Abstract
The novel racemic secondary alcohol (±)-2-{[4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol (12) has been successfully synthesized through S-alkylation of 4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (10) in alkaline medium with 2-bromo-1-phenylethanone followed by reduction of the corresponding ketone 11. All the synthesized compounds were characterized by IR, 1D (1H, 13C, DEPT135) and 2D (1H-1H, 1H-13C and 1H-15N) NMR spectroscopy, elemental analysis and HRMS spectrometry.
1. Introduction
Triazoles are an important class of compounds due to their numerous biomedical applications [], such as antibacterial activity [,], antifungal [] anticancer [,], antioxidant activity and anticonvulsant effects []. Grafting different substituents on the heterocyclic ring leads to a variation of the type of biological activity and the intensity with which it manifests itself []. The recent literature reveals that the presence of 4,5-disubstituted-4H-1,2,4-triazole-3-thiol moiety in chemical compounds is associated with numerous biological properties such as antimicrobial [,,,,], anti-inflammatory [] and antifungal [] activities.
The general method of synthesis of 4,5-disubstituted-4H-1,2,4-triazole-3-thiols 6 is carried out by cyclization of the corresponding 2-acyl-N-(4-aryl)hydrazine-1-carbothioamides 3 [,]. The required 2-acyl-N-(4-aryl)hydrazine-1-carbothioamides 3 can be obtained by reaction of the appropriate carboxylic acid hydrazides 1 with (aryl)isothiocyanates 2 [,] or by a one-pot reaction starting from an aromatic amines 4 by successive reaction with carbon sulphide, sodium chloroacetate and hydrazine with the intermediate obtaining of N-(aryl)hydrazinecarbothioamides 5 [,], followed by their acylation with acyl chlorides (Scheme 1).
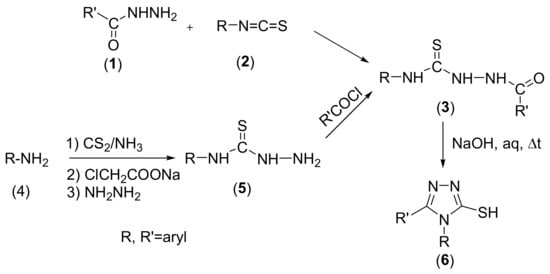
Scheme 1.
Synthetic route to 4,5-disubstituted-4H-1,2,4-triazole-3-thiols.
2. Results and Discussion
4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (10) was synthesized starting from 4-bromoaniline according to the literature methods (Scheme 2). The S-alkylation of the triazole 10 was performed with 2-bromo-1-phenylethanone in the presence of cesium carbonate [,] followed by the reduction of the corresponding ketone 11 with sodium borohydride to give the secondary alcohol 12 [,] (Scheme 2).

Scheme 2.
Synthetic route to 4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol.
Theoretically 4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (10) can have two tautomeric forms: the thiol form 10a and the thione form 10b. As a result, alkylation in a basic medium can occur in fact as S-alkylation at the tautomeric form 10a or as N-alkylation at the tautomeric form 10b (Scheme 3).
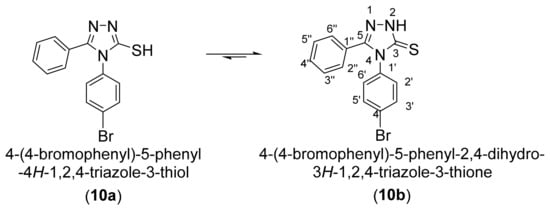
Scheme 3.
Tautomeric equilibrium of 4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (10).
The corresponding 1H NMR and 13C NMR spectra confirmed that the tautomeric equilibri is confirmed by the deshielded signals of the 2-N-H proton at 14.11 ppm and of the 3-C carbon atom at 168.9 ppm which corresponds to a thione-type (C=S) carbon atom.
Following alkylation using cesium carbonate as a base in N,N-dimethylformamide, it has been observed that the alkylation occurs exclusively at the thiol group as S-alkylation [] (Scheme 4). This is observed from 2D NMR spectroscopic analysis by analyzing the couplings over two or three bonds in the HMBC spectrum, as well as by the shift of the signal of the triazole carbon 3-C atom to a lower δ value at 152.0 ppm, corresponding to a thiol (C-SH)-type carbon atom. The alkylation is proved by the existence of a 1H NMR signal at 4.98 ppm corresponding to the methylene proton (S-CH2) and the 13C NMR signal at 193.0 ppm corresponding to the carbonyl carbon atom (C=O) from the ketone (11). The 2D 1H-15N HMBC spectrum does not show the cross-peak over two bonds between the 2-N carbon atom and the methylene protons (-CH2) that could have been observed in the case of N-alkylation, which confirms that S-alkylation has occurred. In the case of S-alkylation the long-range coupling over 4 bonds between the 2-N atom and the methylene protons is not observable.
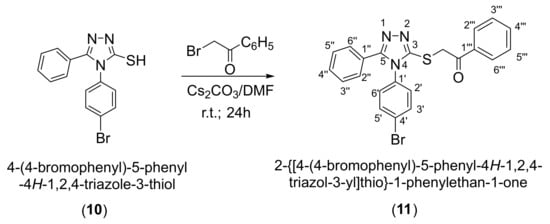
Scheme 4.
Synthetic route to 2-{[4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (11).
Reduction of the carbonyl group to the secondary alcohol group was accomplished with sodium borohydride in ethanol. Secondary alcohol 12 was obtained in a yield of 57.0% after recrystallization from ethanol (Scheme 5).
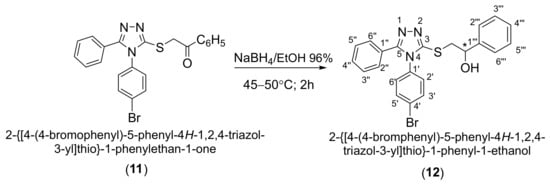
Scheme 5.
Synthetic route to (±)-2-{[4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (12).
From the correlative 1H-15N HMBC spectra the signal for the 4-N nitrogen atom in all the synthesized compounds could be identified, by its coupling over three bonds with hydrogen atoms in the ortho positions of the phenyl ring attached to this atom. This long- range coupling was very useful in the assignment of the corresponding 1H NMR signals for the ortho protons on the phenyl ring bound to the 4-N nitrogen atom. The reduction of ketone 11 to secondary alcohol 12 is evidenced from the 1H NMR spectrum by the doublet at 4.94 ppm attributed to the hydroxyl proton (OH), the multiplet at 5.20–5.18 ppm attributed to the methine proton (CH) and the doublets of doublets at 3.47 and 3.62 respectively attributed to the two diastereotopic protons of the methylene group (S-CH2). The 13C NMR spectrum shows the disappearance of the deshielding signal at 193.0 ppm corresponding to the carbonyl carbon atom and the appearance of the signal at 73.3 ppm attributed to the methine carbon atom (CH-O).
The secondary alcohol 12 has two diastereotopic protons at the methylene group which appear in the 1H NMR spectrum at different δ values as two distinct doublets of doublets. This is specific for a methylene group attached to an asymmetric carbon atom. From the 1H-13C HMBC spectrum, the long-range coupling over three bonds of the methylene diastereotopic protons with the 3-C triazole carbon atom is observed, thus further confirming the S-alkylation.
In conclusion we obtained two novel compounds that have not yet been reported in the literature, 2-{[4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (11) and (±)-2-{[4-(4-bromphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (12) whose structures were confirmed by 1D and 2D NMR spectroscopy.
3. Materials and Methods
The chemical reagents were purchased from commercial sources and used in the various syntheses with no further purification. Melting points were determined on a Böetius PHMK (Veb Analytik. Dresden, Germany) melting point apparatus and are uncorrected. Infrared spectra (IR) were recorded as KBr disks on a Jasco FT/IR-410 spectrometer (JASCO Corporation, Tokyo, Japan). NMR spectra were recorded on a Bruker AVANCE III 500 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany), in DMSO-d6 and CDCl3 using TMS as an internal standard for protons and carbons. Chemical shifts are reported in ppm units and the coupling constants are given in Hz. High resolution MS (HRMS) spectra were recorded on a Bruker Maxis II QTOF spectrometer (Bruker Daltonics, Bremen, Germany) with electrospray ionization (ESI) in positive mode. The compounds have been dissolved in acetonitrile. MS spectra processing and isotope pattern simulations were performed with Compass Data Analysis V.4.4 (Bruker Daltonics).
3.1. NMR Characterization of 4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (10)
1H NMR (500 MHz, DMSO-d6) δ(ppm): 14.18 (s, 1H, -NH); 7.70 (dt, 2H, J = 8.7 Hz, J = 2.0 Hz, 2′-H, 6′-H,); 7.43 (tt, 1H, J = 7.2 Hz, J = 1.34 Hz, 4′-H); 7.39–7.32 (m, 6H, 2″-H, 6″-H, 3″-H, 5″-H, 3′-H, 5′-H);
13C NMR (125 MHz, DMSO-d6) δ(ppm): 168.9 (3-C); 150.9 (5-C); 139.7 (1′-C); 132.3 (2′-C, 6′-C); 130.8 (3′-C, 5′-C); 130.3 (4″-C); 128.5 (3″-C, 5″-C); 128.3 (2″-C, 6″-C); 125.5 (1″-C); 122.5 (4′-C);
15N NMR (50 MHz, DMSO-d6) δ(ppm): 183.1 (4-N); 275.6 (1-N).
3.2. Synthesis of 2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenylethan-1-one (11)
In a round bottom flask 4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-sulfanyl (10, 2.11 g, 0.006 mol) was solubilized in DMF (41 mL). After complete dissolution of the compound, cesium carbonate (1.03 g, 0.0038 mol) was added in small portions. After 15 min. a solution of 2-bromo-1-phenylethanone (1.26 g, 0.006 mol) in DMF (20 mL), was slowly dropped over the reaction mass and then allowed to stir for approximately 24 h. The crude reaction was precipitated in distilled water. Compound 11 was purified by recrystallization from ethanol to give 1.75 g (61% yield) of a white powder pure product. M.p. 152–153 °C. TLC: Rf = 0.51 (n-hexane/ethyl acetate, 3:7). FT-IR (KBr, cm−1): 758 (γsk.ar), 759 (γsk.ar), 1492 (), 1578 (), 1682 (), 2915 (νasCH2), 3058 (νCarH), 3089 (νCarH). 1H NMR (CDCl3, 500 MHz) δ (ppm): 8.04 (d,2H, J = 7.4 Hz, 2‴-H, 6‴-H); 7.65–7.60 (m, 3H, 3′-H, 5′-H, 4‴-H); 7.50 (t, 2H, J = 7.8 Hz, 3‴-H, 5‴-H); 7.41–7.35 (m, 3H, 2″-H, 6″-H, 4″-H); 7.30 (t, 2H, J = 7.6 Hz, 3″-H, 5″-H); 7.15 (d, 2H, J = 8.6 Hz, 2′-H, 6′-H); 4.98 (s, 2H, -CH2); 13C NMR (CDCl3,125 MHz) δ (ppm): 193.0 (C=O); 154.9 (5-C); 152.0 (3-C); 135.2 (1‴-C); 134.0 (4‴-C); 133.3 (3′-C,5′-C); 133.0 (1″-C); 129.9 (4″-C); 128.85 (2′-C, 6′-C); 128.83 (3‴-C, 6‴-C); 128.6 (3″-C, 5″-C); 128.5 (2‴-C, 6‴-C); 128.2 (2″-C, 6″-C); 126.2 (1′-C); 124.2 (4′-C); 41.4 (CH2); 15N NMR (CDCl3,50 MHz) δ (ppm): 175.1 (4-N).
(All spectra are reported in Supplementary Materials) Elemental analysis for C22H16BrN3OS Calcd. (%): C, 58.67; H, 3.58; Br, 17.74; N, 9.33; S, 7.12. Found (%): C, 58.62; H, 3.54; Br, 17.68; N, 9.20; S, 7.02. HRMS: calculated for C22H16BrN3OS+Na: 472.0095; found: 472.0081.
3.3. Synthesis of (±)-2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol (12)
Compound 11 (0.8 g, 0.00177 moles) was dissolved in ethanol (50 mL) with mild heating (45–50 °C. Then NaBH4 (0.096 g, 0.0025 mol) was added in small five portions within 1.25 h. After 30 min from the last portion addition the conversion is monitored by TLC, and the obtained product precipitated in water. The purification was carried out by recrystallization from 96% ethanol, finally giving 0.462 g (a yield of 57%) of 2-{[4-(4-bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenylethane-1-ol as a white powder. M.p. 183.5–185 °C. TLC Rf = 0.4 (n-hexane/ethyl acetate, 3:7). FT-IR (KBr, cm−1): 695 (γsk.ar), 774 (γsk.ar), 826 (γsk.ar), 1269 (), 1427 (), 1492 (), 2853 (νsCH2), 2935 (νasCH2), 3032 (νCarH), 3057 (νCarH), 3087 (νCarH), 3197 (νOH). 1H NMR (500 MHz, DMSO-d6) δ(ppm): 7.61 (d, 2H, J = 8.6Hz, 3′-H, 5′-H); 7.45 (d, 2H, J = 7.4 Hz, 2‴-H, 6‴-H); 7.39–7.26 (m, 8H, 2′-H, 6′-H, 3′-H, 5′-H, 4′-H, 3‴-H, 5‴-H, 4‴-H); 7,10 (d, 2H, J = 8.6 Hz, 2′-H, 6′-H); 5.20–5.18(m, 1H, -CH); 4.94 (d, 1H, J = 3.8 Hz, -OH); 3.62 (dd, 1H, J = 14.4 Hz, J = 3.1Hz, Ha); 3.47 (dd, 1H, J = 8.2 Hz, J = 14.4 Hz, Hb); 13C NMR (125 MHz, DMSO-d6) δ(ppm): 154.9 (5-C); 153.7 (3-C); 142.8 (1‴-C); 133.3 (3′-C, 5′-C); 133.0 (1′-C); 130.1 (4″-C); 128.77 (2′-C, 6′-C); 128.72 (3″-C, 5″-C); 128.5 (3‴-C, 5‴-C); 128.1 (2″-C, 6″-C); 127.8 (4‴-C); 126.0 (1″-C); 125.9 (2‴-C, 6‴-C); 124.0 (4′-C); 73.3 (CH); 41,.6 (CH2); 15N NMR (50 MHz, DMSO-d6) δ(ppm): 175.1 (4-N).
(All spectra are reported in Supplementary Materials) Elemental analysis for C22H18BrN3OS Calcd. (%): C, 58.41; H, 4.01; Br, 17.66; N, 9.29; S, 7.09. Found (%):C, 58.40; H, 3.99; Br, 17,58; N, 9.20; S, 7.01. HRMS: calculated for C22H18BrN3OS+Na: 474.0252; found: 474.0347.
Supplementary Materials
The following are available online, Figure S1. 1H NMR spectrum of compound (10) in DMSO-d6; Figure S2. 13C NMR spectrum of compound (10) in DMSO-d6; Figure S3. HMBC 1H-15N spectrum of compound (10) in DMSO-d6; Figure S4. FT-IR spectrum of compound (11); Figure S5. 1H NMR spectrum of compound (11) in CDCl3; Figure S6. 13C NMR spectrum of compound (11) in CDCl3; Figure S7. COSY 1H-1H spectrum of compound (11) in CDCl3; Figure S8. 13C DEPT135 spectrum of compound (11) in CDCl3; Figure S9. HMBC 1H-13C spectrum of compound (12) in CDCl3 Figure S10. HMBC 1H-15N spectrum of compound (11) in CDCl3; Figure S11. HSQCCED 1H-13C spectrum of compound (11) in CDCl3; Figure S12. FT-IR spectrum of compound (12); Figure S13. 1H NMR spectrum of compound (12) in CDCl3; Figure S14. 13C NMR spectrum of compound (12) in CDCl3; Figure S15. COSY 1H-1H spectrum of compound (12) in CDCl3; Figure S16. 13C DEPT135 spectrum of compound (12) in CDCl3; Figure S17. HMBC 1H-13C spectrum of compound (12) in CDCl3; Figure S18. HMBC 1H-15N spectrum of compound (12) in CDCl3; Figure S19. HSQCED 1H-13C spectrum of compound 6 (12) in CDCl3; Figure S20. HRMS spectrum of compound (11); Figure S21. HRMS spectrum of compound (12).
Author Contributions
Designed the experiments, V.B.; performed the experiments, M.M.V. and V.B.; analyzed the spectral data, V.B.; wrote the manuscript, V.B. and M.M.V.; supervision and funding acquisitions, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P2-2.1-PED-2019-3414, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghanaat, J.; Khalilzadeh, M.A.; Zareyee, D. Molecular docking studies, biological evaluation and synthesis of novel 3-mercapto-1,2,4-triazole derivatives. Mol. Divers. 2020, 25, 223–232. [Google Scholar] [CrossRef]
- Plech, T.; Luszczki, J.J.; Wujec, M.; Flieger, J.; Pizoń, M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Karabasanagouda, T.; Adhikari, A.V.; Shetty, N.S. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Behalo, M.S.; Aly, A.A.; Wasfy, A.F.; Rizk, M.M. Synthesis of some novel 1,2,4-triazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2013, 4, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Holla, B.S.; Veerendra, B.; Shivananda, M.K.; Poojary, B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2003, 38, 759–769. [Google Scholar] [CrossRef]
- Duran, A.; Dogan, H.N.; Rollas, S. Synthesis and preliminary anticancer activity of new 1,4-dihydro-3-(3-hydroxy-2-naphthyl)-4-substituted-5H-1,2,4-triazoline-5-thiones. Farmaco 2002, 57, 559–564. [Google Scholar] [CrossRef]
- Kaproń, B.; Czarnomysy, R.; Wysokiński, M.; Andrys, R.; Musilek, K.; Angeli, A.; Supuran, C.T.; Plech, T. 1,2,4-Triazole-based anticonvulsant agents with additional ROS scavenging activity are effective in a model of pharmacoresistant epilepsy. J. Enzym. Inhib. Med. Chem. 2020, 35, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent advances bioactive 1,2,4-triazole-3-thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef]
- Al-Aabdullah, E.S.; Asiri, H.H.; Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-Inflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols. Drug Des. Dev. Ther. 2014, 8, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Godhani, D.R.; Sanja, D.B.; Sanghani, A.M. Synthesis and antimicrobial elucidation of [1,2,4]-triazole-3-thione derivatives. J. Chem. Pharm. Res. 2013, 5, 240–243. [Google Scholar]
- Goswami, B.N.; Kataky, J.C.S.; Baruah, J.N. Synthesis and antibacterial activity of 1-(2,4-dichlorobenzoyl)-4-substituted thiosemicarbazides, 1,2,4-triazoles and their methyl derivatives. J. Heterocycl. Chem. 1984, 21, 1225–1229. [Google Scholar] [CrossRef]
- Tehranchian, S.; Akbarzadeh, T.; Fazeli, M.; Reza, M.; Jamalifar, H.; Shafiee, A. Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5H)ones. Bioorg. Med. Chem. Lett. 2005, 15, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sul-fonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Łuszczki, J.J.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Zołnierek, M.; Nowak, G. Studies on the anticonvulsant activity of 4-alkyl-1,2,4-triazole-3-thiones and their effect on GABAergic system. Eur. J. Med. Chem. 2014, 86, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Nuțiu, M.; Bercean, V.; Birău, M. Synthesis of some 4-aryl-thiosemicarbazides. Ann. West Univ. Timişoara 1996, 5, 7–10. [Google Scholar]
- Maxwell, J.R.; Wasdahl, D.A.; Wolfson, A.C.; Stenberg, V.I. Synthesis of 5-aryl-2H-tetrazoles, 5-Aryl-2H-tetrazole-2-acetic acids, and [(4-phenyl-5-aryl-4H-1,2,4-triazol-3-yl)thio]acetic acids as possible superoxide scavengers and antiinflammatory agents. J. Med. Chem. 1984, 27, 1565–1570. [Google Scholar] [CrossRef]
- Wang, L.-C.; Tang, J.; Wei, T.-B.; Zhang, Y.-M. Synthesis and biological bctivity of 2-(3-phenoxymethyl-4-phenyl-[1,2,4]triazole-5-thio)acetic acid. Chin. J. Org. Chem. 2008, 28, 343–347. [Google Scholar] [CrossRef]
- Dimri, A.K.; Parmar, S.S. Synthesis of 3-aryl-4-oxothiazolin-2-yl(4-ethoxy-3-methoxy)phenyl hydrazones as possible anti-convulsants. J. Heterocycl. Chem. 1978, 15, 335–336. [Google Scholar] [CrossRef]
- Radl, S. Preparation of some pyrazole derivatives by extrusion of elemental sulfur from 1,3,4-thiadiazines. J. Heterocycl. Chem. 1992, 57, 656–659. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Behyar, T. Fast and efficient method for reduction of carbonyl compounds with NaBH4 /wet SiO2 under solvent free condition. J. Braz. Chem. Soc. 2005, 16, 1200–1209. [Google Scholar] [CrossRef] [Green Version]
- Toda, F.; Kiyoshige, K.; Yagi, M. NaBH4 Reduction of Ketones in the Solid State. Angew. Chem. Int. Ed. Engl. 1989, 28, 320–321. [Google Scholar] [CrossRef]
- Fizer, M.; Slivka, M.; Korol, N.; Fizer, O. Identifying and explaining the regioselectivity of alkylation of 1,2,4-triazole-3-thiones using NMR, GIAO and DFT methods. J. Mol. Struct. 2021, 1223, 128973. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).