549, MCF-7, and HeLa human cancer cell lines as compared to other derivatives and the standard drug cisplatin [21]. Grandolini et al. discovered that tryptanthrin undergoes the Darzens reaction to yield Compound C, which showed antimicrobial activity [22].
However, the use of dicarboxylic acids as acylating agents in reaction with tryptanthrin has not been previously reported. It should be noted that the carboxyl group is an important constituent of organic molecules, such as amino acids and fatty acids, which play essential roles in biosynthesis and cellular respiration. An attachment of the carboxyl group makes a compound very soluble in polar solvents. This polarity, coupled with the presence of a carboxylate fragment at the other end of the molecule, allows dicarboxylic acid derivatives to engage in a strong hydrogen bonding that leads to an effective anchoring of the molecules within binding sites of potential biotargets.
In this paper, we report the previously unknown tryptanthrin derivative which can be classified as the glutamic acid nonsymmetric diester bearing thyptanthrin-6-oxime and ethanol moieties. This derivative can be regarded as a prospective biologically active compound.
2. Results and Discussion
2.1. Synthesis
We have preliminarily obtained tryptanthrin-6-oxime by the condensation of commercially available tryptanthrin with hydroxylamine hydrochloride [16,20] (Scheme 2).
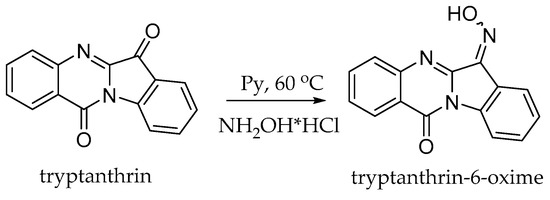
Scheme 2.
Synthesis of tryptanthrin-6-oxime.
Further modification of tryptanthrin-6-oxime by the action of ethyl glutaryl chloride (Compound 1) led to ethyl 5-oxo-5-(((12-oxoindolo[2,1-b]quinazolin-6(12H)-ylidene)amino)oxy)pentanoate (target product 2, Scheme 3). The acylation reaction proceeds for 40 min at 0 °C in pyridine as the solvent and the base. At the end of the process, complete conversion was achieved (control by TLC, eluent chloroform). The expected crude acyl oxime derivative was isolated by filtration with 88% yield. The compound was purified by recrystallization from ethanol.
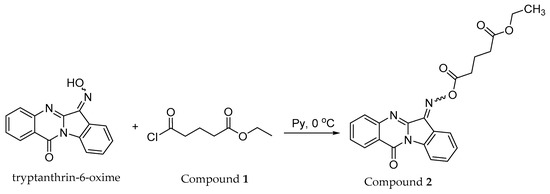
Scheme 3.
Synthesis of title Compound 2.
Title Compound 2 is the first representative of a tryptanthrin-6-oxime derivative containing a dicarboxylic acid residue in the substituent. This compound can be applicable in medicinal, organic, and materials chemistry.
It is known [23] that oximes and their derivatives exist in the forms of Z- or E-isomers that differ in the C=N double bond configuration. According to the NMR data (acetone-d6), the recrystallized Compound 2 presents an individual isomer. The 1H NMR spectrum (Figure S1, Supplementary Materials) contains signals of the ethyl ester group at 4.13 (quartet) and 1.23 (triplet) ppm. Signals of the trimethylene linker within the dicarboxylic moiety are located between 2.10 and 2.93 ppm. Protons of the indolo[2,1-b]quinazoline heterocycle are observed between 7.49 and 8.60 ppm. The signals in the NMR 13C spectrum (Figure S2) also confirm the structure of the resulting product (see NMR results, Section 3). The atom numbering is shown in Scheme 4.
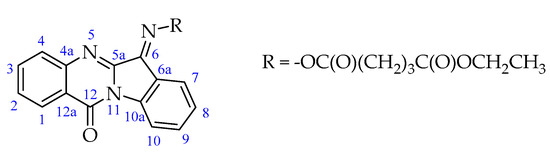
Scheme 4.
Atom numbering for NMR assignments in molecule 2.
The main characteristics of the title Compound 2: yellow crystals, M.p. 124–125 °C, soluble in acetone and chloroform.
2.2. DFT Study of Z,E-Isomerism
We studied the relative stability of two possible geometric isomers of Compound 2 in acetone using the DFT method. The lowest-energy conformations of the isomers were pre-optimized using the r2SCAN-3c composite DFT approach [24] and their single point energies were further evaluated with an M06-2X functional and def2-TZVPD basis set. This basis set includes diffuse functions pertinent for an adequate treatment of oxime lone pair interactions. The optimized structures of the geometric isomers are presented in Figure S3. The E-isomer was found to be more stable, while the Z-isomer has the calculated energy 7.73 kJ/mol above the E-isomer. Based on these results, we propose that the synthesized Compound 2 consists of a relatively more stable E-isomer (see Section 2.1). It should be noted that the side chains in the optimized structures of both Z- and E-isomers are oriented towards the heterocyclic fragment, obviously because of attractive Van der Waals interactions.
To evaluate the energy barrier for Z,E-isomerization about the C=N bond, we applied the climbing image nudged elastic band (CI-NEB) methodology that is efficient in finding a minimum energy path and a transition state (TS) [25]. The climbing image obtained by us was considered a good guess for the saddle point and was refined by the TS optimization procedure with the r2SCAN-3c method. The TS energy calculated further at the M06-2X/def2-TZVPD level led to the value of 208.4 kJ/mol for the E ⇄ Z isomerization barrier of Compound 2. The TS structure (Figure S3) suggests that the interconversion between Z- and E-isomers occurs via in-plane inversion of the nitrogen atom like in other similar compounds [26,27], i.e., without rotation about the C=N double bond. The calculated isomerization barrier is high enough and close to the corresponding values of other oximes [27].
2.3. In Silico ADME Predictions
We have evaluated ADME characteristics of Compound 2 using the SwissADME online tool [28]. The SwissADME program analyzes the structure of a chemical compound and gives a number of values, including physicochemical properties, the value of the lipophilicity coefficient (octanol–water partition coefficient), solubility in water, and some pharmacokinetic parameters (binding to liver enzymes, etc.). The program also checks a compound for compliance with bioavailability criteria, including Lipinski rules [29,30,31,32,33].
We obtained bioavailability radar plots that display an assessment of Compound 2 drug-likeness. Six important physicochemical properties, including lipophilicity, size, polarity, solubility, flexibility (conformational change), and insaturation, were considered. It was obtained that the investigated derivative of tryptanthrin-6-oxime and glutaric acid in general has satisfactory ADME properties as illustrated by a radar representation of bioavailability in Figure S4 (Panel A). Incorporation of the glutaric acid residue significantly improves the insaturation score of Compound 2 with respect to its precursor tryptanthrin-6-oxime and to the known JNK inhibitor SP600125 of the anthrapyrazolone series [34] (see Figure S4, Panels B and C). Compared to tryptanthrin-6-oxime and SP600125, molecule 2 has a higher flexibility and polarity, which gives the ability to address a wider variety of binding sites. According to the calculated ADME parameters (Table 1) and bioavailability radar (Figure S4), the synthesized derivative 2 is expected to be highly bioavailable.

Table 1.
Physicochemical ADME properties of Compound 2.
3. Materials and Methods
3.1. General Information and Compound 2 Synthesis
Elemental analysis was made using a Carlo Erba analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The 1H and 13C NMR spectra were recorded on a Bruker AVANCE III HD instrument (operating frequency 1H—400 MHz; 13C—100 MHz, Bruker Corporation, Billerica, MA, USA). The melting point of the obtained compound was measured using a Melting Point Apparatus SMP30 (Stuart Scientific, Staffordshire, UK), heating rate 3.0 °C/min. IR spectra were recorded on a FT-IR spectrometer Nicolet 5700 (Thermo Fisher Scientific, Waltham, MA, USA) with KBr pellets. The reaction was monitored by thin layer chromatography (TLC) on Silufol UV-254 and Merck plates, silica gel 60, F254.
Tryptanthrin was purchased from Combi-Blocks (San Diego, CA, USA). Tryptanthrin-6-oxime was prepared according to the procedures in the literature [16,20].
Ethyl 5-oxo-5-(((12-oxoindolo[2,1-b]quinazolin-6(12H)-ylidene)amino)oxy)pentanoate (Compound 2). To a solution of tryptanthrin-6-oxime (0.5 mmol, 0.132 g) in 5 mL pyridine, ethyl glutaryl chloride (0.5 mmol, 0.0781 mL) was added on permanent stirring at 0 °C for 40 min. The reaction was monitored by TLC (eluent: chloroform). The reaction mixture was poured in water, the precipitate was filtered out and washed with water. The title Compound 2 was obtained as yellow crystals (yield 88%); M.p. 124–125 °C (from ethanol).
1H NMR (400 MHz, (CD3)2CO), δ, ppm: 1.23 (t, 3H, J 8 Hz, CH2CH3), 2.10 (m, 2H, CH2CH2CH2), 2.53 (t, 2H, J 8 Hz, CH2CH2CH2COOEt), 2.93 (t, 2H, J 6 Hz, CH2CH2CH2COOEt), 4.13 (q, 2H, J 8 Hz, CH2CH3), 7.49 (t, 1H, J 8 Hz, H-8), 7.68 (t, 1H, J 7 Hz, H-9), 7.76 (t, 1H, J 7 Hz, H-2), 7.86 (d, 1H, J 8 Hz, H-10), 7.92 (t, 1H, J 8 Hz, H-3), 8.35 (d, 1H, J 8 Hz, H-4), 8.44 (d, 1H, J 8 Hz, H-7), 8.60 (d, J 8 Hz, H-1) 13C NMR (100 MHz, CDCl3), δ, ppm: 14.38; 20.03; 31.67; 33.07; 60.74; 117.72; 118.23; 122.62; 127.23; 127.36; 129.03; 129.55; 129.60; 135.11; 135.17; 141.57; 146.91; 147.15; 150.02; 158.95; 169.65; 172.94. IR (KBr), cm−1: ν(CH3) 2981; ν(CH2) 2874; ν(C=O) 1784, 1728, 1680; ν(C=N) 1647, 1614; ν(C(O)-O) 1199. Found, % C, 65.37; H, 4.58; N, 10.31, C22H19N3O5 Calculated, %: C, 65.18; H, 4.72; N, 10.37.
3.2. DFT Calculations
The ORCA 5.0 computational chemistry software [35] was used for DFT calculations of E- and Z-isomers of Compound 2. Before the calculations, conformation searches were performed for geometric isomers using the VConf 2.0 program of the VeraChem suite of software (VeraChem LLC, Germantown, MD, USA). For the best (Top 10) conformations found for each isomer, singlet state geometry optimizations were carried out with ORCA 5.0 employing the r2SCAN-3c composite method [24] and the CPCM solvation model with acetone as a solvent. Afterwards, for the lowest-energy conformation of each isomer, single point energy calculations were performed with an M06-2X functional [36], def2-TZVPD basis set [37,38], and SMD solvation model [39]. For CI-NEB calculations of the isomerization paths, the PBEh-3c method [40] was applied. The obtained climbing image was optimized with the OptTS keyword in ORCA 5.0 using the r2SCAN-3c method, and a single point energy evaluation was made for the obtained TS at the M06-2X/def2-TZVPD/SMD level of theory. The energy values of the E-isomer and TS calculated with M06-2X functional were used for the estimation of the isomerization barrier. Frequency calculations were performed for all the geometries optimized with r2SCAN-3c in order to establish the nature of the stationary points. Analysis and visualization of the DFT results were made with the Chemcraft 1.8 program. The ORCA 5.0 output files for the lowest-energy conformations and the TS are available in Supplementary Materials.
3.3. ADME Predictions
The physicochemical properties of selected compounds were computed using SwissADME (http://www.swissadme.ch, accessed on 7 July 2022).
4. Conclusions
In this work, we presented a synthesis of the previously unknown Compound 2 (Ethyl 5-oxo-5-(((12-oxoindolo[2,1-b]quinazolin-6(12H)-ylidene)amino)oxy)pentanoate). The compound structure was confirmed by NMR, IR spectrometry, and elemental analysis. According to the DFT results, Compound 2 has a more favorable E-configuration. The calculated isomerization path suggest that the E,Z-isomerization occurs via in-plane inversion of the oxime nitrogen atoms. An in silico estimation of ADME characteristics indicates that Compound 2 should be highly bioavailable and thus has the prospective to be used as a biologically active compound like other indoloquinazoline analogues [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. While classical methods were used to obtain the O-substituted oxime 2, this compound is the first representative of tryptanthrin derivatives with dicarboxylic acid residue, which are of great interest for further drug design.
Supplementary Materials
The following are available online. Figures: the NMR 1H and 13C of Compound 2; the DFT-optimized conformations of Z- and E-isomers of the title compound; the structure of the TS for Z,E-isomerization; ADME radar plots for the title compound, SP600125, and tryptanthrin-6-oxime. Files: ORCA 5.0 output files for geometric isomers of Compound 2 and for the transition state optimized by r2SCAN-3c method.
Author Contributions
Conceptualization was conducted by A.R.K. and A.I.K.; methodology and experimental works were conducted by A.R.K., A.A.K. (Alina A. Kolpakova), and A.A.K. (Andrei A. Kuznetzov); data analysis, writing, and editing of the paper were conducted by A.R.K. and A.I.K.; project administration and supervision were conducted by A.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. Nauka FSWW-2020-0011). Synthesis and the DFT study of Compound 2 were funded by the Tomsk Polytechnic University Development Program (Project Priority-2030-NIP/IZ-009-0000-2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brufani, M.; Fedeli, W.; Mazza, F.; Gerhard, A.; Kellersc, W. Structure of tryptanthrin. Experientia 1971, 27, 1249–1250. [Google Scholar] [CrossRef]
- Schindler, F.; Zähner, H. Mitteilung. Tryptanthrin, ein von Tryptophan abzuleitendes Antibioticum aus Dandida Lipolytica. Arch. Mikrobiol. 1971, 79, 187. [Google Scholar] [CrossRef]
- Bergman, J.; Egestad, B.; Lindström, J.-O. The structure of some indolic constituents in Couroupita Guaianensis Aubl. Tetrahedron Lett. 1977, 18, 2625. [Google Scholar] [CrossRef]
- Honda, G.; Tosirisuk, V.; Tabata, M. Isolation of an Antidermatophytic, Tryptanthrin, from Indigo Plants, Polygonum tinctorium and Isatis tinctoria. Planta Med. 1980, 38, 275. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Döbler, I.; Rheims, H.; Felske, A.; El-Ghezal, A.; Flade-Schröder, D.; Laatsch, H.; Lang, S.; Pukall, R.; Tindall, B.J. Oceanibulbus indolifex gen. nov., sp. nov., a North Sea alphaproteobacterium that produces bioactive metabolites. Int. J. Syst. Evol. Microbiol. 2004, 54, 1177. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.-C.; Cerdá-Nicolás, M.; Potterat, O.; Hamburger, M.; Ríos, J.-L. Anti-Inflammatory and Antiallergic Activity in Vivo of Lipophilic Isatis tinctoria Extracts and Tryptanthrin. Planta Med. 2006, 72, 670. [Google Scholar] [CrossRef]
- Bandekar, P.P.; Roopnarine, K.A.; Parekh, V.J.; Mitchell, T.R.; Novak, M.J.; Sinden, R.R. Antimicrobial Activity of Tryptanthrins in Escherichia coli. J. Med. Chem. 2010, 53, 3558. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Reddy, B.V.S.; Sridevi, B.; Ravikumar, A.; Venkateswarlu, A.; Sravanthi, G.; Sridevi, J.P.; Yogeeswari, P.; Sriram, D. Synthesis and biological evaluation of phaitanthrin congeners as anti-mycobacterial agents. Bioorg. Med. Chem. Lett. 2015, 25, 3867. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef]
- Vedula Sharma, M.; Prasanna, P.; Adi Seshu, K.V.; Renuka, B.; Laxman Rao, C.V.; Sunil Kumar, G.; Prasad Narasimhulu, C.; Aravind Babu, P.; Puranik, R.C.; Subramanyam, D.; et al. Novel Indolo[2,1-b]quinazoline Analogues as Cytostatic Agents: Synthesis, Biological Evaluation and Structure–Activity Relationship. Bioorg. Med. Chem. Lett. 2002, 12, 2303. [Google Scholar] [CrossRef]
- Kimoto, T.; Hino, K.; Koya-Miyata, S.; Yamamoto, Y.; Takeuchi, M.; Nishizaki, Y.; Micallef, M.J.; Ushio, S.; Iwaki, K.; Ikeda, M.; et al. Cell differentiation and apoptosis of monocytic and promyelocytic leukemia cells (U-937 and HL-60) by tryptanthrin, an active ingredient of Polygonum tinctorium Lour. Pathol. Int. Cell 2001, 51, 315. [Google Scholar] [CrossRef]
- Miao, S.; Shi, X.; Zhang, H.; Wang, S.; Sun, J.; Hua, W.; Miao, Q.; Zhao, Y.; Zhang, C. Proliferation-Attenuating and Apoptosis-Inducing Effects of Tryptanthrin on Human Chronic Myeloid Leukemia K562 Cell Line in Vitro. Int. J. Mol. Sci. 2011, 12, 3831. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.T.; Chen, T.M.; Chern, J.W.; Tseng, S.Y.; Chen, Y.; Chen, H. Downregulation of GSTpi expression by tryptanthrin contributing to sensitization of doxorubicin-resistant MCF-7 cells through c-jun NH2-terminal kinase-mediated apoptosis. Anti-Cancer Drugs 2009, 20, 382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, X.; Ma, G.J.; Wang, H.; Yang, Q.J. Transport Characteristics of Tryptanthrin and its Inhibitory Effect on P-gp and MRP2 in Caco-2 Cells. Pharm. Pharm. Sci. 2011, 14, 325. [Google Scholar] [CrossRef]
- Kim, W.; Youn, H.; Kwon, T.; Kang Kim, E.; Son, B.; Yang, H.J.; Youn, Y. PIM1 kinase inhibitors induce radiosensitization in non-small cell lung cancer cells. Pharmacol. Res. 2013, 70, 90. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Khlebnikov, A.I.; Potapov, A.S.; Kovrizhina, A.R.; Matveevskaya, V.V.; Belyanin, M.L.; Quinn, M.T. Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors. Eur. J. Med. Chem. 2019, 161, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.-W.; Lin, W.-C.; Wu, Y.-T.; Wu, P.-L. Isolation, Structure Elucidation, and Synthesis of Cytotoxic Tryptanthrin Analogues from Phaius mishmensis. J. Nat. Prod. 2008, 71, 1275. [Google Scholar] [CrossRef]
- Chang, C.-F.; Hsu, Y.-L.; Lee, C.-Y.; Wu, C.-H.; Wu, Y.-C.; Chuang, T.-H. Isolation and cytotoxicity evaluation of the chemical constituents from Cephalantheropsis gracilis. Int. J. Mol. Sci. 2015, 16, 3980–3989. [Google Scholar] [CrossRef]
- Jao, C.-W.; Hung, T.-H.; Chang, C.-F.; Chuang, T.-H. Chemical constituents of Phaius mishmensis. Molecules 2016, 21, 1605. [Google Scholar] [CrossRef] [PubMed]
- Krivogorsky, B.; Nelson, A.C.; Douglas, K.A.; Grundt, P. Tryptanthrin derivatives as Toxoplasma gondii inhibitors-Structure-activity-relationship of the 6-position. Bioorg. Med. Chem. Lett. 2013, 23, 1032–1035. [Google Scholar] [CrossRef]
- Guda, R.; Korra, R.; Balaji, S.; Palabindela, R.; Eerla, R.; Lingabathula, H.; Yellu, N.R.; Kumar, G.; Kasula, M. Design, synthesis and biological evaluation of 8-substituted-6-hydrazonoindolo[2,1-b]quinazolin-12(6H)-one scaffolds as potential cytotoxic agents: IDO-1 targeting molecular docking studies. Bioorg. Med. Chem. Lett. 2017, 27, 4741–4748. [Google Scholar] [CrossRef] [PubMed]
- Grandolini, G.; Ambrogi, V.; Perioli, L.; Giannangeli, M.; Jovicevic, L.; Rossi, V. Synthesis and antimicrobial activity of some new derivatives of 6,12-dihydroindolo[2,1-b]quinazolin-6,12-dione. Farmaco 1997, 52, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Karabatsos, G.J.; Taller, R.A. Structural studies by nuclear magnetic resonance—XV: Conformations and configurations of oximes. Tetrahedron 1968, 24, 3347–3360. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef] [PubMed]
- Ásgeirsson, V.; Birgisson, B.O.; Bjornsson, R.; Becker, U.; Neese, F.; Riplinger, C.; Jónsson, H. Nudged Elastic Band Method for Molecular Reactions Using Energy-Weighted Springs Combined with Eigenvector Following. J. Chem. Theory Comput. 2021, 17, 4929–4945. [Google Scholar] [CrossRef]
- Weiss, K.; Warren, C.H.; Wettermark, G. cis-trans isomerization about the carbon-nitrogen double bond. Structures of the isomers of N-benzylideneaniline. J. Am. Chem. Soc. 1971, 93, 4658–4663. [Google Scholar] [CrossRef]
- Blanco, F.; Alkorta, I.; Elguero, J. Barriers about Double Carbon-Nitrogen Bond in Imine Derivatives (Aldimines, Oximes, Hydrazones, Azines). Croat. Chem. Acta. 2009, 82, 173–183. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Oprea, T.I.; Davis, A.M.; Teague, S.J.; Leeson, P.D. Is there a difference between leads and drugs? A historical perspective. J. Chem. Inf. Comput. Sci. 2001, 41, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account 2008, 120, 215–241. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Grimme, S.; Brandenburg, J.-G.; Bannwarth, C.; Hansen, A. Consistent structures and interactions by density functional theory with small atomic orbital basis sets. J. Chem. Phys. 2015, 143, 054107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).