Abstract
We report a synthesis of 3-bromo-9-N′-(N,N-dimethylformamidino)benzanthrone in good yield by a condensation reaction of 3-bromo-9-aminobenzanthrone with dimethylformamide in the presence of phosphorous oxychloride. The structure of the synthesized amidine was characterized by FTIR-ATR, NMR experiments, and elemental analysis. The title compound was analyzed by UV-Vis and fluorescence spectroscopy in various organic solvents.
1. Introduction
7H-Benzo[de]anthracen-7-one (benzanthrone) derivatives are currently known to be used in various applications such as dyes, luminophores, photochromic materials, fluorescent markers, and probes [1]. Due to the high photostability and low toxicity of benzanthrone derivatives, many of these dyes can be used as fluorescent probes for the study of proteins, lipids, and living cells [2,3,4,5]. The most common substituted benzanthrone derivatives contain an amino group at various positions in the aromatic system. The possibility of replacing hydrogen atoms in the amino group makes it possible to obtain various N-containing derivatives, which are already or may find practical use in the future.
Among benzanthrone monoamines, the greatest interest has been attracted by 3-, 4-, 6- and 8-amino derivatives as precursors for dyes with interesting optoelectronic properties [6,7,8,9]. In contrast to the widely studied various monoamine derivatives of benzanthrone, disubstituted benzanthrone derivatives have been insufficiently studied. For example, we have synthesized and studied derivatives of 2-bromo-3-aminobenzantrone [10]. The literature describes only a few 3,9-disubstituted derivatives [11]. These heterocyclic derivatives were synthetized by nucleophilic substitution of bromine atoms in 3,9-dibromobenzanthrone and exhibit thermally activated delayed deep-red fluorescence [11]. Another possible method for the preparation of 3,9-disubstituted derivatives could be the use of 3-bromo-9-aminobenzantrone. Although 3-bromo-9-aminobenzantrone was obtained as early as the 1940s by nitration of 3-bromobenzanthrone followed by reduction of the nitro group [12], no further studies related to the synthesis and study of 3-bromo-9-aminobenzantrone derivatives have been carried out. Therefore, we decided to use 3-bromo-9-aminobenzantrone for synthesis of the first amidino derivative and to study its photophysical properties.
2. Results and Discussion
2.1. Synthesis
We used the traditional method for the preparation of the first amidino derivative of 3-bromo-9-aminobenzanthrone (1), which involves condensation of the primary amine and the corresponding amide in the presence of phosphorus oxychloride. The synthetic route for the target dye 2 is outlined in Scheme 1. We found that 3-bromo-9-aminobenzantrone is more active than 3-aminobenzanthrone and 2-bromo-3-aminobenzanthrone [10] because the reaction takes less time (about 1.5 h) and proceeds at a lower temperature (80 °C). For 3-aminobenzanthrone and 2-bromo-3-aminobenzanthrone, a reaction temperature of 100–110 °C and a time of at least 3 hours are required [10].
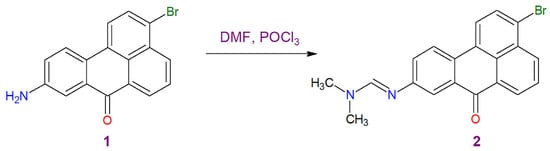
Scheme 1.
Synthesis of 3-bromo-9-N′-(N,N-dimethyl-formamidino)benzanthrone (2).
FT-IR and NMR spectroscopic study confirmed the chemical structure of the new compound 2. In the 1H-NMR spectra of the derivative 2 (Supplementary Materials), the signals of appropriate methyl groups and multiplet signals (from δ 6.99 to 8.90 ppm) of nine aromatic core protons and imide group proton were found. Methyl groups give two singlet signals at 3.13 and 3.19 ppm. The signal of the proton of the CH=N group is located in the multiplet signal at 7.74–7.63 ppm.
We tried to obtain the mass spectrum of the synthesized compound by chromatomass spectrometry, but only two molecular ions of bromobenzanthrone (with M = 308 and M = 310) were detected in the mass spectrum of the analyzed compound, which indicates the low stability of the compound 2 under these conditions.
The synthesized dye exhibits pronounced luminescent properties; therefore, we investigated its luminescence characteristics in organic solvents.
2.2. Photophysical Properties
The photophysical properties of the obtained amidine 2 were evaluated, and the corresponding data are summarized in Table 1. The absorption and emission spectra were recorded in eight organic solvents with a wide range of polarities (see Figure 1). The position of absorption maximum is situated between 430 nm and 490 nm. As the polarity of the solvent increased from hexane to dimethyl sulfoxide, a bathochromic shift (about 50 nm) of absorption band was observed. Interestingly, the bathochromic shift of absorption band for amidines of 2-bromo-3-aminobenzanthrone is smaller: 20–30 nm [10]. Furthermore, the obtained luminophore has slightly higher molar absorption coefficients.

Table 1.
Photophysical parameters of studied derivative 2 in various solvents (concentration is 1 × 10−5 mol L−1).
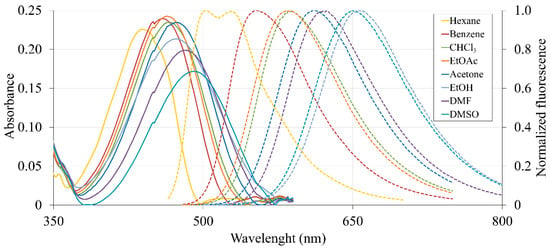
Figure 1.
The UV-Vis absorption and fluorescence emission (dashed lines) spectra of amidine 2 in various organic solvents (concentration is 1 × 10−5 mol L−1).
The studied dye luminesces at 500–660 nm, showing a double emission band in hexane solution and a wide band for other solvents. The luminescence quantum yield is higher than that of the previously obtained derivatives of 2-bromo-3-aminobenzanthrone and approximately the same as that of appropriate 3-amidines. However, the highest quantum yield is observed in solutions of ethyl acetate and chloroform, and not in benzene, as for the other above-mentioned amidines. The dye has relatively large Stokes shifts (up to 5920 cm−1 in ethanol). These characteristics of the prepared amidine demonstrate its potential as a biomedical probe for proteins, lipids, and cells. This dye can be used as a suitable sensitive probe for checking the polarity of the solvent.
3. Materials and Methods
3.1. Materials and Basic Measurements
All reagents were of analytical grade (Aldrich Chemical Company, St. Louis, MO, USA) and were used as received. The progress of the chemical reactions and the purity of products were monitored via TLC on silica gel plates (Fluka F60254, 20 × 10, 0.2 mm, ready-to-use), using C6H6-CH3CN (3:1) as an eluent and visualization under UV light. Melting points were determined on an MP70 Melting Point System apparatus and are not corrected.
The identification of the chemical bonds was performed by means of Fourier-transform infrared (FTIR) spectrometry. A Bruker Vertex 70v vacuum spectrometer equipped with an attenuated total reflection (ATR) accessory was used in this study. 1H-NMR spectra were recorded on Bruker equipment, operating at 400 MHz in CDCl3 (with TMS as the internal standard) at ambient temperature. Elemental analysis was performed on a Euro Vector EA-3000 CHNS analyzer.
The absorption spectra were obtained using the UV–Visible spectrophotometer “Specord’s UV/VIS”. Fluorescence spectra were recorded on a FLSP920 (Edinburgh Instruments Ltd., Livingston, UK) spectrofluorometer in the visible range (450–800 nm). The studies were performed in quartz cuvettes with an absorbing layer thickness of 1 cm at a concentration of solutions in organic solvents of 1 × 10−5 mol L−1; the solvents used were of spectroscopic or equivalent grade.
3.2. Synthesis and Characterization
3-Bromo-9-aminobenzanthrone (1) was prepared by nitration of 3-bromobenzanthrone and subsequent reduction of the obtained 9-nitroderivative according to the procedure in the literature procedure [1].
3-Bromo-9-N′-(N,N-dimethylformamidino)benzanthrone (2).
To the solution of 0.50 g (2.0 mmol) 3-bromo-9-aminobenzanthrone (1) in 3 mL of N,N-dimethylformamide, the phosphorus oxychloride (0.2 mL, 2.1 mmol) was added dropwise under stirring. The resulting mixture was heated for 2 h at 80 °C. After cooling to ambient temperature, the crude product that precipitated on pouring into 50 mL of 2% NaOH water solution was filtered, washed with water, dried, recrystallized from benzene, and dried to obtain pure compound 2 in 86% yield as an orange-colored solid (m.p. 185.5–186.5 °C). FT-IR (KBr): 3042 (w), 2939 (w), 2807 (w), 1670 (s), 1521 (s), 1508 (m), 1447 (m), 1376 (m), 1310 (m), 1266 (m), 1199 (m), 1102 (s), 988 (m), 845 (s), 771 (s), 740 (s), 702 (s) cm−1. 1H-NMR (400 MHz, CDCl3, δ): 8.90 (dd, J = 8.2, 1.5 Hz, 1H), 8.79 (dd, J = 7.3, 1.5 Hz, 1H), 8.48 (ddd, J = 7.9, 1.5, 0.5 Hz, 1H), 8.32 (d, J = 8.1 Hz, 1H), 8.25 (dd, J = 8.1, 0.6 Hz, 1H), 7.74–7.63 (m, 2H), 7.45 (ddd, J = 8.0, 7.1, 1.1 Hz, 1H), 6.99 (d, J = 8.0 Hz, 1H), 3.19 (s, 3H), 3.13 (s, 3H). 13C-NMR (100 MHz, CDCl3, δ): 183.94; 143.80; 136.78; 134.20; 133.17; 132.02; 130.03; 129.57; 129.26; 129.07; 128.67; 127.89; 126.53; 126.07; 125.45; 123.34; 122.30; 122.26; 54.85. Anal. Calcd. for C20H15BrN2O: C, 63.34; H, 3.99; N, 7.39; found: C, 63.18; H, 4.01; N, 7.27.
Supplementary Materials
The following are available online. Figure S1: 1H-NMR spectrum of compound 2; Figure S2: 13C NMR spectrum of compound 2; Figure S3: IR spectrum of compound 2.
Author Contributions
E.R. designed the chemical synthesis, T.G. performed structure determination, A.P. performed spectroscopic experiments and analyzed results, and E.K. analyzed results and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krasovitskii, B.M.; Bolotin, B.M. Organic Luminescent Materials; Wiley-VCH: New York, NY, USA, 1988; pp. 149–152. [Google Scholar]
- Tarabara, U.; Kirilova, E.; Kirilov, G.; Vus, K.; Zhytniakivska, O.; Trusova, V.; Gorbenko, G. Benzanthrone dyes as mediators of cascade energy transfer in insulin amyloid fibrils. J. Mol. Liq. 2021, 324, 115102. [Google Scholar] [CrossRef]
- Rubenina, I.; Gavarane, I.; Kirilova, J.; Mezaraupe, L.; Kirjusina, M. Comparison of the benzanthrone luminophores: They are not equal for rapid examination of parafasciolopsis fasciolaemorpha (trematoda: Digenea). Biomolecules 2021, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Gavarane, I.; Kirilova, J.; Rubenina, I.; Mezaraupe, L.; Deksne, G.; Puckins, A.; Kokina, I.; Bulanovs, A.; Kirjusina, M.; Osipovs, S. A simple and rapid staining technique for sex determination of Trichinella larvae parasites by confocal laser scanning microscopy. Microsc. Microanal. 2019, 25, 1491–1497. [Google Scholar] [CrossRef]
- Vus, K.; Trusova, V.; Gorbenko, G.; Sood, R.; Kirilova, E.; Kirilov, G.; Kalnina, I.; Kinnunen, P. Fluorescence investigation of interactions between novel benzanthrone dyes and lysozyme amyloid fibrils. J. Fluoresc. 2014, 24, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Altaf, Y.; Ullah, S.; Khan, F.A.; Maalik, A.; Rubab, S.L.; Hashmi, M.A. Finding New Precursors for Light Harvesting Materials: A Computational Study of the Fluorescence Potential of Benzanthrone Dyes. ACS Omega 2021, 6, 32334–32341. [Google Scholar] [CrossRef] [PubMed]
- Belov, S.P.; Burdukova, O.A.; Komlev, I.V.; Petukhov, V.A.; Povedailo, V.A.; Semenov, M.A. New efficient laser dyes for the red region of the spectrum. Part 1. Peri-indenones. Quantum Elec. 2016, 46, 589. [Google Scholar] [CrossRef]
- Umeda, R.; Namba, T.; Yoshimura, T.; Nakatsukasa, M.; Nishiyama, Y. Selective introducing of aryl and amino groups: Reaction of benzanthrone and organometallic reagents. Tetrahedron 2013, 69, 1526–1531. [Google Scholar] [CrossRef]
- Bentley, P.; McKellar, J.F. The Photochemistry of Benz[de]anthracen-7-ones. Part III. Fluorescence Quantum Yield and Continuous Photolysis Studies. J. Chem. Soc. 1976, 15, 1850–1854. [Google Scholar] [CrossRef]
- Kirilova, E.M.; Puckins, A.I.; Romanovska, E.; Fleisher, M.; Belyakov, S.V. Novel amidine derivatives of benzanthrone: Effect of bromine atom on the spectral parameters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 202, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tsiko, U.; Bezvikonnyi, O.; Volyniuk, D.; Minaev, B.F.; Keruckas, J.; Cekaviciute, M.; Jatautiene, E.; Andruleviciene, V.; Dabuliene, A.; Grazulevicius, J.V. TADF quenching properties of phenothiazine or phenoxazine-substituted benzanthrones emitting in deep-red/near-infrared region towards oxygen sensing. Dyes Pigm. 2022, 197, 109952. [Google Scholar] [CrossRef]
- Day, F.H. Nitration of the 13-halogenobenzanthrones. J. Chem. Soc. 1940, 1474–1475. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvents Effects in Organic Chemistry, 2nd ed.; Verlag Chemie: Weinheim, Germany, 1988. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).