Abstract
Allobetulin was synthesized at room temperature, starting from betulin by Wagner–Meerwein rearrangement in the presence of tetrafluoroboric acid diethyl ether complex. The structure of the compound obtained was confirmed by spectroscopic methods (1H, 13C NMR and IR).
1. Introduction
The five-membered triterpene system of betulin 1 undergoes numerous, often uncontrolled, rearrangements under an acidic environment. Changes in the structure of triterpenes most often consist of the formation of a carbocation and 1,2-migration of hydrogen atoms or alkyl groups (the Wagner–Meerwein rearrangement). For example, the betulin E five-membered ring can be transformed into the E’ six-membered system, forming 19β,28-epoxy-18α-olean-3β-ol, called allobetulin 2 (Scheme 1) [1].
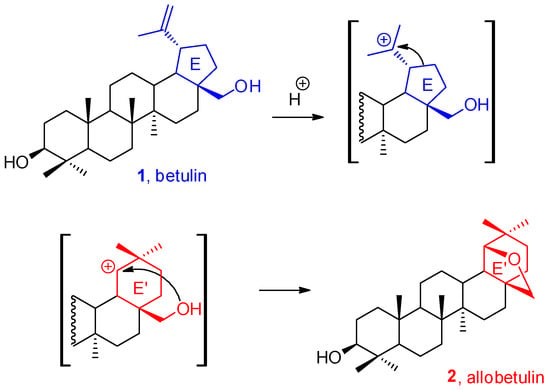
Scheme 1.
The Wagner–Meerwein rearrangement of betulin 1 within the E ring.
The intramolecular etherification reaction between the isopropenyl group and the primary hydroxyl group of betulin 1, in trans configuration to each other, was first described by Schulze and Pieroh in 1922 [2]. It was catalyzed with hot, concentrated formic acid. However, the lack of appropriate analytical techniques allowed the researchers to identify the product only as a “monoalcohol containing an ether group”. The chemical structure of allobetulin 2 was elucidated and verified several years later [3,4]. There are many procedures in the literature for the intramolecular Wagner–Meerwein-type rearrangement of betulin 1 into the constitutional isomer-allobetulin 2. Sulfuric acid in acetic acid [5], hydrogen chloride solution in ethanol [6], trifluoroacetic acid [7], or p-toluenesulfonic acid in chloroform [8] may be the catalyst system for this transformation. It is also possible to use a solid-supported acid catalyst, such as silica gel with p-toluenesulfonic acid, sulfuric acid, iron(III) nitrate or iron(III) chloride, kaolinite, bentonite, KSF and K10 montmorillonites, etc. Reactions of this type are carried out in boiling DCM for 0.5–6 h, and their yield ranges from 91–99% [9]. The most important procedures are summarized in Table 1 [5,6,7,8,9,10,11,12].

Table 1.
Selected, most important methods for the intramolecular Wagner–Meerwein-type rearrangement of betulin 1 into allobetulin 2.
In general, all of the methods described so far rely on dissolving betulin 1 in a suitable medium and treatment with an acid catalyst. They often require elevated temperatures and aqueous work-up of the reaction mixture (multiple washing with water to remove the catalyst), which generates a large amount of waste. Therefore, we decided to develop a new method aimed at using mild reaction conditions (room temperature, short reaction time) and a simplified, non-aqueous work-up procedure.
2. Results and Discussion
Due to the fact that the Wagner-Meerwein-type rearrangement of betulin 1 to allobetulin 2 requires an acidic environment, we decided to use tetrafluoroboric acid diethyl ether complex. The reaction was carried out in dichloromethane. It can be monitored by spectroscopic methods (e.g., 1H NMR) as well as TLC (DCM/AcOEt, 8:1, v:v). As we observed, it proceeds efficiently already at room temperature, and the minimum reaction time is 1 h. Next, instead of the aqueous work-up of the reaction mixture, an acetone washing was performed (room temperature, 2 times). During this procedure, allobetulin was slurried in acetone (where it is insoluble), and then the acetone solution containing the impurities was decanted with a Pasteur pipette. The measured melting point of the obtained crystalline product was 266.5–268 °C (266–268 °C [9]).
The course of the reaction and the structure of the obtained compound were determined by spectroscopic methods (1H, 13C NMR, and IR; Figure 1 and Supplementary Materials).
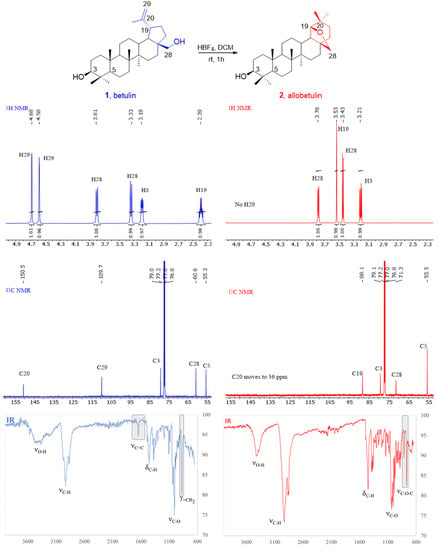
Figure 1.
Transformation of betulin 1 into allobetulin 2—changes in the spectroscopic characteristics.
In the 1H NMR spectrum, signals from the proton of the CHOH group at C3 (3.21 ppm, dd, J1 = 4.8 Hz, J2 = 11.4 Hz, 1H) and protons of the CH2OC at position C28 (3.78 ppm, d, J = 7.6 Hz and 3.43 ppm, d, J = 7.6 Hz) were observed. However, the doublet of triplets (dt) characteristic for betulin at 2.39 ppm, corresponding to the protons at the C19 position, and the signals of the isopropenyl group disappeared. At the same time, three new singlets appeared: 3.53 ppm (1H, H-19), 0.93 ppm (3H, Me), and 0.77 ppm (3H, Me).
In turn, the absence (13C NMR spectrum) of signals at 47.78 ppm (C19), 150.46 ppm (C20), 109.67 ppm (C29), and 19.08 ppm (C30) confirmed the disappearance of the isopropenyl group. There was also no signal from C28 at 60.57 ppm (it moves to 71.3 ppm). The presence of the signal at 79.14 ppm was evidence of the preservation of the hydroxyl group at C3. In addition, a new signal (C19) appeared at 88.15 ppm, and it was noticed that the arrangement of the peaks in the area of the triterpene rings had changed.
In the FT-IR spectrum, a decrease in the intensity of the extended absorption band in the range of hydroxyl groups (3500–3200 cm−1, νO-H) was observed, which could be evidence of the disappearance of one of the OH groups. Additionally, the disappearance of the band corresponding to the stretching vibrations of the double bonds (1645 cm−1, νC=C) suggests the absence of the isopropenyl group. The arrangement of absorption bands in the fingerprint region (1300–900 cm−1) also changed.
Allobetulin obtained in this way can be used without any further purification (e.g., as a substrate in the synthesis of its analogs or derivatives showing anti-inflammatory, immunotropic, antibacterial, or antifungal properties) [1].
3. Materials and Methods
3.1. General
All commercially available reagents and solvents were used without further purification. Melting points were determined in capillaries and were uncorrected. 1H-, and 13C NMR spectra were recorded at operating frequencies of 600 and 150 MHz, respectively, using TMS as the resonance shift standard. All chemical shifts (δ) are reported in ppm and coupling constants (J) in Hz. IR spectra were recorded using an FT-IR spectrometer (ATR method).
3.2. Allobetulin (2)
Betulin (0.226 mmol, 100 mg) and 1.5 cm3 of DCM were placed in a 10 cm3 round-bottom flask equipped with a CaCl2 tube and a magnetic stirrer. The suspension was cooled in an ice-water bath for 10 min, then tetrafluoroboric acid diethyl ether complex (0.15 mmol, 0.02 cm3) was added dropwise. After a few minutes, from an initially clear solution, a white precipitate separated. The reaction was carried out with continuous stirring at room temperature for 1 h. The solvent was then evaporated and the residue, in the form of a pale pink solid, was dried in vacuo for 1 h. The crude allobetulin was slurried in acetone (1.5 cm3), and the mixture was stirred for 15 min at room temperature. Next, the acetone solution containing the impurities was decanted with a Pasteur pipette. The operation was repeated twice. The obtained residue (white solid) was dried under vacuum to give allobetulin, m.p. 266.5–268.0 °C in 85% yield. 1H NMR (600 MHz, CDCl3): δ 0.77 (s, 3H, CH3), 0.80 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.93 (s, 3H, CH3), 0.98 (s, 6H, 2 × CH3), 1.20-1.72 (m, 24H, CH, CH2), 3.20 (dd, J1 4.8 Hz, J2 11.4 Hz, 1H, H-3), 3.44 (d, J 7.6 Hz, 1H, H-28a), 3.53 (s, 1H, H-19), 3.78 (d, J 7.6 Hz, 1H, H-28b); 13C NMR (150 MHz, CDCl3): δ 13.48, 15.37, 15.68, 16.47, 18.25, 20.96, 24.52 (C-29 or C-30), 26.20, 26.42, 27.95 (C-2), 28.78 (C-29 or C-30), 32.15, 32.68, 33.91, 34.10, 35.12, 36.22, 36.68, 37.25, 38.86, 38.93, 40.71, 41.49, 43.29, 46.81, 51.09, 55.50, 71.26 (C-28), 79.14 (C-3), 88.15 (C-19). IR (ATR): 3423, 2927, 2860, 1751, 1448, 1384, 1375, 1033, 1007, 767, 730 cm−1. The spectroscopic data is in agreement with the literature data [9,12,13].
4. Conclusions
A new and effective method for the synthesis of allobetulin from betulin by the Wagner–Meerwein rearrangement was developed. The reaction proceeds smoothly at room temperature in DCM in the presence of tetrafluoroboric acid diethyl ether complex, and the isolation of allobetulin does not require an aqueous work-up.
Supplementary Materials
Copies of 1H NMR, 13C NMR, and IR spectra.
Author Contributions
Conceptualization: M.G. and J.A.; methodology: M.G. and J.A.; formal analysis: M.G. investigation: M.G. writing—original draft preparation: J.A.; writing—review and editing: M.G. and J.A.; supervision: M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Silesian University of Technology (Poland) Grant BK No. 04/050/BK_22/0139.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dehaen, W.; Mashentseva, A.; Seitembetov, T. Allobetulin and Its Derivatives: Synthesis and Biological Activity. Molecules 2011, 16, 2443–2466. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.; Pieroh, K. Zur Kenntnis des Betulins. Chem. Ber. 1922, 55, 2322–2346. [Google Scholar] [CrossRef]
- Davy, G.S.; Halsall, T.G.; Jones, E.R.H.; Meakins, G.D. The chemistry of the triterpenes. Part X. The structures of some isomerisation products from betulin and betulinic acid. J. Chem. Soc. 1951, 2702–2705. [Google Scholar] [CrossRef]
- Santos, R.C.; Pinto, R.M.A.; Beja, A.M.; Salvador, J.A.R.; Paixão, J.A. 19β,28-Epoxy-18α-olean-3β-ol. Acta Cryst. 2009, E65, o2088–o2089. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.H.R.; Holness, N.J.; Triterpenoids, V. Some relative configurations in rings C, D, and E of the β -amyrin and the lupeol group of triterpenoids. J. Chem. Soc. 1952, 78–92. [Google Scholar] [CrossRef]
- Errington, S.G.; Ghisalberti, E.L.; Jefferies, P.R. The chemistry of the Euphorbiaceae. XXIV. Lup-20(29)-ene-3β,16β,28-triol from Beyeria brevifolia var brevifolia. Aust. J. Chem. 1976, 29, 1809–1814. [Google Scholar] [CrossRef]
- Medvedeva, N.I.; Flekhter, O.B.; Kukovinets, O.S.; Galin, F.Z.; Tolstikov, G.A.; Baglin, I.; Cavé, C. Synthesis of 19β,28-epoxy-23,24-dinor-A-neo -18α-olean-4-en-3-one from betulin. Russ. Chem. Bull. 2007, 56, 835–837. [Google Scholar] [CrossRef]
- Green, B.; Bentley, M.D.; Chung, B.Y.; Lynch, N.G.; Jensen, B.L. Isolation of Betulin and Rearrangement to Allobetulin, A Biomimetic Natural Product Synthesis. J. Chem. Ed. 2007, 84, 1985–1987. [Google Scholar] [CrossRef]
- Li, T.-S.; Wang, J.-X.; Zheng, X.-J. Simple synthesis of allobetulin, 28-oxyallobetulin and related biomarkers from betulin and betulinic acid catalysed by solid acids. J. Chem. Soc. Perkin Trans. 1 1998, 3957–3965. [Google Scholar] [CrossRef]
- Lavoie, S.; Pichette, A.; Garneau, F.-X.; Girard, M.; Gaudet, D. Synthesis of Betulin Derivatives with Solid Supported Reagents. Synth. Commun. 2001, 31, 1565–1571. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Pinto, R.M.A.; Santos, R.C.; Le Roux, C.; Beja, A.M.; Paixão, J.A. Bismuth triflate-catalyzed Wagner-Meerwein rearrangement in terpenes. Application to the synthesis of the 18α-oleanane core and A-neo-18α-oleanene compounds from lupanes. Org. Biomol. Chem. 2009, 7, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Filippova, E.A.; Shakhmaev, R.N.; Zorin, V.V. Convenient Synthesis of Allobetulin. Russ. J. Gen. Chem. 2013, 83, 1633–1634. [Google Scholar] [CrossRef]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Dufour, P.; Pichette, A. Synthesis and structure-activity relationship study of cytotoxic germanicane- and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorg. Med. Chem. 2007, 15, 6144–6157. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).