Abstract
Herein, we report a bisoxazole derivative as well as a bromo-substituted oxazole derivatives via a simple approach. The synthesis begins with an inexpensive and readily available starting material, such as 2,5-dimethoxybenzaldehyde, hydroquinone, and p-toluenesulfonylmethyl isocyanide (TosMIC). This approach relies on the Van Leusen oxazole method and electrophilic aromatic bromination. The structures of bisoxazole and bromosubstituted aryloxazoles were fully supported by spectroscopic methods (IR, NMR, and HRMS) and further established using single crystal X-ray diffraction studies.
1. Introduction
The synthesis and structural modification of N,O-heteroarenes have attracted a large amount of attention from the synthetic community due to their many latent applications, which lead to additional research activity toward expanding new pharmaceuticals. Additionally, these heteroaryl and aryl bromides are valuable intermediates in organic synthesis. Heterocycles are considered as useful scaffolds that induce physicochemical properties, such as lipophilicity, polarity and hydrogen bonding, which lead to the enhanced pharmacological and pharmacokinetic properties of molecules. Among heterocycles, the five-membered heteroaromatic oxazole scaffolds exhibit significant medicinal and pharmacological applications. The presence of heteroatoms in their core structure is involved in hydrogen bonding, pi–pi stacking and their interactions with various enzymes and receptors of biological systems. These are relatively stable, active moieties in many pharmaceutical ingredients and extensively observed in nature [1,2,3,4,5]. Additionally, these heterocyclic cores have shown potent activity against drug-susceptible, drug-resistant and multidrug-resistant cancer cell lines [6]. These motifs are capable of binding with various enzymes and receptors, such as histone deacetylase (HDAC), cyclooxygenase-2 (COX-2), and human epidermal growth factor receptor (EGFR) kinase in cancer cells through various non-covalent interactions [7,8,9]. Furthermore, oxazoles are identified as valuable synthons and have and therapeutic potential for the development of NCEs (new chemical entities) to cure many diseases of clinical importance [10]. Additionally, heterocycles containing oxazole motifs have many applications, particularly for pharmaceuticals [11]. Therefore, the synthesis of heterocycles bearing oxazole moieties is an intriguing aspect in the scientific community.
Oxazole derivatives are found to be an essential constituents in some drugs and further considered as beneficial in modern drug design. Due to their structural and chemical diversity, along with their abundance in natural oxazole motifs, they are considered as a potentially suitable template for the development of anti-cancer research and drug discovery [12,13]. Some representative examples of biologically relevant compounds, such as 1, 2, 5, and 6 bearing an oxazole framework [13,14,15,16], are depicted in Figure 1. These compounds [13,14,15,16] serve as antibacterial inhibitors of FtSz, COX-inhibitors, antiviral activity against HCV and CVB, as well as act as potent and selective agonists of free fatty acid receptor 1 (GPR 40).
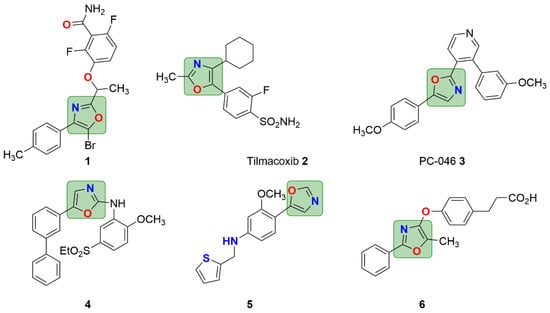
Figure 1.
Representative examples of biological relevant compounds (1–6) bearing an oxazole framework.
For example, diaryl oxazole-based compound 3 (Figure 1) was reported as a potential agent for the treatment of pancreatic cancer [17]. 2-Anilino-5-aryloxazole derivative 4 was identified as a novel class of potent VEGFR2 kinase inhibitor with good enzymatic and cellular activities [18]. Several compounds containing oxazole moieties have served as clinical drugs or candidates and display a key role in the treatment of various types of diseases, such as antibacterial, antidepressant, antifungal, anti-inflammatory, antimicrobial, antiviral, antitubercular, anticancer, antiparasitic, and antidiabetic roles, and act as adrenergic receptor ligands, T-type calcium channel blockers, prostacyclin receptor antagonists, and transthyretin amyloid fibril inhibitors [19,20,21]. Therefore, considerable effort has been exerted to construct this privileged heterocyclic motif [22,23].
2. Results and Discussion
To design various heterocycles, we established new synthetic approaches toward a sequence of heterocycles, such as C3-symmetric-based heterocycles including carbocyclic cages via the Van Leusen oxazole protocol, through the usage of Toluenesulfonylmethyl isocyanide (TosMIC) as a key reagent [24,25,26,27,28]. TosMIC is a stable, odorless, and colorless solid at room temperature, and it was introduced by the Dutch professor Van Leusen in 1972, named Van Leusen’s reagent, and applied in organic synthesis. It is well known as one of the most common building blocks in organic synthesis and is successfully used in the preparation of pyrrole-, imidazole-, and oxazole-based five-membered heterocycles [29,30]. Due to the considerable interest in oxazole motifs as an important structural core in many marketed drugs and its pharmaceutical importance in the new millennium, herein, we conceived a short synthetic sequence to bisoxazole, as well as bromo-oxazoles, under Van Leusen reaction conditions (Scheme 1 and Scheme 2).
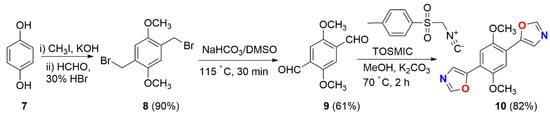
Scheme 1.
Synthesis of bisoxazole derivative 10.
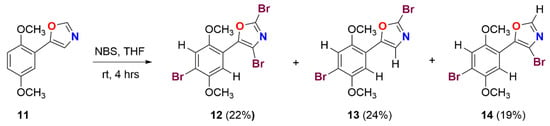
Scheme 2.
Synthesis of bromo-substituted-oxazole motifs 12–14.
In view of its importance in diverse fields, we prepared a bisoxazole derivative starting with an inexpensive and readily accessible starting material, such as hydroquinone 7 (Scheme 1). In this context, compound 8 was prepared in excellent yields in two steps (80–90%) through methylation in the presence of CH3I and KOH followed by bromomethylation with paraformaldehyde in 30% HBr in AcOH. Further, dibromo compound 8 [31,32] was treated with NaHCO3 at 115 °C in DMSO to produce the 2,5-dimethoxyterephthalaldehyde 9 [31] in a 61% yield. Finally, dialdehyde 9 was exposed to TosMIC in the presence of K2CO3 in methanol at reflux (Van Leusen oxazole conditions) to afford bisoxazole 10 in a 78% yield. The bisoxazole compound 10 was fully characterized via spectroscopic parameters (1H NMR, 13C NMR, DEPT-135 NMR, and HRMS data).
Next, we extended our strategy to prepare a different substituted bromo aryloxazoles. In this context, dimethoxy oxazole compound 11 [26] was subjected to bromination. The electrophilic aromatic bromination of oxazole 11 in the presence of NBS in THF at room temperature for 4 h to deliver the different substituted bromooxazoles 14, 12, and 13 in 19, 22, and 24% yields, respectively. The structures of bromo-substituted oxazoles 12–14 were established based on the spectral data. The tribromo oxazole compound 12 was unambiguously characterized through single-crystal X-ray diffraction analysis (Figure 2). These brominated oxazole scaffolds can be modified further as polyoxazoles useful as a biologically relevant molecules via a well-known cross-coupling protocol. These substituted oxazoles are also of interest in the polymers, agrochemicals, drug discovery research, and aryl bromides are useful intermediates in organic synthesis.
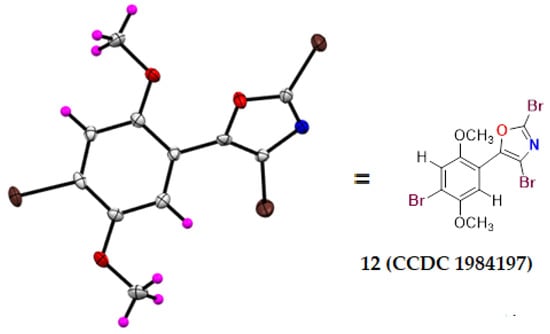
Figure 2.
Single-crystal X-ray structure of compound 12.
The 1H NMR spectrum of bisoxazole 10 revealed the presence of two singlets (two oxazolic protons) at δ 7.91 (1H) indicates the neighboring proton of nitrogen and oxygen and δ 7.61 (1H) represents the adjacent proton of quaternary carbon and nitrogen for the heteroaryl ring system. In addition, the 13C NMR spectrum of 10 showed three characteristic peaks at δ 149.7, 147.6, and 126.5 of the oxazole ring system, which represents the –CH and quaternary carbon of the oxazole contiguous to N & O atoms (Figure 3). The 1H NMR spectrum of compound 13 displayed three typical aromatic and heteroaromatic chemical shifts at δ 7.16, 7.20, and 7.48 as singlets. Similarly, compound 14 also showed three singlets at δ 7.06, 7.21, and 7.90. We clearly identified the substitution pattern of bromine in the oxazole moiety based on chemical shift values (1H and 13C), such as δ 7.48, 129.0, 7.90, and 150.5, which are adjacent to N, O and N, and the quaternary center of both aryl and heteroaryl systems, respectively. Further, we support the substitution pattern with the DEPT/HMBC method (Figure 3). The NMR spectra of these all are provided in the Supplementary Material.
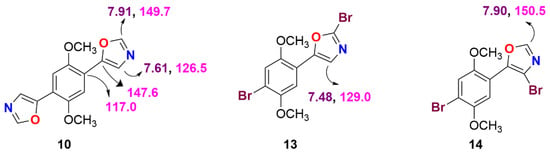
Figure 3.
Correlation of 1H and 13C NMR value (s) of different oxazole frameworks.
3. Materials and Methods
3.1. General Information
2,5-Dimethoxybenzaldehyde, TosMIC, hydroquinone, NBS, and other essential reagents, chemicals, and necessary solvents were used as received and directly obtained from commercial suppliers without any further purifications. Thin-layer chromatography (TLC) plates were made on 10 × 5 cm glass plates layered with commercial-grade Acme’s silica gel (GF-254) containing 13% CaSO4, which acts as a binder. Reaction progress was analyzed using a chromatographic technique (TLC analysis) with suitable solvent systems (EtOAc/Pet ether), and observation was performed using UV, iodine spray and immersion in KMnO4 solution. Column purification was performed using 100–200 mesh silica gel in all cases with suitable solvent systems. The boiling range of petroleum ether used in column purification was around 60–80 °C. All IR samples were recorded using DCM and chloroform as solvents on a Nicolet Impact-400 FTIR spectrometer. Nuclear magnetic resonance (NMR) spectra (1H, 13C and DEPT 135) were recorded on 400 and 500 MHz spectrometers (Bruker) with CDCl3 solvent, and chemical shifts (δ ppm) were reported relative to internal standards, such as TMS. The J values (coupling constants) are given in Hz. Mass spectra (HRMS) were recorded under positive ion electrospray ionization (ESI, Q-TOF) mode. Compounds 8 [32], 9 [31], and 11 [26] were prepared according to the literature, and the data were consistent with the reported values.
3.2. Synthesis and Characterization
3.2.1. 5,5′-(2,5-Dimethoxy-1,4-phenylene)Bis(oxazole) (10)
TosMIC (220 mg, 1.13 mmol, 1.1 equiv. for each formyl group) and K2CO3 (568 mg, 4.11 mmol, 4 equiv for each formyl group) in MeOH (10 mL) were added to a stirred solution 2,5-dimethoxyterephthalaldehyde (9) [31] (100 mg, 0.51 mmol, 1.0 equiv.). Later, the resulting reaction mixture was heated to 70 °C for 2 h. At the end of the reaction, which was monitored using TLC, methanol was removed under vacuum conditions, and an additional crude reaction mixture was purified via column chromatography with a 100–200 mesh silica gel column. Elution of the silica gel column with 40% ethyl acetate in petroleum ether gave the desired dimethoxy oxazole 10 in pure form as colorless needles; TLC Rf = 0.57 (petroleum ether/ethyl acetate = 50:50); yield: 115 mg (82%); Mp: 239–241 °C; IR (neat, cm−1): υmax = 3115, 2932, 2847, 1696, 1503, 1456, 1376, 1330, 1403, 1229, 1210, 1114, 1051, 1032, 944, 851, 638; 1H NMR (500 MHz, CDCl3):δ (ppm) = 7.91 (s, 2H), 7.61 (s, 2H), 7.35 (s, 2H), 3.98 (s, 6H); 13C NMR (125.7 MHz, CDCl3): δ (ppm) = 150.0, 149.7, 147.6, 126.5, 117.0, 108.6, 56.1; HRMS (ESI, Q-ToF): m/z calcd. for C14H13N2O4 [M + H]+ 273.0870, found: 273.0864.
3.2.2. Synthesis of Bromo-Oxazoles 12–14
NBS (690 mg, 3.80 mmol) was added to a stirred solution of dimethoxy oxazole 11 [26] (500 mg, 2.43 mmol) in THF (20 mL). Afterward, the resulting mixture was stirred at room temperature for 4 h. The solvent was evaporated under reduced pressure, and the crude product was purified via silica gel column chromatography using 0–1% ethyl acetate in petroleum ether as an eluent to deliver the bromo-substituted aryloxazoles 12, 13, and 14 in 22%, 24%, and 19% yields, respectively.
3.2.3. 2,4-Dibromo-5-(4-Bromo-2,5-Dimethoxyphenyl)oxazole (12)
Colorless needles; TLC Rf = 0.92 (petroleum ether); yield: 237 mg (22%); Mp: 164–166 °C; IR (neat, cm−1): υmax = 3250, 2905, 2827, 2803, 1690, 1498, 1488, 1388, 1285, 1242, 1215, 1195, 1070, 1041, 1025, 997, 856, 771, 568; 1H NMR (500 MHz, CDCl3): δ (ppm) = 7.20 (s, 1H), 7.00 (s, 1H), 3.88 (s, 3H), 3.83 (s, 3H); 13C NMR (125.7 MHz, CDCl3): δ (ppm) = 151.5, 150.1, 149.3, 133.1, 117.5, 115.6, 115.0, 114.2, 113.7, 57.1, 56.5; HRMS (ESI, Q-ToF): m/z calcd. for C11H10Br2NO3 [M + H]+ 361.9004, found: 361.9022.
3.2.4. 2-Bromo-5-(4-Bromo-2,5-Dimethoxyphenyl)oxazole (13)
Colorless needles; TLC Rf = 0.85 (petroleum ether); yield: 215 mg (24%); Mp: 137–139 °C; IR (neat, cm−1): υmax = 2911, 2664, 2413, 2315, 1501, 1491, 1369, 1290, 1247, 1217, 1186, 1161, 1117, 1061, 1032, 961, 949, 868, 768; 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.48 (s, 1H), 7.20 (s, 1H), 7.16 (s, 1H), 3.92 (s, 3H), 3.90 (s, 3H); 13C NMR (125.7 MHz, CDCl3): δ (ppm) = 151.5, 150.5, 149.9, 132.2, 129.0, 116.7, 115.8, 112.4, 109.1, 57.1, 56.3; HRMS (ESI, Q-ToF): m/z calcd. for C11H10Br2NO3 [M + H]+ 361.9022, found: 361.9021.
3.2.5. 4-Bromo-5-(4-Bromo-2,5-Dimethoxyphenyl)oxazole (14)
Colorless needles; TLC Rf = 0.76 (petroleum ether); yield: 175 mg (19%); Mp: 123–125 °C; IR (neat, cm−1): υmax = 3132, 2969, 2840, 2325, 1490, 1386, 1369, 1315, 1290, 1217, 1180, 1161, 1115, 1061, 1029, 986, 861, 766; 1H NMR (500 MHz, CDCl3): δ (ppm) = 7.90 (s, 1H), 7.21 (s, 1H), 7.06 (s, 1H), 3.88 (s, 3H), 3.83 (s, 3H); 13C NMR (125.7 MHz, CDCl3): δ (ppm) = 151.6, 150.6, 150.1, 145.2, 117.5, 115.1, 114.4, 114.2, 114.0, 57.1, 56.6; HRMS (ESI, Q-ToF): m/z calcd. for C11H10Br2NO3 [M + H]+ 361.9022, found: 361.9027.
3.3. Data Collection and Refinement Details
The X-ray structure of compound 12 was determined using single-crystal X-ray diffraction. Single crystals of compound 12 were obtained from ethyl acetate in hexane solvent at room temperature. Crystal data were collected with graphite monochromatized M0Kα radiation (λ = 0.71073) on a Rigaku Saturn 724+ diffractometer using ω scans at a temperature of 293 K. The structures were solved via direct methods using Olex-2 [33] and ShelXL-97 [34] and refined using full-matrix least-square minimization based on F2. ORTEPs were drawn using the Mercury program [35]. X-Ray crystallographic data and refinement parameters for compound 12 were tabulated in an SI file and deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC No: CCDC-1984197. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 15 February 2020). Selected X-ray data of 12: C11H8Br3NO3, M = 441.91, orthorhombic space group = Pca21, unit cell: a = 20.626 (8) Å3, b = 9.0594 (5) Å3, c = 7.1357 (3) Å3, α = 90°, β = 90 °, γ = 90°, Z = 4, ρcald = 2.201 mg/m3, F(000) = 840, λ = 0.71073 Å, µ = 9.074 mm−1, total/unique reflections = 6776/2346, final R indices [I > 2sigma (I)]: R1 = 0.0293, ωR2 = 0.0572, R indices (all data): R1 = 0.0345, ωR2 = 0.0598.
4. Conclusions
In summary, we successfully synthesized a bisoxazole derivative and various bromo-substituted aryloxazoles in good yields. These compounds were synthesized through the combination of Van Leusen reaction and bromination with NBS used as key steps. Finally, the structures of these oxazoles were confirmed using NMR, HRMS, and X-ray diffraction. It is worth noting that the oxazole frameworks may become valuable candidates for applications in medicinal/pharmaceutical and drug delivery.
Supplementary Materials
The following are available online, 1H, 13C, and DEPT NMR spectra of compounds 8, 9, 10, 11, 12, 13, and 14. Check CIF report and cif file for the title compound 12.
Author Contributions
Investigation, data curation, and writing—original draft preparation, S.R.C.; resources and writing—review and editing, S.R.C. and S.K.; conceptualization, data curation, and writing—original draft preparation, S.R.C. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Defense Research and Development Organization (DRDO; ARDB/01/1041849/M/I), New Delhi, India. The APC was sponsored by MDPI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and the Supplementary Materials.
Acknowledgments
S.R.C. received the UGC-New Delhi research fellowship award and thanks IRCC-IIT Bombay for their RA financial support. The authors are grateful to Darshan S Mhatre for his help in collecting the X-ray data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kakkar, S.; Narasimhan, B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.C. (Ed.) . Oxazoles: Synthesis, Reactions and Spectroscopy, Part B; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Jin, Z. Muscarine, imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2005, 22, 196–229. [Google Scholar] [CrossRef] [PubMed]
- Gomstyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef]
- Rodriquez, M.; Aquino, M.; Bruno, I.; De Martino, G.; Taddei, M.; Gomez-Paloma, L. Chemistry and biology of chromatin remodeling agents: State of art and future perspectives of HDAC inhibitors. Curr. Med. Chem. 2006, 13, 1119–1139. [Google Scholar] [CrossRef]
- Sharma, V.; Bhatia, P.; Alam, O.; Javed Naim, M.; Nawaz, F.; Ahmad Sheikh, A.; Jha, M. Recent advancement in the discovery and development of COX-2 inhibitors: Insight into biological activities and SAR studies (2008–2019). Bioorg. Chem. 2019, 89, 103007. [Google Scholar] [CrossRef]
- Dayam, R.; Grande, F.; Al-Mawsawi, L.Q.; Neamati, N. Recent advances in the design and discovery of small-molecule therapeutics targeting HER2/neu. Expert Opin. Ther. Pat. 2007, 17, 83–103. [Google Scholar] [CrossRef]
- Kaur, R.; Palta, K.; Kumar, M.; Bhargava, M.; Dahiya, L. Therapeutic potential of oxazole scaffold: A patent review (2006–2017). Expert Opin. Ther. Pat. 2018, 28, 783–812. [Google Scholar] [CrossRef]
- Joshi, S.; Bisht, A.S.; Juyal, D. Systematic scientific study of 1,3-oxazole derivatives as a useful lead for pharmaceuticals: A review. Pharma Innovation 2017, 6, 109–117. [Google Scholar]
- Zhou, H.; Cheng, J.-Q.; Wang, Z.-S.; Chen, F.-H.; Liu, X.-H. Oxazole: A Promising Building Block for the Development of Potent Antitumor Agents. Curr. Top. Med. Chem. 2016, 16, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Stokes, N.R.; Baker, N.; Bennett, J.M.; Chauhan, P.K.; Collins, I.; Davies, D.T.; Gavade, M.; Kumar, D.; Lancett, P.; Macdonald, R.; et al. Design, synthesis and structure–activity relationships of substituted oxazole–benzamide antibacterial inhibitors of FtsZ. Bioorg. Med. Chem. Lett. 2014, 24, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Imamura, K.; Haruta, J.; Wakitani, K. 4-(4-Cycloalkyl/aryl-oxazol-5-yl)benzenesulfonamides as Selective Cyclooxygenase-2 Inhibitors: Enhancement of the Selectivity by Introduction of a Fluorine Atom and Identification of a Potent, Highly Selective, and Orally Active COX-2 Inhibitor JTE-522. J. Med. Chem. 2002, 45, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.J.; Zhang, D.J.; Peng, Z.G.; Li, Y.H.; Shan, G.Z.; Zuo, L.M.; Wu, L.T.; Li, S.Y.; Gao, R.M.; Li, Z.R. Synthesis and antiviral activity of a novel class of (5-oxazolyl)phenyl amines. Eur. J. Med. Chem. 2013, 69, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zahanich, I.; Kondratov, I.; Naumchyk, V.; Kheylik, Y.; Platonov, M.; Zozulya, S.; Krasavin, M. Phenoxymethyl 1,3-oxazoles and 1,2,4-oxadiazoles as potent and selective agonists of free fatty acid receptor 1 (GPR40). Bioorg. Med. Chem. Lett. 2015, 25, 3105–3111. [Google Scholar] [CrossRef]
- Shaw, A.Y.; Henderson, M.C.; Flynn, G.; Samulitis, B.; Han, H.; Stratton, S.P.; Chow, H.H.S.; Hurley, L.H.; Dorr, R.T. Characterization of novel diaryl oxazole-based compounds as potential agents to treat pancreatic cancer. J. Pharmacol. Exp. Ther. 2009, 331, 636–647. [Google Scholar] [CrossRef]
- Harris, P.A.; Cheung, M.; Hunter, R.N., III; Brown, M.L.; Veal, J.M.; Nolte, R.T.; Wang, L.; Liu, W.; Crosby, R.M.; Johnson, J.H.; et al. Discovery and evaluation of 2-anilino-5-aryloxazoles as a novel class of VEGFR2 kinase inhibitors. J. Med. Chem. 2005, 48, 1610–1619. [Google Scholar] [CrossRef]
- Palmer, D.C. (Ed.) Oxazoles: Synthesis, Reactions and Spectroscopy, Part A; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Yan, X.; Wen, J.; Zhou, L.; Fan, L.; Wang, X.; Xu, Z. Current Scenario of 1,3-oxazole Derivatives for Anticancer Activity. Curr. Top. Med. Chem. 2020, 20, 1916–1937. [Google Scholar] [CrossRef]
- Rymbai, E.M.; Chakraborty, A.; Choudhury, R.; Verma, N.J.; De, B. Review on Chemistry and Therapeutic Activity of the Derivatives of Furan and Oxazole: The Oxygen Containing Heterocycles. Pharma Chem. 2019, 11, 20–41. [Google Scholar]
- Kadu, V.D. Recent Advances for Synthesis of Oxazole Heterocycles via C-H/C-N Bond Functionalization of Benzylamines. ChemistrySelect 2022, 7, e202104028. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, W.; Zhang, D. Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules 2020, 25, 1594. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Shah, V. Synthesis of bis- and trisoxazole derivatives via Suzuki-Miyaura cross-coupling reaction and van Leusen oxazole synthesis. Synthesis 2007, 23, 3653–3658. [Google Scholar] [CrossRef]
- Kotha, S.; Todeti, S.; Gopal, M.B.; Datta, A. Synthesis and photophysical properties of c 3-symmetric star-shaped molecules containing heterocycles such as furan, thiophene, and oxazole. ACS Omega 2017, 2, 6291–6297. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Cheekatla, S.R. Design and Synthesis of Pentacycloundecane Cage Compound Containing Oxazole Moiety. Heterocycles 2020, 100, 1623–1632. [Google Scholar] [CrossRef]
- Kotha, S.; Sreenivasachary, N. A new synthetic approach to 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) derivatives via a [2+2+2] cycloaddition reaction. Bioorg. Med. Chem. Lett. 2000, 10, 1413–1415. [Google Scholar] [CrossRef]
- Kotha, S.; Sreenivasachary, N. Synthesis of 1,2,3,4-Tetrahydroisoquinoline-3-carboxylic Acid (Tic) Derivatives by Cycloaddition Approaches. Eur. J. Org. Chem. 2001, 18, 3375–3383. [Google Scholar] [CrossRef]
- Van Leusen, D.; Van Leusen, A.M. Synthetic Uses of Tosylmethyl Isocyanide (TosMIC). Org. React. 2001, 57, 417–666. [Google Scholar]
- Mathiyazhagan, A.D.; Anilkumar, G. Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2019, 17, 6735–6747. [Google Scholar] [CrossRef]
- Shao, P.; Li, Z.; Luo, J.; Wang, H.; Qin, J. A Convenient Synthetic Route to 2,5 Dialkoxyterephthalaldehyde. Synth. Commun. 2005, 35, 49–53. [Google Scholar] [CrossRef]
- Roviello, A.; Borbone, F.; Carella, A.; Diana, R.; Roviello, G.; Panunzi, B.; Ambrosio, A.; Maddalena, P. High quantum yield photoluminescence of new polyamides containing oligo-PPV amino derivatives and related oligomers. J. Polym. Sci. A Polym. Chem. 2009, 47, 2677–2689. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Bruno, L.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, L.R.; Van de Streek, J.; Wood, P.A. Mercury CSD 2.0 -New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).