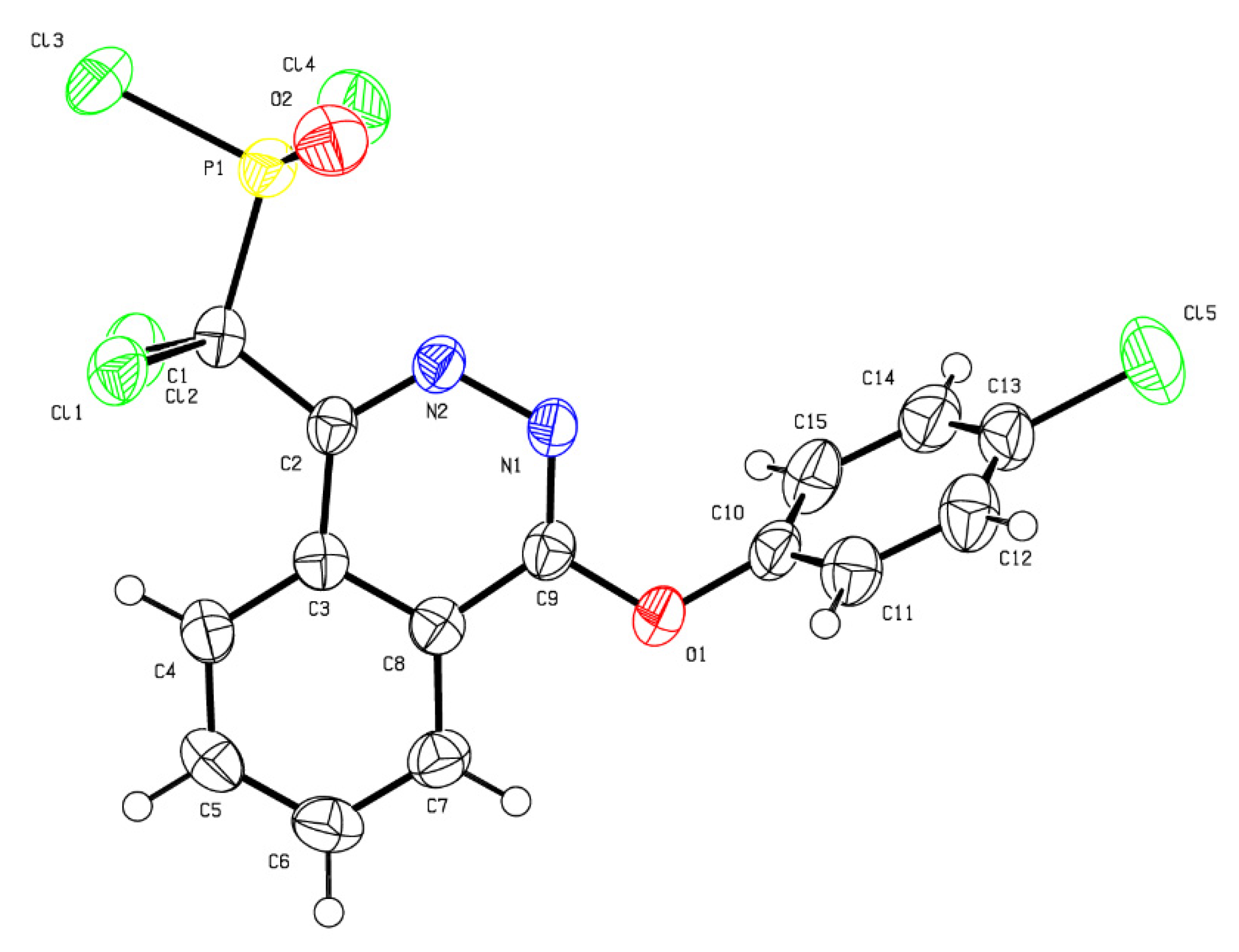
Dichloro{4-(4-chlorophenoxy)phthalazin-1-yl} methylphosphonic dichloride
Abstract
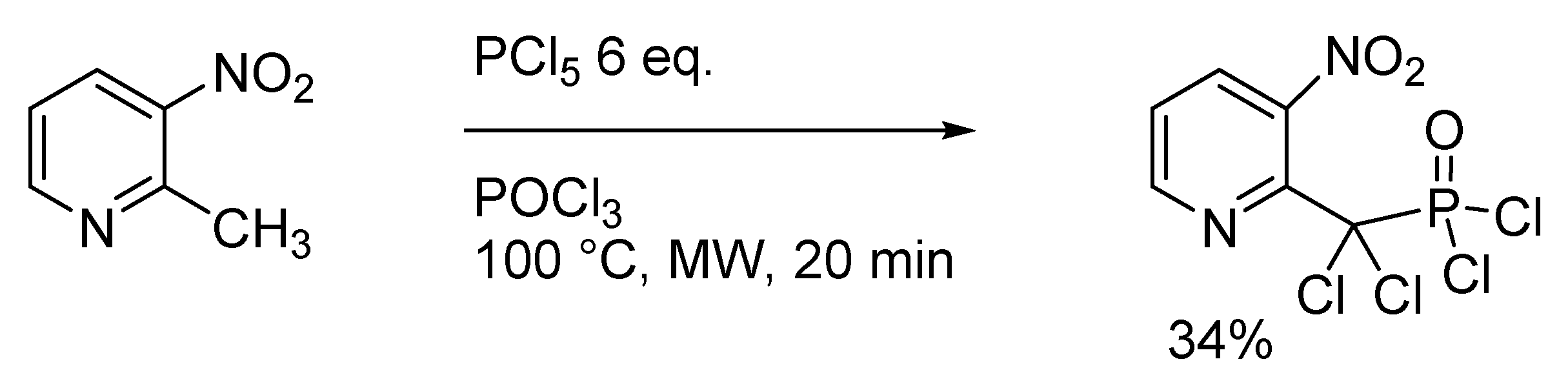
:1. Introduction
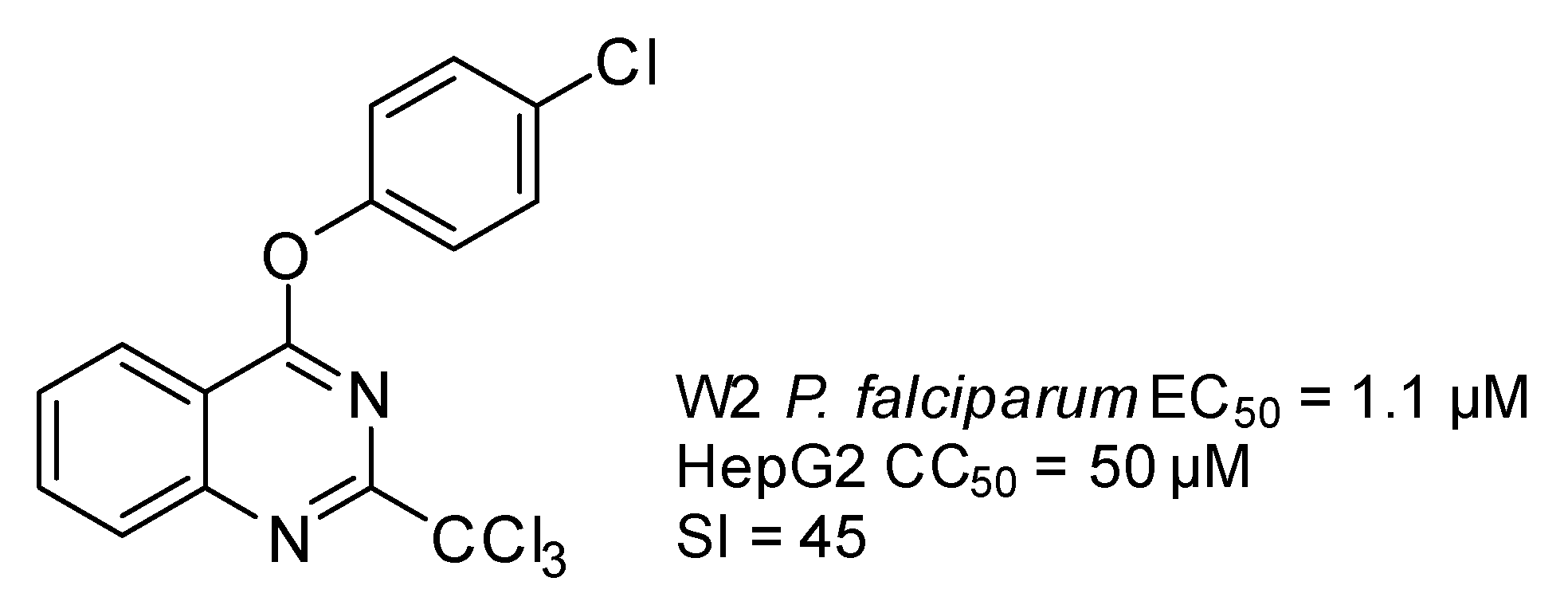
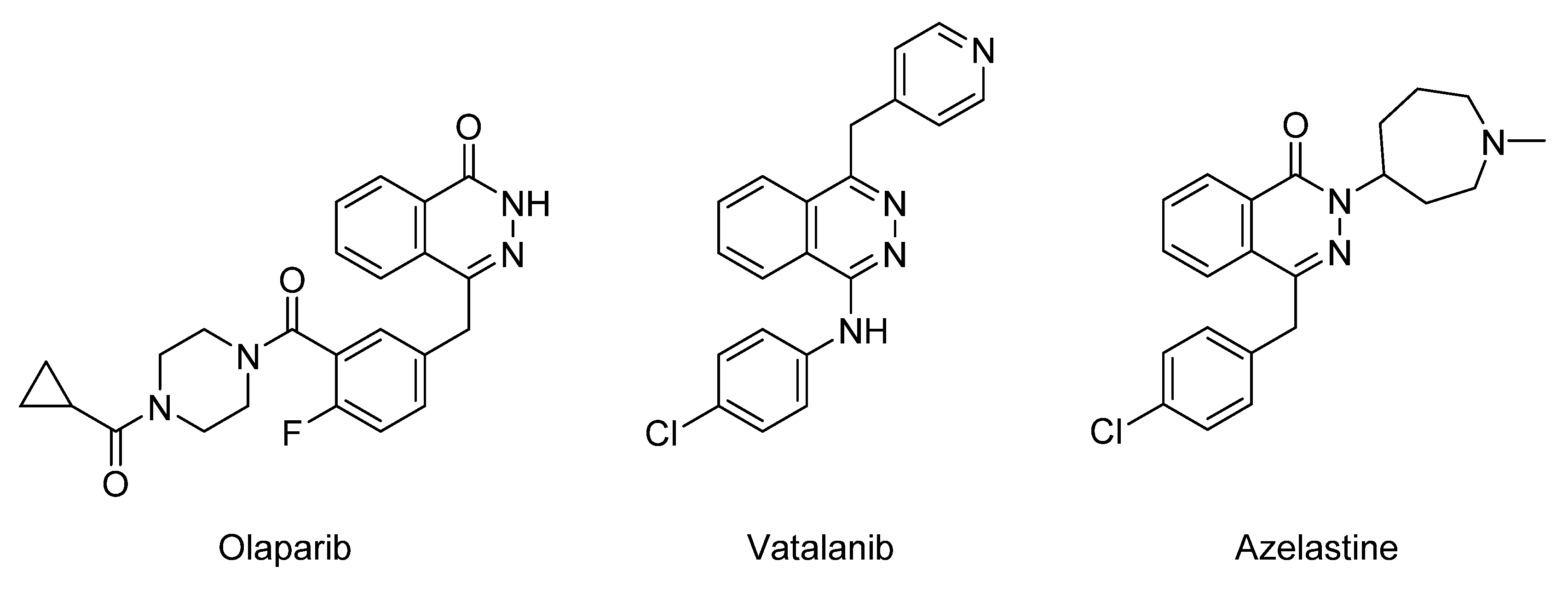
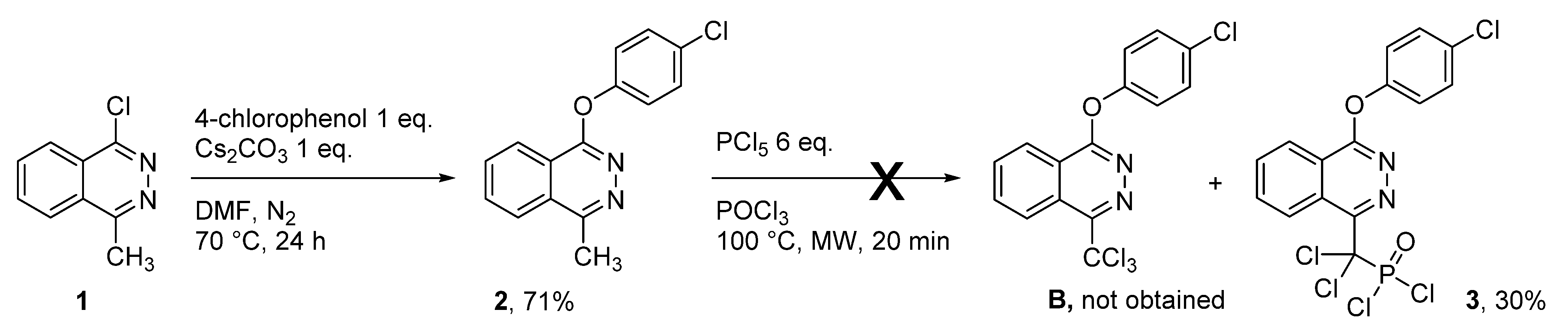
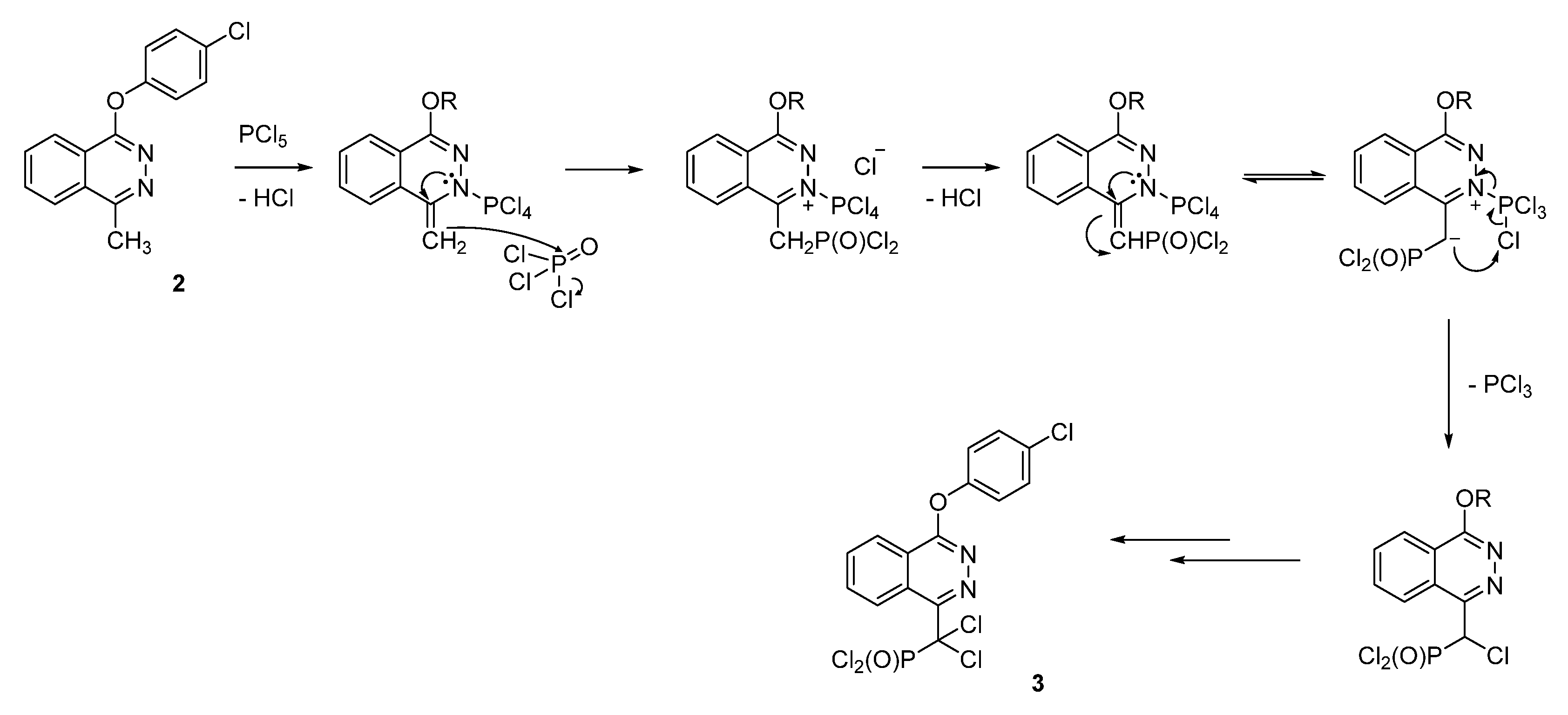
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Burrows, J.N.; Duparc, S.; Gutteridge, W.E.; Hooft van Huijsduijnen, R.; Kaszubska, W.; Macintyre, F.; Mazzuri, S.; Möhrle, J.J.; Wells, T.N.C. New developments in anti-malarial target candidate and product profiles. Malar. J. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Amrane, D.; Gellis, A.; Hutter, S.; Prieri, M.; Verhaeghe, P.; Azas, N.; Vanelle, P.; Primas, N. Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines. Molecules 2020, 25, 3929. [Google Scholar] [CrossRef] [PubMed]
- Amrane, D.; Arnold, C.-S.; Hutter, S.; Sanz-Serrano, J.; Collia, M.; Azqueta, A.; Paloque, L.; Cohen, A.; Amanzougaghene, N.; Tajeri, S.; et al. 2-Phenoxy-3-Trichloromethylquinoxalines Are Antiplasmodial Derivatives with Activity against the Apicoplast of Plasmodium Falciparum. Pharmaceuticals 2021, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Amrane, D.; Primas, N.; Arnold, C.-S.; Hutter, S.; Louis, B.; Sanz-Serrano, J.; Azqueta, A.; Amanzougaghene, N.; Tajeri, S.; Mazier, D.; et al. Antiplasmodial 2-Thiophenoxy-3-Trichloromethyl Quinoxalines Target the Apicoplast of Plasmodium Falciparum. Eur. J. Med. Chem. 2021, 224, 113722. [Google Scholar] [CrossRef] [PubMed]
- Castera-Ducros, C.; Azas, N.; Verhaeghe, P.; Hutter, S.; Garrigue, P.; Dumètre, A.; Mbatchi, L.; Laget, M.; Remusat, V.; Sifredi, F.; et al. Targeting the Human Malaria Parasite Plasmodium Falciparum: In Vitro Identification of a New Antiplasmodial Hit in 4-Phenoxy-2-Trichloromethylquinazoline Series. Eur. J. Med. Chem. 2011, 46, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Meinhardt, G.; Jacques, C.; Laurent, D.; Thomas, A.L. Vatalanib: The Clinical Development of a Tyrosine Kinase Inhibitor of Angiogenesis in Solid Tumours. Expert Opin. Investig. Drugs 2007, 16, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ratner, P.H.; Findlay, S.R.; Hampel, F.; van Bavel, J.; Widlitz, M.D.; Freitag, J.J. A Double-Blind, Controlled Trial to Assess the Safety and Efficacy of Azelastine Nasal Spray in Seasonal Allergic Rhinitis. J. Allergy Clin. Immunol. 1994, 94, 818–825. [Google Scholar] [CrossRef]
- Zaib, S.; Khan, I. Synthetic and Medicinal Chemistry of Phthalazines: Recent Developments, Opportunities and Challenges. Bioorg. Chem. 2020, 105, 104425. [Google Scholar] [CrossRef]
- Elmeligie, S.; Aboul-Magd, A.M.; Lasheen, D.S.; Ibrahim, T.M.; Abdelghany, T.M.; Khojah, S.M.; Abouzid, K.A.M. Design and Synthesis of Phthalazine-Based Compounds as Potent Anticancer Agents with Potential Antiangiogenic Activity via VEGFR-2 Inhibition. J. Enzyme Inhib. Med. Chem. 2019, 34, 1347–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaeghe, P.; Rathelot, P.; Gellis, A.; Rault, S.; Vanelle, P. Highly Efficient Microwave Assisted α-Trichlorination Reaction of α-Methylated Nitrogen Containing Heterocycles. Tetrahedron 2006, 62, 8173–8176. [Google Scholar] [CrossRef]
- Primas, N.; Vanelle, P.; Amrane, D. CCDC 2074292: Experimental Crystal Structure Determination. 2021. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc27mgn0&sid=DataCite (accessed on 11 August 2022).
- Kato, T.; Katagiri, N.; Wagai, A. Synthesis of Methylpyridine Derivatives—XXXIII Phosphonylation and Chlorination of Methylpyridine and 3-Nitro-Methylpyridine Derivatives. Tetrahedron 1978, 34, 3445–3449. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrane, D.; Khoumeri, O.; Vanelle, P.; Primas, N. Dichloro{4-(4-chlorophenoxy)phthalazin-1-yl} methylphosphonic dichloride. Molbank 2022, 2022, M1439. https://doi.org/10.3390/M1439
Amrane D, Khoumeri O, Vanelle P, Primas N. Dichloro{4-(4-chlorophenoxy)phthalazin-1-yl} methylphosphonic dichloride. Molbank. 2022; 2022(3):M1439. https://doi.org/10.3390/M1439
Chicago/Turabian StyleAmrane, Dyhia, Omar Khoumeri, Patrice Vanelle, and Nicolas Primas. 2022. "Dichloro{4-(4-chlorophenoxy)phthalazin-1-yl} methylphosphonic dichloride" Molbank 2022, no. 3: M1439. https://doi.org/10.3390/M1439
APA StyleAmrane, D., Khoumeri, O., Vanelle, P., & Primas, N. (2022). Dichloro{4-(4-chlorophenoxy)phthalazin-1-yl} methylphosphonic dichloride. Molbank, 2022(3), M1439. https://doi.org/10.3390/M1439








