Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with 2-(Phenylsulfonyl)acetonitrile
Abstract
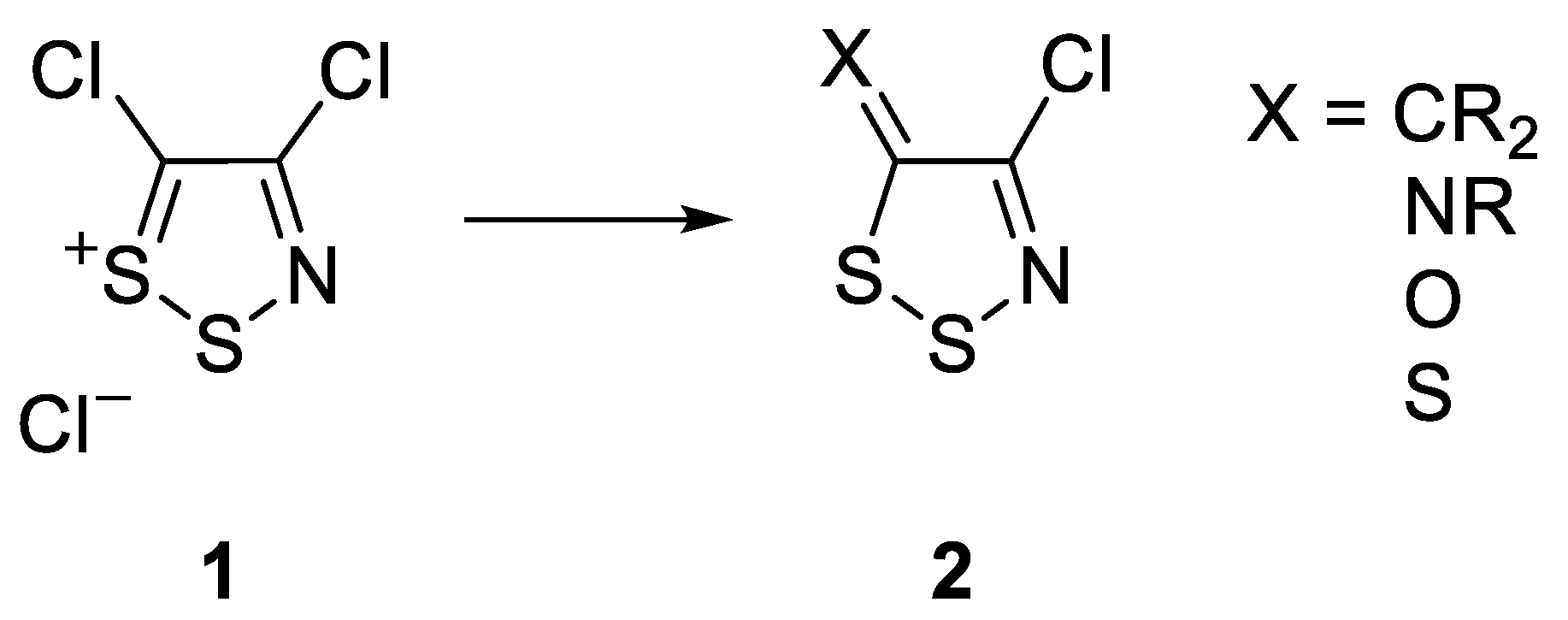
:1. Introduction
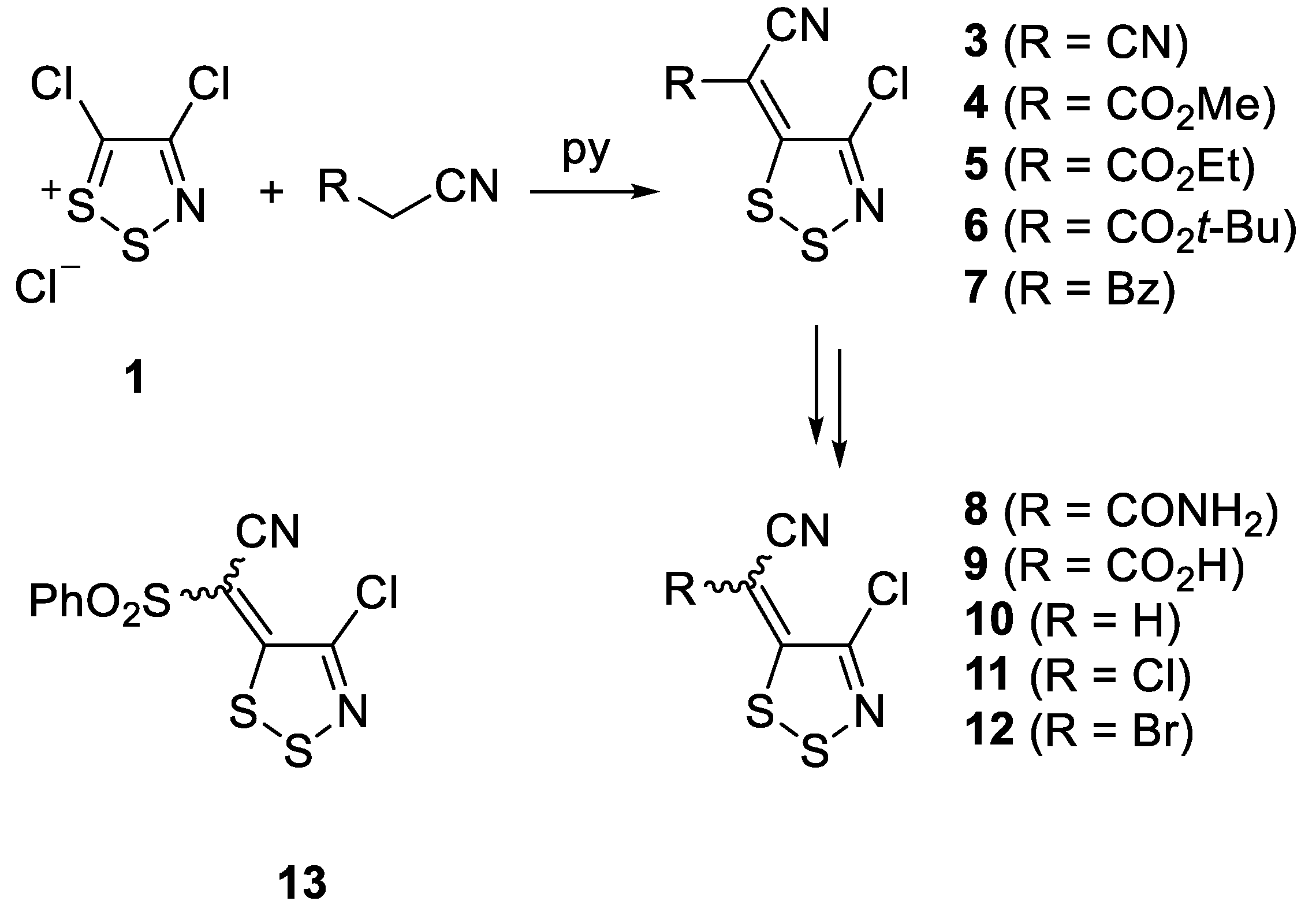
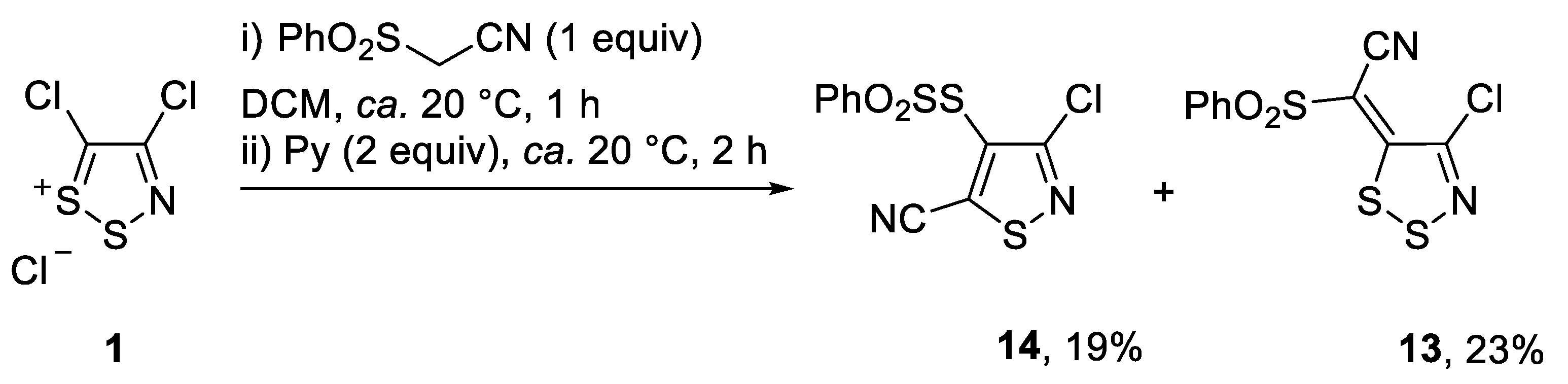
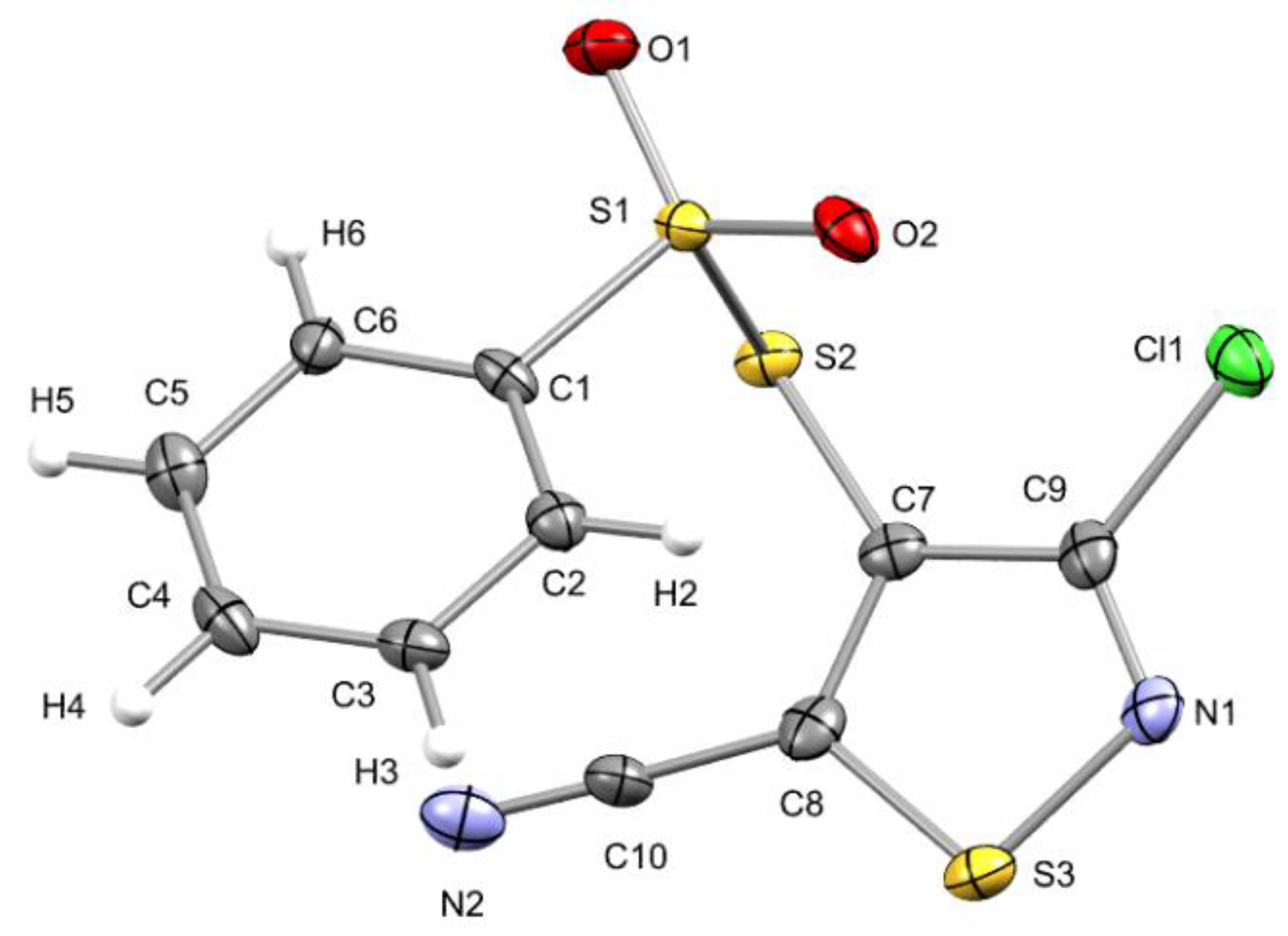
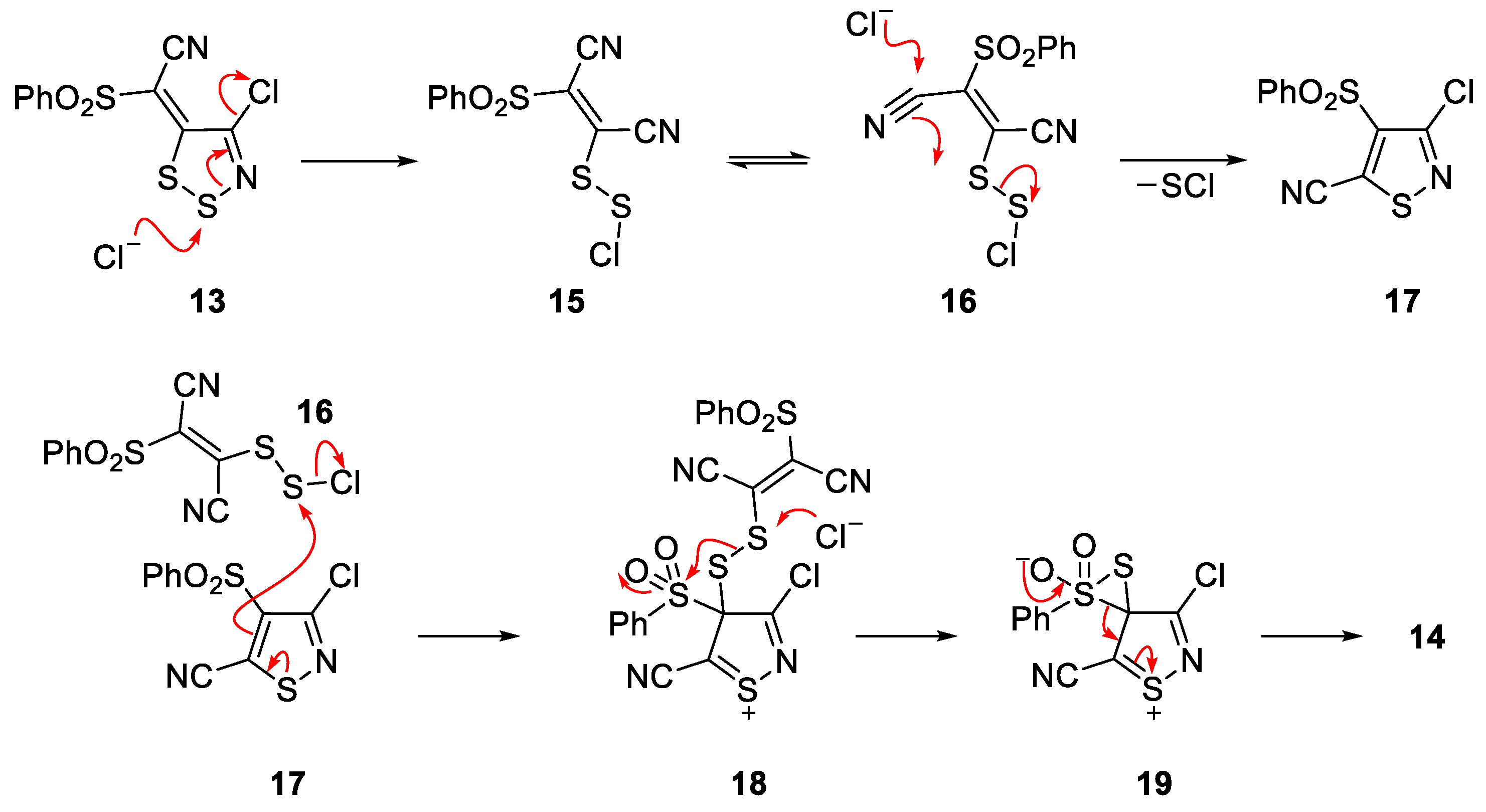
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, J.E. Certain 4-Halo-5-Aryl-1,2,3-Dithiazole Compounds and Their Preparation. U.S. Patent 4,059,590, 22 November 1977. [Google Scholar]
- Appel, R.; Janssen, H.; Haller, I.; Plempel, M. 1,2,3-Dithiazolderivate, Verfahren zu ihrer Herstellung Sowie ihre Verwendung als Arzneimittel. DE Patent 2,848,221, 14 May 1980. [Google Scholar]
- Besson, T.; Rees, C.W.; Cottenceau, G.; Pons, A.-M. Antimicrobial evaluation of 3,1-benzoxazin-4-ones, 3,1-benzothiazin-4-ones, 4-alkoxyquinazolin-2-carbonitriles and N-arylimino-1,2,3-dithiazoles. Bioorg. Med. Chem. Lett. 1996, 6, 2343–2348. [Google Scholar] [CrossRef]
- Cottenceau, G.; Besson, T.; Gautier, V.; Rees, C.W.; Pons, A.-M. ChemInform Abstract: Antibacterial Evaluation of Novel N-Arylimino-1,2,3-dithiazoles and N-Arylcyanothioformamides. Bioorg. Med. Chem. Lett. 1996, 6, 529–532. [Google Scholar] [CrossRef]
- Thiéry, V.; Rees, C.W.; Besson, T.; Cottenceau, G.; Pons, A.-M. Antimicrobial activity of novel N-quinolinyl and N-naphthylimino-1,2,3-dithiazoles. Eur. J. Med. Chem. 1998, 33, 149–153. [Google Scholar] [CrossRef]
- Joseph, R.W.; Antes, D.L.; Osei-Gyimah, P. Antimicrobial Compounds with Quick Speed of Kill. U.S. Patent 5,688,744, 3 November 1995. [Google Scholar]
- Asquith, C.R.M.; Konstantinova, L.S.; Laitinen, T.; Meli, M.L.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R.; Hilton, S.T. Evaluation of Substituted 1,2,3-Dithiazoles as Inhibitors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein via a Proposed Zinc Ejection Mechanism. ChemMedChem 2016, 11, 2119–2126. [Google Scholar] [CrossRef]
- Asquith, C.R.; Meili, T.; Laitinen, T.; Baranovsky, I.V.; Konstantinova, L.S.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R. Synthesis and comparison of substituted 1,2,3-dithiazole and 1,2,3-thiaselenazole as inhibitors of the feline immunodeficiency virus (FIV) nucleocapsid protein as a model for HIV infection. Bioorg. Med. Chem. Lett. 2019, 29, 1765–1768. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bol’Shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, S.; Thiéry, V.; et al. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar] [CrossRef]
- Oppedisano, F.; Catto, M.; Koutentis, P.A.; Nicolotti, O.; Pochini, L.; Koyioni, M.; Introcaso, A.; Michaelidou, S.S.; Carotti, A.; Indiveri, C. Inactivation of the glutamine/amino acid transporter ASCT2 by 1,2,3-dithiazoles: Proteoliposomes as a tool to gain insights in the molecular mechanism of action and of antitumor activity. Toxicol. Appl. Pharmacol. 2012, 265, 93–102. [Google Scholar] [CrossRef]
- Napolitano, L.; Scalise, M.; Koyioni, M.; Koutentis, P.; Catto, M.; Eberini, I.; Parravicini, C.; Palazzolo, L.; Pisani, L.; Galluccio, M.; et al. Potent inhibitors of human LAT1 (SLC7A5) transporter based on dithiazole and dithiazine compounds for development of anticancer drugs. Biochem. Pharmacol. 2017, 143, 39–52. [Google Scholar] [CrossRef]
- Barclay, T.M.; Cordes, A.W.; Beer, L.; Oakley, R.T.; Preuss, K.E.; Taylor, N.J.; Reed, R.W. Sterically protected 1,2,3-dithiazolyl radicals: Preparation and structural characterization of 4-chloro-5-pentafluorophenyl-1,2,3-dithiazolyl. Chem. Commun. 1999, 531–532. [Google Scholar] [CrossRef]
- Beer, L.; Cordes, A.W.; Haddon, R.C.; Itkis, M.E.; Oakley, R.T.; Reed, R.W.; Robertson, C.M. A π-stacked 1,2,3-dithiazolyl radical. Preparation and solid state characterization of (Cl2C3NS)(ClC2NS2). Chem. Commun. 2002, 1872–1873. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Koutentis, P.A. The Preparation and Characterization of 5-Substituted-4-chloro-1,2,3-dithiazolium Salts and their Conversion into 4-Substituted-3-chloro-1,2,5-thiadiazoles. Molecules 2005, 10, 346–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakitin, O.A. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Zhdankin, V.V., Eds.; Elsevier: Oxford, UK, 2008; Volume 6, Chapter 6.01; pp. 1–36. [Google Scholar]
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008, 77, 521–546. [Google Scholar] [CrossRef]
- Kim, K. Recent Advances in 1,2,3-Dithiazole Chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1997, 120, 229–244. [Google Scholar] [CrossRef]
- Kim, K. Synthesis and Reactions of 1,2,3-Dithiazoles. Sulfur Rep. 1998, 21, 147–207. [Google Scholar] [CrossRef]
- Emayan, K.; English, R.F.; Koutentis, P.A.; Rees, C.W. New routes to benzothiophenes, isothiazoles and 1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1997, 3345–3350. [Google Scholar] [CrossRef]
- Clarke, D.; Emayan, K.; Rees, C.W. New synthesis of isothiazoles from primary enamines. J. Chem. Soc. Perkin Trans. 1 1998, 77–82. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. The reaction of 4,5-dichloro-1,2,3-dithiazolium chloride with dimethylsulfonium dicyanomethylide: An improved synthesis of (4-chloro-1,2,3-dithiazolylidene)malononitrile. Tetrahedron 2009, 65, 6850–6854. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reactions of tetracyanoethylene oxide with 1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1998, 2505–2510. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.J.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2014, 4, 7735–7748. [Google Scholar] [CrossRef]
- Rees, C.W. Polysulfur-nitrogen heterocyclic chemistry. J. Heterocycl. Chem. 1992, 29, 639–651. [Google Scholar] [CrossRef]
- Jeon, M.-K.; Kim, K. Synthesis of new5-alkylidene-4-chloro-5H-1,2,3-dithiazoles and their stereochemistry. Tetrahedron 1999, 55, 9651–9667. [Google Scholar] [CrossRef]
- Chang, Y.-G.; Cho, H.S.; Kim, K. Novel Synthesis and Reactions of 5,7-Dialkyl-4,6-dioxo-4,5,6,7-tetrahydro-isothiazolo[3,4,-d]pyrimidine-3-carbonitriles and 6-Methyl-4-oxo-4H-1-aza-5-oxa-2-thiaindene-3-carbonitrile. Org. Lett. 2003, 5, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Koyioni, M.; Manoli, M.; Manolis, M.J.; Koutentis, P.A. Reinvestigating the Reaction of 1H-Pyrazol-5-amines with 4,5-Dichloro-1,2,3-dithiazolium Chloride: A Route to Pyrazolo[3,4-c]isothiazoles and Pyrazolo[3,4-d]thiazoles. J. Org. Chem. 2014, 79, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Gaillot, J.-M.; Gelas-Mialhe, Y.; Vessiere, R. Synthèse et réactivité des halogéno-2 sulfonyl-2 aziridines. Can. J. Chem. 1979, 57, 1958–1966. [Google Scholar] [CrossRef]
- El-Sayed, I.; Belsky, V.K.; Zavodnik, V.E.; Jørgensen, K.A.; Senning, A. Chlorotropic rearrangements of an α-sulfanyl-α-sulfonylalkanesulfenyl chloride to an α-chloroalkyl disulfide and an S-(α-chloroalkyl) thiosulfonate. J. Chem. Soc. Perkin Trans. 1 1994, 1251–1252. [Google Scholar] [CrossRef]
- Bruni, M.C.; Ponterini, G.; Scoponi, M. Photophysics and photochemistry of diphenyl sulfone. 2. Investigation of the S1 decay pathways. J. Phys. Chem. 1989, 93, 678–683. [Google Scholar] [CrossRef]
- CrysAlis CCD and CrysAlis RED, version 1.171.32.15; Oxford Diffraction Ltd.: Abingdon, UK, 2008.
- Sheldrick, G.M. SHELXL-97: A Program for the Refinement of Crystal Structure; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Farrugia, L.J. WinGXsuite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, version 3.1d; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plakas, K.; Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with 2-(Phenylsulfonyl)acetonitrile. Molbank 2022, 2022, M1322. https://doi.org/10.3390/M1322
Plakas K, Kalogirou AS, Kourtellaris A, Koutentis PA. Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with 2-(Phenylsulfonyl)acetonitrile. Molbank. 2022; 2022(1):M1322. https://doi.org/10.3390/M1322
Chicago/Turabian StylePlakas, Konstantinos, Andreas S. Kalogirou, Andreas Kourtellaris, and Panayiotis A. Koutentis. 2022. "Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with 2-(Phenylsulfonyl)acetonitrile" Molbank 2022, no. 1: M1322. https://doi.org/10.3390/M1322
APA StylePlakas, K., Kalogirou, A. S., Kourtellaris, A., & Koutentis, P. A. (2022). Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with 2-(Phenylsulfonyl)acetonitrile. Molbank, 2022(1), M1322. https://doi.org/10.3390/M1322






