Abstract
The X-ray structures of three simple heterocyclic compounds have been obtained for the first time. Structures of both 3-acetyloxazolidin-2-one 1 and its unsaturated analogue 3-acetyloxazolin-2-one 3 show a planar imide nitrogen with the exocyclic C=O oriented anti to the ring N–C(=O) bond and negligible intermolecular interactions, a pattern consistent with previously reported analogues. In contrast the parent NH heterocycle, oxazolin-2(3H)-one 4, exists as hydrogen bonded dimers of two closely similar independent molecules but an unusual type of disorder involving exchange of the ring O and NH positions results in a very high R factor.
1. Introduction
The 1,3-oxazolidin-2-ones are of considerable synthetic value with N-acyl derivatives, particularly those with stereogenic centres at position 4 and/or 5 derived from amino acids and other natural products playing a key role in the chiral auxiliary approach to asymmetric synthesis [1]. Even an achiral oxazolidin-2-one can be used to promote various organic transformations [2]. Despite the high level of synthetic interest, the amount of structural information on simple heterocycles of this type is quite limited. Thus, although there are several reports on the X-ray structure of the parent 1,3-oxazolidin-2-one [3,4], only a few simple N-acyl derivatives have been structurally characterised. By a sequence of ring chlorination and thermal dehydrochlorination [5,6,7], the simple N-acetyloxazolidin-2-one 1 may be converted via 2 into the unsaturated derivative 3 which is then N-deprotected by methanolysis to afford the parent heterocycle oxazolin-2(3H)-one 4 (Scheme 1). As far as we are aware there is only a single X-ray structure in the literature containing the oxazolin-2(3H)-one ring system and this is a rather complex camphor derivative [8]. In this paper we report the X-ray structure determination of compounds 1, 3 and 4.
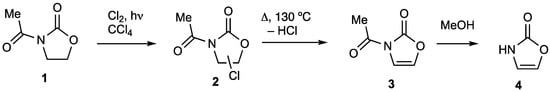
Scheme 1.
Synthetic route from compound 1 to 3 and 4.
The starting N-acetyloxazolidin-2-one 1 was readily prepared by reaction of oxazolidin-2-one with acetic anhydride in the presence of catalytic sodium acetate [6]. Oxazolidin-2-one itself is commercially available and can also be easily prepared from ethanolamine and diethyl carbonate [6]. The chlorination [5,6] proceeded readily to give 2 as a mixture of regioisomers as confirmed by the NMR data [7]. Simply heating this under an inert atmosphere led to efficient loss of HCl and formation of the oxazolinone 3 [5,6]. The final deprotection of nitrogen was accomplished in low yield by methanolysis [5,9], to give a sample of 4 whose identity was confirmed by comparison of its 1H NMR data (see Supplementary Materials) with literature values for samples prepared using the route of Scheme 1 [5] and a completely independent method [10].
2. Results
Both compound 1 and its unsaturated analogue 3 gave good quality X-ray structures and the resulting molecular structures are shown (Figure 1) with molecular dimensions compared in Table 1. Comparison of these with normal values [11] shows that, as well as the expected changes in bond lengths and angles resulting from the introduction of the CH=CH double bond in 3 in place of CH2–CH2 in 1, there are some more unexpected differences. Compared to 1, not only the C(4)–C(5) bond is shorter in 3 but also the O(1)–C(5) and N(3)–C(4) bonds, reflecting some significant enamine/enol ether delocalisation of electrons. Perhaps linked to this, both the C(2)–O(1) and exocyclic N(3)–C(6) bonds are significantly longer in 3 than in 1.
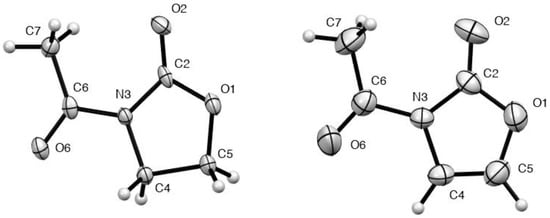
Figure 1.
Structures of compounds 1 and 3 (thermal ellipsoids at 50% probability).

Table 1.
Bond lengths and angles for 1 and 3.
A further point to note is that, in both 1 and 3, the nitrogen is quite accurately planar with its two adjacent carbonyl groups aligned anti to one another. A survey of a range of simple N-acyloxazolidiones 5a–h for which X-ray structures have been published (Table 2, [12,13,14,15,16,17,18,19]) shows that while planarity at nitrogen is the norm, the degree of anti alignment of the two carbonyls varies, with torsion angles as low as 149–154° in some cases. The structure of the only previously determined N-acyloxazolin-2(3H)-one 6 (Figure 2) agrees well with that of 3 in these respects [8].

Table 2.
Geometrical parameters for 1 and 3 and comparison compounds 5a–h and 6.
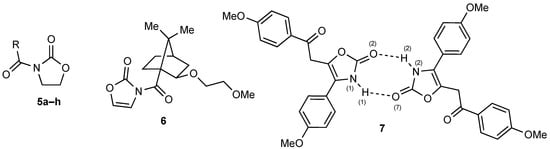
Figure 2.
Structures of comparison compounds 5, 6 and 7.
When we come to the parent oxazolin-2(3H)-one 4, the structure solution raised an unexpected problem. The structure consisted of two independent molecules with very similar geometries (Figure 3) but the R factor was very high due to disorder in the crystal between the location of O(1)/N(3)H(3) or O(11)/N(13)H(13). This is especially significant since, with correct alignment, there is strong hydrogen bonding between these and in fact the crystal structure consists of alternating rows of hydrogen bonded dimers of the two independent molecules exhibiting an R22(8) interaction [20] (Figure 4, Table 3).
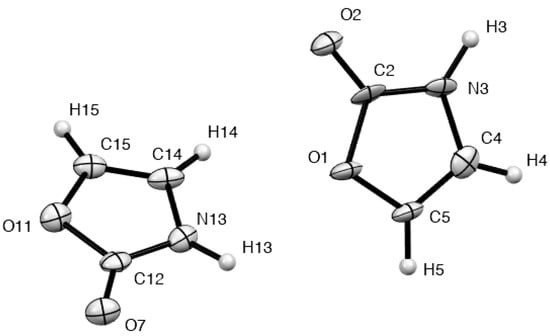
Figure 3.
Structure and numbering of the two independent molecules of compound 4.
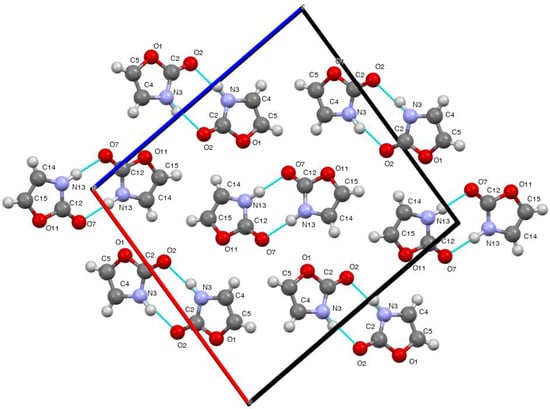
Figure 4.
Hydrogen bonding pattern for compound 4 viewed along the b axis.

Table 3.
Hydrogen bonding parameters for 4 and comparison compound 7 (Å, °).
When we consider the possible interactions between adjacent molecules combined with the positional disorder, it is clear that there can with equal probability be four situations involving either two, one or no hydrogen bonds (Figure 5). The resulting high R factor means that unfortunately little meaningful data can be obtained on the precise molecular dimensions of this fundamental heterocyclic molecule in the crystalline state.
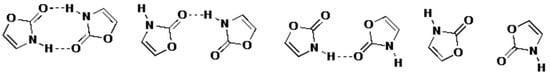
Figure 5.
Different hydrogen bonding situations resulting from positional disorder in the crystal between ring O and NH.
We have located one published X-ray structure of a 4,5-disubstituted-3-unsubstituted oxazolin-2(3H)-one, compound 7 (CCDC Ref Code ENALEM) [21] which forms hetero-dimers between two slightly different independent molecules (Figure 2), and its hydrogen bonding parameters are comparable with those for 4 (Table 3).
In summary, the X-ray crystal structures of the two simple N-acetyl heterocycles 1 and 3 have been obtained for the first time and are compared with each other and with related literature structures. In the case of the deacetylated oxazolin-2(3H)-one 4 disorder between the positions of the ring O and NH resulted in a very high R factor meaning no accurate structural information could be obtained.
3. Experimental
Samples of 1, 3 and 4 were prepared by the reported methods [5,6]. While crystals of 1 obtained by addition of diethyl ether to the toluene product extract as described [6] followed by cooling, and of 3 obtained after kugelrohr distillation followed by silica gel chromatography (hexanes/ethyl acetate, 3:1) were directly suitable for X-ray diffraction, suitable crystals of 4 were obtained by vacuum sublimation.
Crystal data for 1: C5H7NO3, M = 129.12 g mol–1, colourless plate, crystal dimensions 0.10 mm × 0.10 mm × 0.01 mm, monoclinic, space group Pn (No. 7), a = 6.9924(9), b = 5.1635(4), c = 8.1820(10) Å, β = 108.8310(14)°, V = 279.60(5) Å3, Z = 2, Dcalc = 1.534 g cm–3, T = 93 K, R1 = 0.0419, Rw2 = 0.1038 for 1053 reflections with I > 2σ(I), and 83 variables. Data were collected using graphite monochromated Mo Kα radiation λ = 0.71073 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2194101.
Crystal data for 3: C5H5NO3, M = 127.10 g mol–1, colourless plate, crystal dimensions 0.20 mm × 0.10 mm × 0.01 mm, orthorhombic, space group Pbca (No. 61), a = 6.8375(6), b = 12.0213(10), c = 14.2226(13) Å, V = 1169.04(18) Å3, Z = 8, Dcalc = 1.444 g cm–3, T = 173 K, R1 = 0.0327, Rw2 = 0.0967 for 986 reflections with I > 2σ(I), and 83 variables. Data were collected using graphite monochromated Mo Kα radiation λ = 0.71073 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2194102.
Crystal data for 4: C3H3NO2, M = 85.06 g mol–1, colourless plate, crystal dimensions 0.20 mm × 0.02 mm × 0.01 mm, monoclinic, space group P21/n (No. 14), a = 13.5387(19), b = 3.6822(5), c = 14.1637(19) Å, β = 94.683(12)°, V = 702.39(17) Å3, Z = 8, Dcalc = 1.609 g cm–3, T = 93 K, R1 = 0.1351, Rw2 = 0.3104 for 1149 reflections with I > 2σ(I), and 117 variables. Data were collected using graphite monochromated Mo Kα radiation λ = 0.71073 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2194103.
The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures. The structures were solved by direct methods and refined by full-matrix least-squares against F2 (SHELXL, Version 2018/3 [22]).
Supplementary Materials
The following is available online, H NMR data, cif and check-cif files for 1, 3 and 4.
Author Contributions
J.S.L. prepared the compounds and obtained the NMR data; A.M.Z.S. collected the X-ray data and solved the structures; R.A.A. designed the study, analysed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ager, D.J.; Prakash, I.; Schaad, D.R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996, 96, 835–875. [Google Scholar] [CrossRef]
- Murakata, M.; Tsutsui, H.; Hoshino, O. Unprecedented effect of achiral oxazolidinones on enantioselective radical-mediated conjugate additions using a chiral zinc triflate. Org. Lett. 2001, 3, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Turley, J.W. The crystal structure of 2-oxazolidinone. Acta Crystallogr. Sect. B 1972, 28, 140–143. [Google Scholar] [CrossRef]
- Wouters, J.; Ooms, F.; Durant, F. 2-Oxazolidinone. Acta Crystallogr. Sect. C 1997, 53, 895–897. [Google Scholar] [CrossRef]
- Scholz, K.-H.; Heine, H.-G.; Hartmann, W. Eine einfach Synthese von 4-Oxazolin-2-on. Liebigs Ann. Chem. 1976, 1319–1322. [Google Scholar] [CrossRef]
- Scholz, K.-H.; Heine, H.-G.; Hartmann, W. Synthesis and Diels-Alder reactions of 3-acetyl-2(3H)-oxazolone: 6-Amino-3,4-dimethyl-cis-3-cyclohexen-1-ol. Org. Synth. 1984, 62, 149–153. [Google Scholar] [CrossRef]
- Gaenzler, F.C.; Smith, M.B. A dichlorination-reductive-dechlorination route to N-acetyl-2-oxazolone. Synlett 2007, 1299–1301. [Google Scholar] [CrossRef]
- Ishizuka, T.; Ishibuchi, S.; Kunieda, K. New camphor-derived auxiliaries in methoxyselenation and methoxybromination with opposite diastereofacial selectivity. Preparation of β-amino alcohol chiral synthons. Tetrahedron Lett. 1989, 30, 3449–3452. [Google Scholar] [CrossRef]
- Scholz, K.-H.; Hartmann, W.; Heine, H.-G. Δ4-Oxazolin-2. German Patent 2610676, 15 September 1977. [Chem. Abstr. 1978, 88, 6860]. [Google Scholar]
- Tavernier, D.; Van Damme, S.; Ricquier, P.; Anteunis, M.J.O. A Convenient Preparation of 3H-1,3-oxazol-2-one and its N-Formyl Derivative. Bull. Soc. Chim. Belg. 1988, 97, 859–865. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc., Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Cheng, J.-L.; Tan, C.-X.; Zhu, G.-N. 3-Benzoyl-1,3-oxazolidin-2-one. Acta Crystallogr. Sect. E 2005, 61, o3194–o3195. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Cai, C.; Hruby, V.J.; Van Meervelt, L.; Yamazaki, T. Rational design of highly diastereoselective, organic base-catalyzed, room-temperature Michael addition reactions. J. Org. Chem. 2000, 65, 6688–6696. [Google Scholar] [CrossRef]
- Shen, Y.-D.; Wang, Y.; Xiao, Z.-L.; Lei, H.-T.; Sun, Y.-M. (Z)-3-(2-Oxo-1,3-oxazolidin-3-ylcarbonyl)prop-2-enoic acid. Acta Crystallogr. Sect. E 2007, 63, o710–o711. [Google Scholar] [CrossRef]
- Skelton, B.W.; Pyne, S.G. CCDC 1543018: Experimental Crystal Structure Determination; The Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Yamada, S.; Misono, T.; Tsuzuki, S. Cation–π interactions of a thiocarbonyl group and a carbonyl group with a pyridinium nucleus. J. Am. Chem. Soc. 2004, 126, 9862–9872. [Google Scholar] [CrossRef]
- Marsh, R.E.; Clemente, D.A. A survey of crystal structures published in the Journal of the American Chemical Society. Inorg. Chim. Acta 2007, 360, 4017–4024. [Google Scholar] [CrossRef]
- Shen, Y.; Chai, J.; Yang, G.; Chen, W.; Chai, Z. Stereocontrolled synthesis of trans/cis-2,3-disubstituted cyclopropane-1,1-diesters and applications in the synthesis of furanolignans. J. Org. Chem. 2018, 83, 12549–12558. [Google Scholar] [CrossRef]
- Li, L.; Guo, J.-Y.; Liu, X.-G.; Chen, S.; Wang, Y.; Tan, B.; Liu, X.-Y. Amide groups switch selectivity: CH trifluoromethylation of α,β-unsaturated amides and subsequent asymmetric transformation. Org. Lett. 2014, 16, 6032–6035. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Görgen née Boersch, C.; Lutsenko, K.; Merkul, E.; Frank, W.; Müller, T.J.J. Catalytic one-pot synthesis of 4-(hetero)aryl substituted 5-(2-oxoethyl)oxazol-2(3H)-ones by coupling–isomerization–elimination (CIE) sequence. Org. Chem. Front. 2016, 3, 887–896. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).