2-Diphenylphosphinomethyl-3-methylpyrazine
Abstract
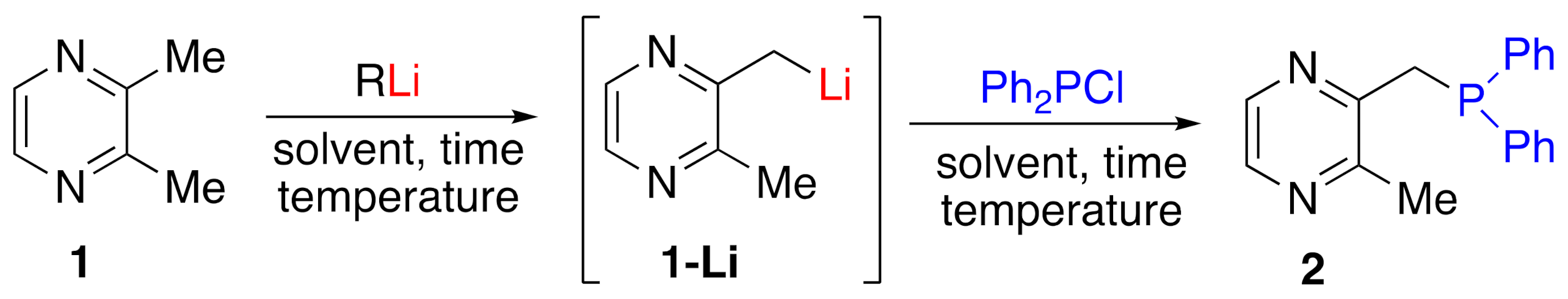
:1. Introduction
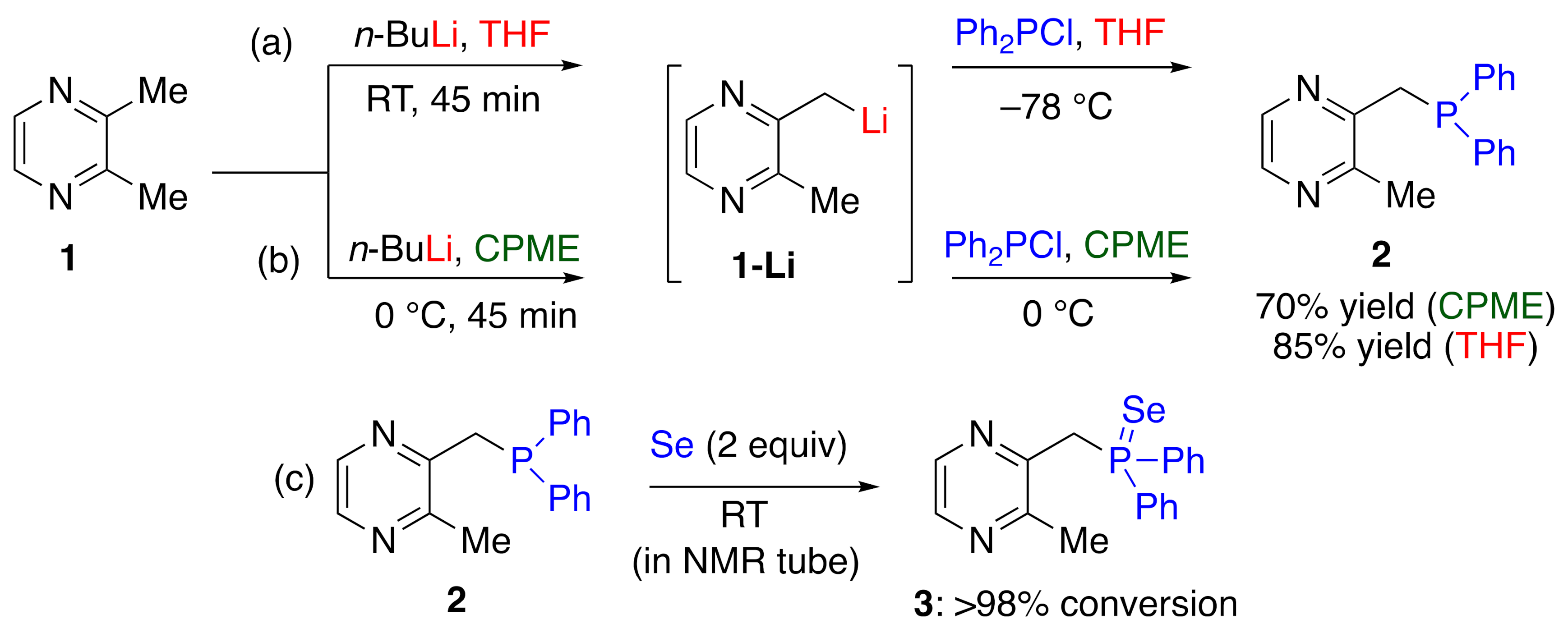
2. Results and Discussions
3. Materials and Methods
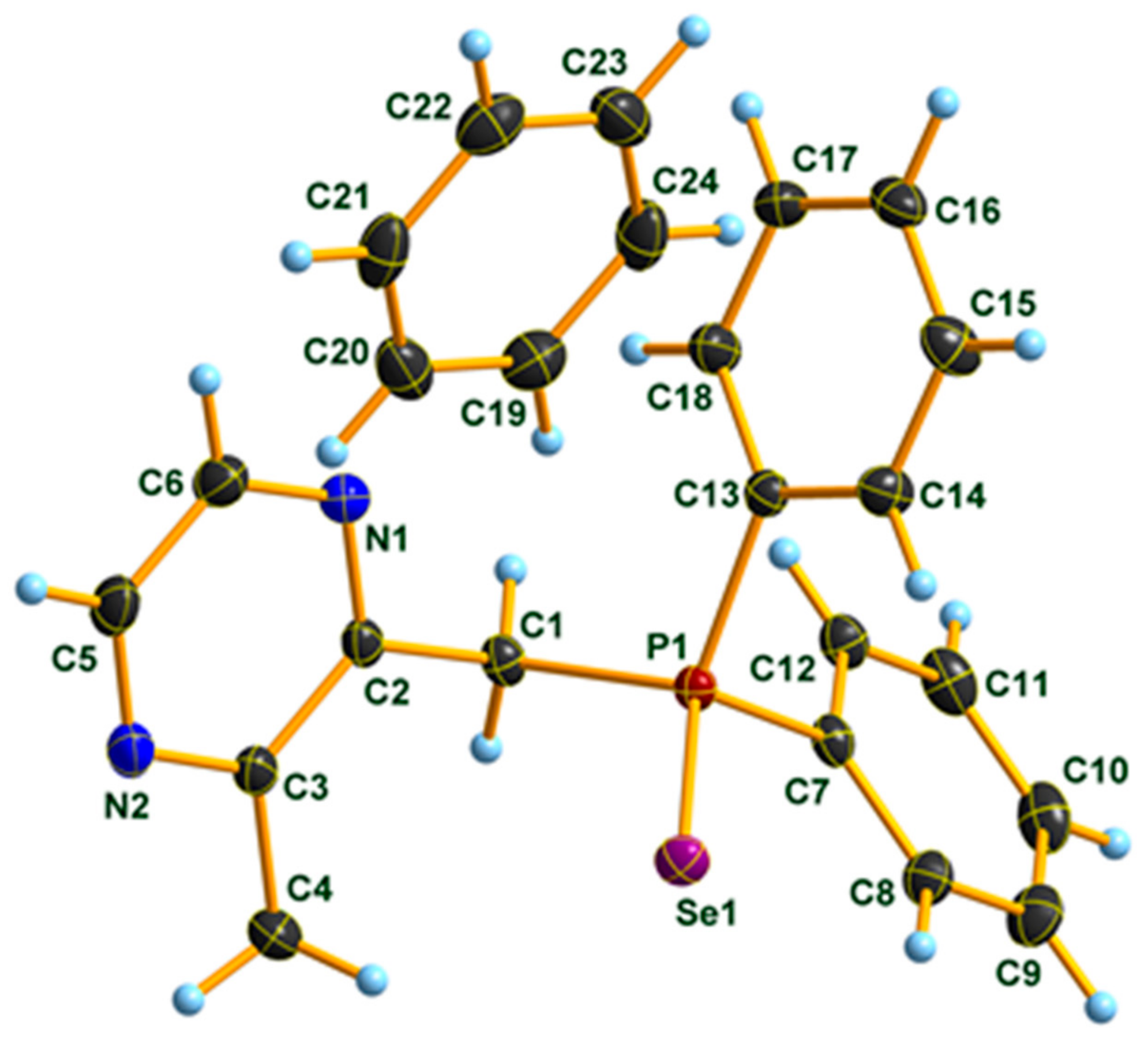
3.1. X-ray Diffraction Studies on Compound 3 · C6D6
3.2. Synthetic Procedure for the Synthesis of 2 in THF
3.3. Synthetic Procedure for the Synthesis of 2 in CPME
3.4. Synthetic Procedure for the Synthesis of 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miniyar, P.B.; Murumkar, P.R.; Patil, P.S.; Barmade, M.A.; Bothara, K.G. Unequivocal role of pyrazine ring in medicinally important compounds: A review. Mini. Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [CrossRef]
- Asif, M. Piperazine and Pyrazine containing molecules and their diverse pharmacological activities. Int. J. Adv. Sci. Res. 2015, 1, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Dolezal, M.; Zitko, J. Pyrazine derivatives: A patent review (June 2012–present). Expert Opin. Ther. Pat. 2015, 25, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An antibiotic agent pyrrolo [1,2-a] pyrazine-1,4-dione, hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphilococcus Aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef] [Green Version]
- Srinivasarao, S.; Nandikolla, A.; Suresh, A.; Van Calster, K.; De Voogt, L.; Cappoen, D.; Ghosh, B.; Aggarwal, H.; Murugesan, S.; Sekhar, K.V.G.C. Seeking potent anti-tubercolar agents: Design and synthesis of substituted-N-(6-(4-(pyrazine-2-carbonyl)piperazine/homopiperazine-1-yl)pyridin-3-yl)benzamide derivatives as anti-tubercolar agents. RSC Adv. 2020, 10, 12272–12288. [Google Scholar] [CrossRef]
- Patel, A.; Kumar, A.; Sheoran, N.; Kumar, M.; Sahu, P.K.; Ganeshan, P.; Ashajyothi, M.; Gopalakrishnan, S.; Gogoi, R. Antifungal and defense elicitor activities of pyrazines identified in endophytic Pseudomonas putida BP25 against fungal blast incited by Magnaporthe oryzae in rice. J. Plant Dis. Protect. 2021, 128, 261–272. [Google Scholar] [CrossRef]
- Achelle, S.; Baudequin, C.; Plé, N. Luminescent materials incorporating pyrazine or quinoxaline moieties. Dye. Pigment. 2013, 98, 575–600. [Google Scholar] [CrossRef] [Green Version]
- Walton, R.A.; Matthewa, R.W. Coordination compounds of silver(II). Preparation and characterization of new pyrazine and pyrazine carboxylate complexes and some related silver(I), copper(II), cobalt(II) and nichel(II) derivatives. Inorg. Chem. 1971, 10, 1433–1438. [Google Scholar] [CrossRef]
- Fitchett, C.M.; Steel, P.J. Chiral heterocyclic ligands. XII. Metal complexes of a pyrazine ligand derived from camphor. Arkivoc 2006, 3, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Ogyzek, M.; Chylewska, A.; Królicka, A.; Banasiuk, R.; Turecka, K.; Lesiak, D.; Nidzworski, D.; Makowski, M. Coordination chemistry of pyrazine derivatives analogues of PZA: Design, synthesis, characterization and biological activity. RSC Adv. 2016, 6, 52009–52025. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-Z.; Xu, Y.-C.; Wang, L.; Li, L.-L.; Jia, X.-G.; Lee, G.-H.; Peng, S.-M. Transition metal complexes with pyrazine amine ligand: Preparation, structure and carbon dioxide copolymerization behavior. J. Mol. Struct. 2019, 1193, 280–285. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Y.; Ran, J.; Li, L. Synthesis, crystal structure, photoluminescence and catalytic properties of a novel cuprous complex with 2,3-pyrazinedicarboxylic acid ligands. Sci. Rep. 2020, 10, 6723. [Google Scholar] [CrossRef]
- Clark, R.D.; Jahangir, A. Lateral Lithiation Reactions Promoted by Heteroatomic Substituents. In Organic Reactions; Paquette, L.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1995; Volume 47, Chapter 1; pp. 1–314. [Google Scholar] [CrossRef]
- Clayden, J. Organolithiums: Selectivity for Synthesis. In Tetrahedron Organic Chemistry Series; Pergamon: Amsterdam, The Netherlands, 2002; Volume 23. [Google Scholar]
- Luisi, R.; Capriati, V. Lithiums Compounds in Organic Synthesis—From Fundamentals to Applications; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Di Nunno, L.; Scilimati, A.; Vitale, P. Regioselective synthesis and side-chain metallation and elaboration of 3-alkyl-5-alkylisoxazoles. Tetrahedron 2002, 58, 2659–2665. [Google Scholar] [CrossRef]
- Kaiser, E.M. Lateral Metallation of Methylated Nitrogenous Heterocycles. Tetrahedron 1983, 39, 2055–2064. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Alshammari, M.B.; Fekri, A. Control of Site of Lithiation of 3-(Aminomethyl)pyridine derivatives. Synthesis 2013, 45, 3426–3464. [Google Scholar] [CrossRef]
- Palao, E.; de la Moya, S.; Agarrabeitia, A.R.; Esnal, I.; Bañuelos, J.; López-Arbeloa, I.; Ortiz, M.J. Selective Lateral Lithiation of Methyl BODIPYs: Synthesis, Photophysics, and Electrochemistry of New Meso Derivatives. Org. Lett. 2014, 16, 4364–4367. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Tou, J.M. Synthesis of Highly Functionalized Pyrazines by Ortho-Lithiation Reactions. Pyrazine Ladder Polymers. J. Am. Chem. Soc. 1999, 121, 8783–8790. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Smith, K.; Hegazy, A.S.; Alshammari, M.B.; Masmali, A.M. Directed lithiation of simple aromatics and heterocycles for synthesis of substituted derivatives. Arkivoc 2015, 4, 19–47. [Google Scholar] [CrossRef]
- Kamal, M.; Levine, R. Chemistry of Pyrazine and Its Derivatives. V. Acylation and Alkylation of 2,6-Dimethylpyrazine and Certain Other Pyrazine Derivatives. J. Org. Chem. 1962, 27, 1355–1359. [Google Scholar] [CrossRef]
- Kamal, M.; Levine, R. The Chemistry of Pyrazine and Its Derivatives. VI. The Synthesis of Carbinols Containing the Pyrazine Nucleus. J. Org. Chem. 1962, 27, 1360–1363. [Google Scholar] [CrossRef]
- Bassfield, R.; Houminer, Y. Selectivity in the Metalation of Polymethylpyrazines. J. Org. Chem. 1983, 48, 2130–2133. [Google Scholar] [CrossRef]
- Houminer, Y.; Fenner, R.A.; Secor, H.V.; Seeman, J.I. Steric Effects on Pyrolysis Reactions. The Thermal Retro-Ene Reaction of Pyrazineethanols. J. Org. Chem. 1987, 52, 3971–3974. [Google Scholar] [CrossRef]
- Capriati, V.; Florio, S.; Luisi, R.; Perna, F.M.; Spina, A. 2-Lithio-3,3-dimethyl-2-oxazolinyloxirane: Carbanion or Azaenolate? Structure, Configurational Stability, and Stereodynamics in Solution. J. Org. Chem. 2008, 73, 9552–9564. [Google Scholar] [CrossRef] [PubMed]
- Capriati, V.; Florio, S.; Perna, F.M.; Salomone, A.; Abbotto, A.; Amedjkouh, M.; Nilsson Lill, S.O. On the Dichotomic Reactivity of Lithiated Styrene Oxide: A Computational and Multinuclear Magnetic Resonance Investigation. Chem. Eur. J. 2009, 15, 7958–7979. [Google Scholar] [CrossRef]
- Capriati, V.; Florio, S.; Perna, F.M.; Salomone, A. Lithiated Fluorinated Styrene Oxides: Configurational Stability, Synthetic Applications, and Mechanistic Insight. Chem. Eur. J. 2010, 16, 9778–9788. [Google Scholar] [CrossRef]
- Perna, F.M.; Salomone, A.; Dammacco, M.; Florio, S.; Capriati, V. Solvent and TMEDA Effects on the Configurational Stability of Chiral Lithiated Aryloxiranes. Chem. Eur. J. 2011, 17, 8216–8225. [Google Scholar] [CrossRef]
- Mansueto, R.; Mallardo, V.; Perna, F.M.; Salomone, A.; Capriati, V. Gated access to α-lithiated phenyltetrahydrofuran: Functionalisation via direct lithiation of the parent oxygen heterocycle. Chem. Commun. 2013, 49, 10160–10162. [Google Scholar] [CrossRef] [PubMed]
- Cicco, L.; Addante, V.; Temperini, A.; Donau, C.A.; Karaghiosoff, K.; Perna, F.M.; Capriati, V. Toward Customized Tetrahydropyran Derivatives through Regioselective α-Lithiation and Functionalization of 2-Phenyltetrahydropyran. Eur. J. Org. Chem. 2016, 2016, 3157–3161. [Google Scholar] [CrossRef]
- Di Nunno, L.; Vitale, P.; Scilimati, A. Effect of the aryl group substituent in the dimerization of 3-arylisoxazoles to syn 2,6-diaryl-3,7-diazatricyclo[4.2.0.02,5]octan-4,8-diones induced by LDA. Tetrahedron 2008, 64, 11198–11204. [Google Scholar] [CrossRef]
- Di Nunno, L.; Scilimati, A.; Vitale, P. 5-Hydroxy-2-phenyl-5-vinyl-2-isoxazoline and 3-phenyl-5-vinylisoxazole: Synthesis and reactivity. Tetrahedron 2005, 61, 11270–11278. [Google Scholar] [CrossRef]
- Coppi, D.I.; Salomone, A.; Perna, F.M.; Capriati, V. Exploiting the Lithiation-Directing Ability of Oxetane for the Regioselective Preparation of Functionalized 2-Aryloxetane Scaffolds under Mild Conditions. Angew. Chem. Int. Ed. 2012, 30, 7650–7654. [Google Scholar] [CrossRef]
- Perna, F.M.; Falcicchio, A.; Salomone, A.; Milet, A.; Rizzi, R.; Hamdoun, G.; Barozzino-Consiglio, G.; Stalke, D.; Oulyadi, H.; Capriati, V. First Direct Evidence of an ortho-Lithiated Aryloxetane: Solid and Solution Structure, and Dynamics. Eur. J. Org. Chem. 2019, 2014, 5549–5556. [Google Scholar] [CrossRef]
- Mansueto, R.; Perna, F.M.; Salomone, A.; Perrone, S.; Florio, S.; Capriati, V. Efficient Regioselective Synthesis of 3,4,5-Trisubstituted 1,2,4-Triazoles on the Basis of a Lithiation-Trapping Sequence. Eur. J. Org. Chem. 2014, 2014, 6653–6657. [Google Scholar] [CrossRef]
- Sassone, F.C.; Perna, F.M.; Salomone, A.; Florio, S.; Capriati, V. Unexpected lateral-lithiation-induced alkylative ring opening of tetrahydrofurans in deep eutectic solvents: Synthesis of functionalized primary alcohols. Chem. Commun. 2015, 51, 9459–9462. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, J. Palladium in Organic Synthesis; Springer: Berlin, Germany, 2005. [Google Scholar] [CrossRef]
- Tsuji, J. Palladium Reagents and Catalysts-New Perspectives for the 21st Century; Wiley: Chichester, UK, 2004. [Google Scholar]
- Hartwig, J. Organotransition Metal Chemistry; University Science Books: Sausalito, CA, USA, 2010; pp. 745–824. [Google Scholar]
- Konrad, T.M.; Fuentes, J.A.; Slawin, A.M.Z.; Clarke, M.L. Highly Enantioselective Hydroxycarbonylation and Alkoxycarbonylation of Alkenes using Dipalladium Complexes as Precatalysts. Angew. Chem. Int. Ed. 2010, 49, 9197–9200. [Google Scholar] [CrossRef]
- Pews-Davtyan, A.; Fang, X.; Jackstell, R.; Spannenberg, A.; Baumann, W.; Franke, R.; Beller, M. Synthesis of New Diphosphine Ligands and their Application in Pd-Catalyzed Alkoxycarbonylation Reactions. Chem. Asian J. 2014, 9, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Duddeck, H. Sulfur, Selenium, and Tellurium NMR. In Encyclopedia of NMR, 2nd ed.; Harris, R.K., Wasylishen, R.E., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; Volume 8, pp. 4920–4933. [Google Scholar]
- Azzena, U.; Carraro, M.; Pisano, L.; Monticelli, S.; Bartolotta, R.; Pace, V. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083–2097. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond 3.2 h; Crystal Impact GbR: Bonn, Germany, 2012. [Google Scholar]
- CrysAlis RED; Version 1.171.27p5 beta; Oxford Diffraction Ltd: Abingdon, UK, 2005.
- SCALE3 ABSPACK—An Oxford Diffraction Program; Version 1.0.4, gui: 1.0.3 (C); Oxford Diffraction, Ltd: Abingdon, UK, 2005.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97: Program for Crystal Structure Solution; University of Göttingen: Wilhelmsplatz, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON: A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1999. [Google Scholar]
- The Cambridge Crystallographic Data Centre (CCDC). Available online: https://www.ccdc.cam.ac.uk/data_request/cif (accessed on 3 August 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccuzzi, T.; Cicco, L.; Quivelli, A.F.; Vitale, P.; Perna, F.M.; Karaghiosoff, K.; Capriati, V. 2-Diphenylphosphinomethyl-3-methylpyrazine. Molbank 2021, 2021, M1267. https://doi.org/10.3390/M1267
Boccuzzi T, Cicco L, Quivelli AF, Vitale P, Perna FM, Karaghiosoff K, Capriati V. 2-Diphenylphosphinomethyl-3-methylpyrazine. Molbank. 2021; 2021(3):M1267. https://doi.org/10.3390/M1267
Chicago/Turabian StyleBoccuzzi, Tiziana, Luciana Cicco, Andrea Francesca Quivelli, Paola Vitale, Filippo Maria Perna, Konstantin Karaghiosoff, and Vito Capriati. 2021. "2-Diphenylphosphinomethyl-3-methylpyrazine" Molbank 2021, no. 3: M1267. https://doi.org/10.3390/M1267
APA StyleBoccuzzi, T., Cicco, L., Quivelli, A. F., Vitale, P., Perna, F. M., Karaghiosoff, K., & Capriati, V. (2021). 2-Diphenylphosphinomethyl-3-methylpyrazine. Molbank, 2021(3), M1267. https://doi.org/10.3390/M1267







