3-Carbamoylmethyl-Indole-1-Carboxylic Acid Ethyl Ester
Abstract
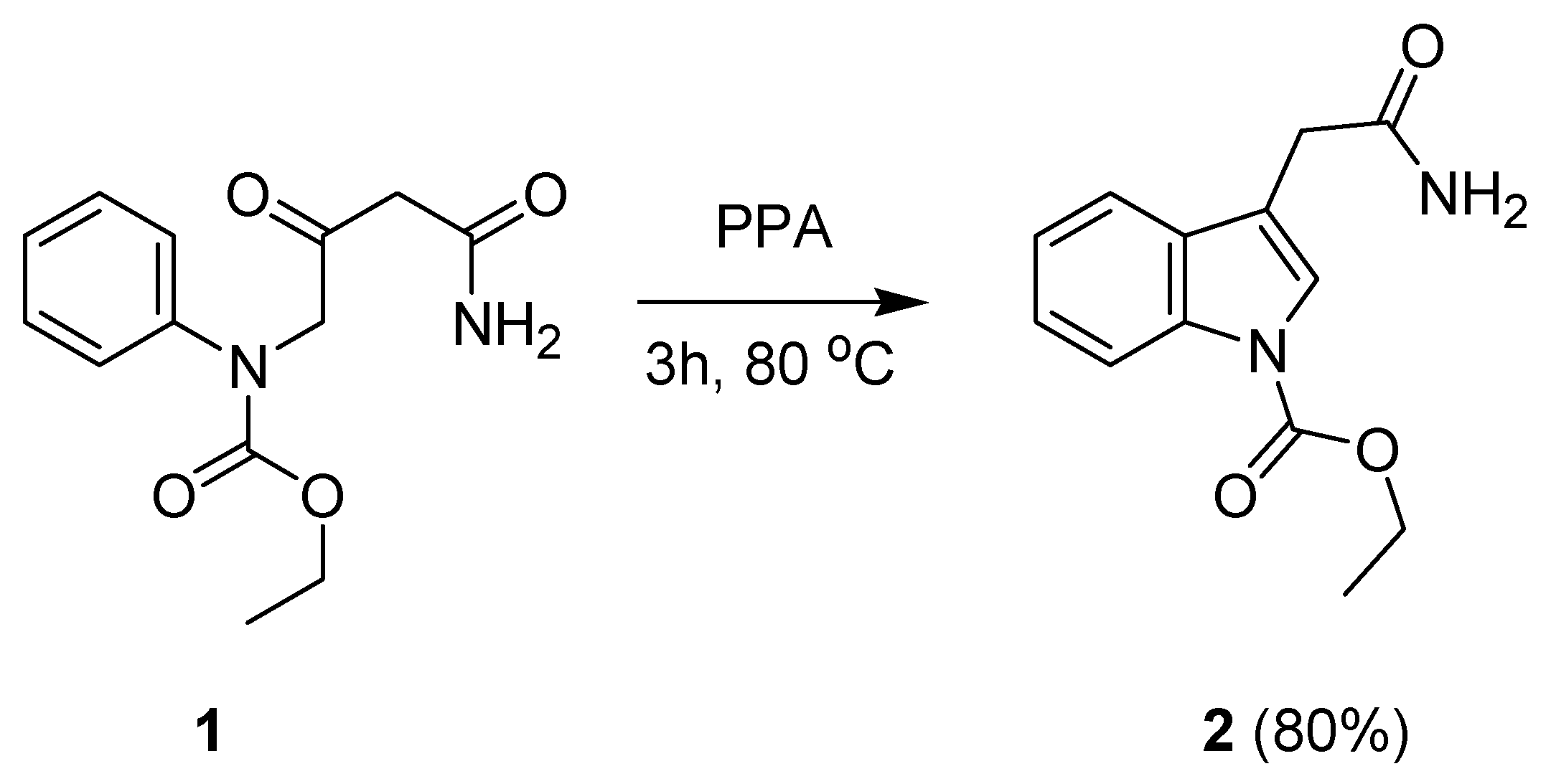
:1. Introduction
2. Results
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heravi, M.M.; Amiri, Z.; Kafshdarzadeh, K.; Zadsirjan, V. Synthesis of indole derivatives as prevalent moieties present in selected alkaloids. RSC Adv. 2021, 11, 33540–33612. [Google Scholar] [CrossRef]
- Taber, D.F.; Tirunahari, P.K. Indole synthesis: A review and proposed classification. Tetrahedron 2011, 67, 7195–7210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, S.; Petrášek, J. Why plants need more than one type of auxin. Plant Sci. 2011, 180, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, D.G.; Boyd, J.B.; Johnson, H.E. Indole-3-alkanamides. J. Org. Chem. 1960, 25, 1826–1827. [Google Scholar] [CrossRef]
- Karigiannis, G.; Papaioannou, D. Structure, Biological Activity and Synthesis of Polyamine Analogues and Conjugates. Eur. J. Org. Chem. 2000, 2000, 1841–1863. [Google Scholar] [CrossRef]
- Kalantzi, S.; Athanassopoulos, C.M.; Ruonala, R.; Helariutta, Y.; Papaioannou, D. A general approach for the liquid phase fragment synthesis of orthogonally protected naturally occurring polyamines and applications thereof. J. Org. Chem. 2019, 84, 15118–15130. [Google Scholar] [CrossRef] [PubMed]
- Winter-Vann, A.M.; Baron, R.A.; Wong, W.; Cruz, J.; York, J.D.; Gooden, D.M.; Bergo, M.O.; Young, S.G.; Toone, E.J.; Casey, P.J. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4336–4341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.-Y.; Li, X.; Zhu, H.-P.; Huang, W. Regulation of the Ras-Related Signaling Pathway by Small Molecules Containing an Indole Core Scaffold: A Potential Antitumor Therapy. Front. Pharmacol. 2020, 11, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, K.N.; Lewis-Ballester, A.; Yeh, S.-R. Structural Basis of Inhibitor Selectivity in Human Indoleamine 2,3-Dioxygenase 1 and Tryptophan Dioxygenase. J. Am. Chem. Soc. 2019, 141, 18771–18779. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, G.; Liu, R.; Chen, D.; Chen, Z. Divergent oriented synthesis (DOS) of aza-heterocyclic amides through palladium-catalyzed ketenimination of 2-iodo-N-(propa-1,2-dien-1-yl)anilines. Org. Chem. Front. 2020, 7, 890–895. [Google Scholar] [CrossRef]
- Julia, M.; Igolen, J. Bull. De La Soc. Chim. De Fr. 1962, pp. 1056–1060. Available online: https://www.reaxys.com/#/hopinto?context=C&query=CNR.CNR%3D116200&database=RX&origin=ReaxysOutput&ln= (accessed on 23 January 2022).
- Yanev, P.; Angelov, P. Synthesis of functionalised β-keto amides by aminoacylation/domino fragmentation of β-enamino amides. Beilstein J. Org. Chem. 2018, 14, 2602–2606. [Google Scholar] [CrossRef] [PubMed]
- Angelov, P.; Velichkova, S.; Yanev, P. 4-Aminoalkyl Quinolin-2-one Derivatives via Knorr Cyclisation of ω-Amino-β-Keto Anilides. Molbank 2021, 2021, M1266. [Google Scholar] [CrossRef]
- Suarez-Castillo, O.R.; Montiel-Ortega, L.A.; Melendez-Rodrıguez, M.; Sanchez-Zavala, M. Cleavage of alkoxycarbonyl protecting groups from carbamates by t-BuNH2. Tetrahedron Lett. 2007, 48, 17–20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollova, Y.; Angelov, P.; Yanev, P. 3-Carbamoylmethyl-Indole-1-Carboxylic Acid Ethyl Ester. Molbank 2022, 2022, M1324. https://doi.org/10.3390/M1324
Mollova Y, Angelov P, Yanev P. 3-Carbamoylmethyl-Indole-1-Carboxylic Acid Ethyl Ester. Molbank. 2022; 2022(1):M1324. https://doi.org/10.3390/M1324
Chicago/Turabian StyleMollova, Yordanka, Plamen Angelov, and Pavel Yanev. 2022. "3-Carbamoylmethyl-Indole-1-Carboxylic Acid Ethyl Ester" Molbank 2022, no. 1: M1324. https://doi.org/10.3390/M1324
APA StyleMollova, Y., Angelov, P., & Yanev, P. (2022). 3-Carbamoylmethyl-Indole-1-Carboxylic Acid Ethyl Ester. Molbank, 2022(1), M1324. https://doi.org/10.3390/M1324





