Abstract
4-(4-Methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-thiol (4) was alkylated to 2-{[4-(4-methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5) in alkaline conditions using 2-bromo-1-phenylethanone. The alkylated compound (5) was reduced at the carbonyl group to the corresponding racemic secondary alcohol with an asymmetric carbon, (R,S)-2-{[4-(4-methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (6). Both synthesized compounds, ketone (5) and secondary alcohol (6), are new and have not yet been reported in the literature. All the synthesized compounds were characterized by IR, 1D and 2D 1H-1H, 1H-13C and 1H-15N NMR spectroscopy and by elemental analysis.
1. Introduction
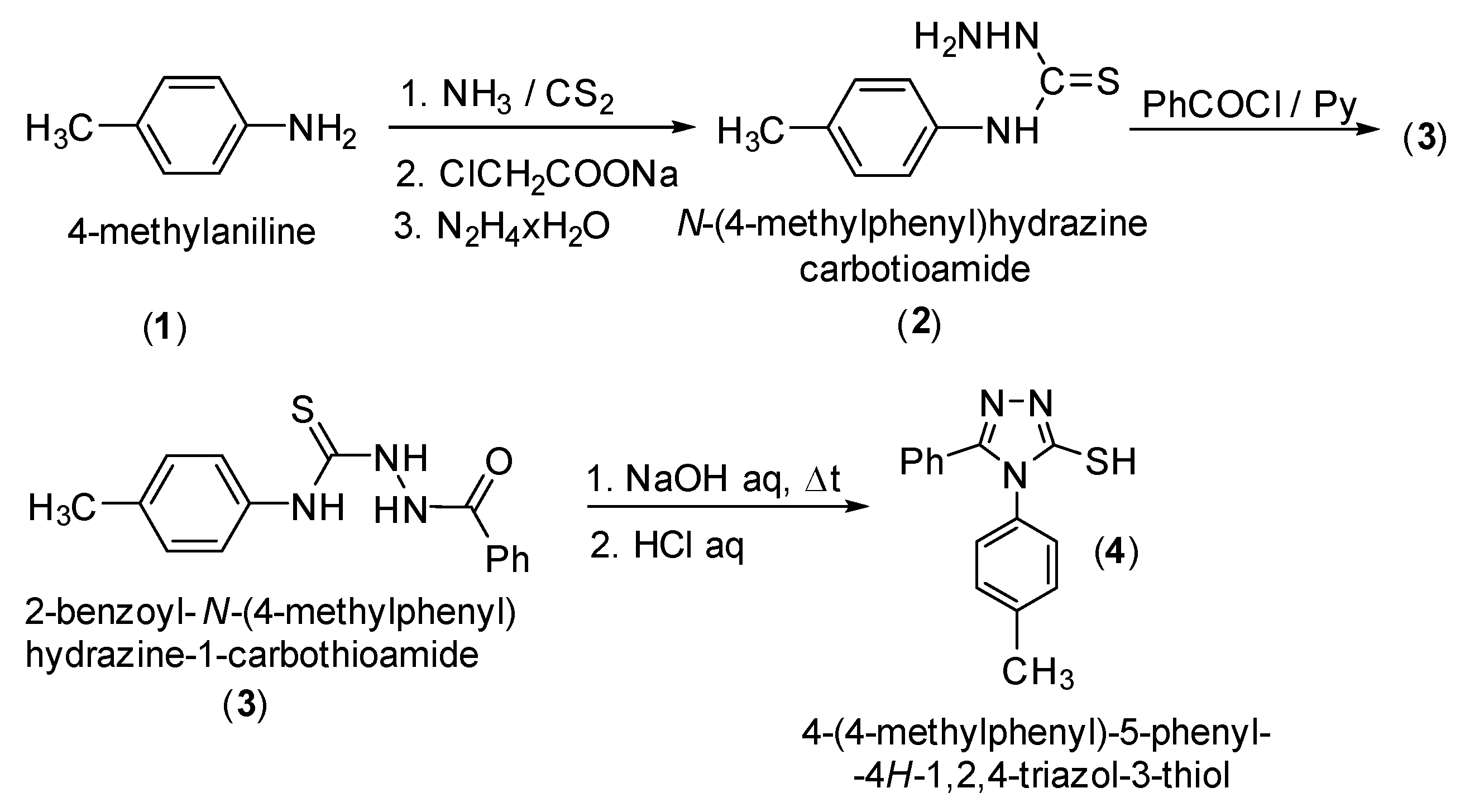
4-(4-Methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-thiol (4) was synthesized starting from 4-methylaniline (1) via the corresponding N-(4-methylphenyl)hydrazinecarbothioamide (2), followed by acylation to 2-benzoyl-N-(4-methylphenyl)hydrazine-1-carbothioamide (3) and cyclization of (3) to 4-(4-methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-thiol (4) according to the literature methods (Scheme 1) [1,2,3,4,5]. The S-alkylation was performed using cesium carbonate as a base [6,7], and the reduction of ketones group to the corresponding secondary alcohol was carried out with sodium borohydride [8].
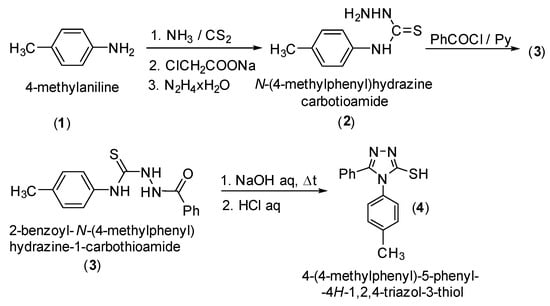
Scheme 1.
Synthetic route to 4-(4-methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-thiol (4).
Nowadays, the 4,5-disubstituted-3-mercaptotriazolic nucleus is considered an important element in the synthesis and design of bioactive compounds, which are associated with numerous biological activities. Some of these biological activities are cytotoxic against different cell lines [9]: antibacterial, antifungal [10,11] (Scheme 2), anti-HIV [12] and anti-inflammatory [13,14].
Scheme 2.
Compounds with antibacterial activity based on 1,2,4 triazoles.
2. Results and Discussion
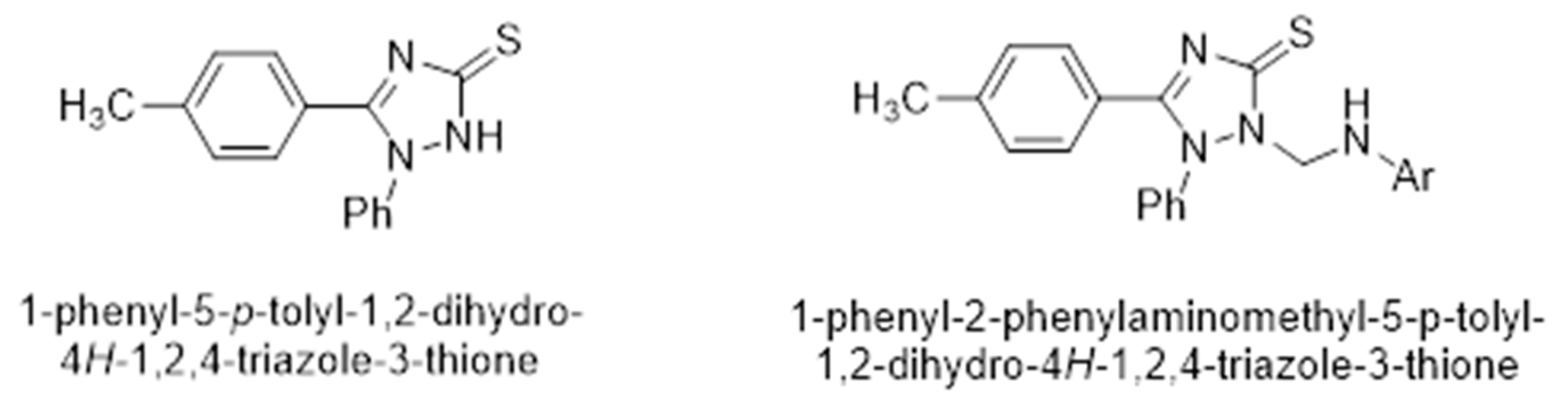
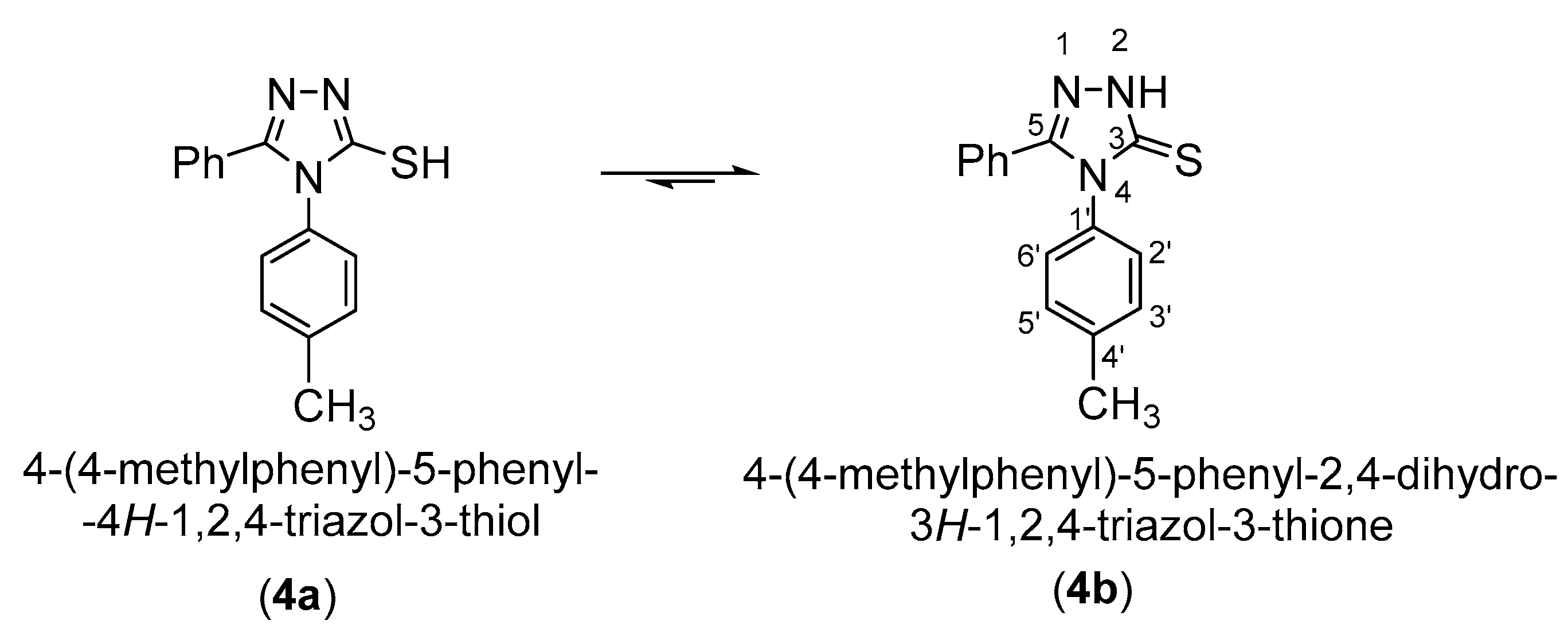
3-Mercaptotriazole (4) can theoretically have two tautomeric forms: the thiol form (4a) and the thione form (4b). As a result, alkylation in a basic medium can theoretically occur as S-alkylation at the tautomeric form (4a) or as N-alkylation at the tautomeric form (4b).
The equilibrium shift to tautomeric thione form 4b was determined by the comparison of experimentally observed NMR chemical shifts of the product 4 to those predicted by density functional theory (DFT) calculations via the gage independent atomic orbital (GIAO) method.
NMR chemical shifts were calculated in DMSO phase, using the optimized structures by density functional theory (DFT) calculations via the Gauge Independent Atomic Orbital (GIAO) method at the B3LYP/6-31+G (d, p) level of theory.
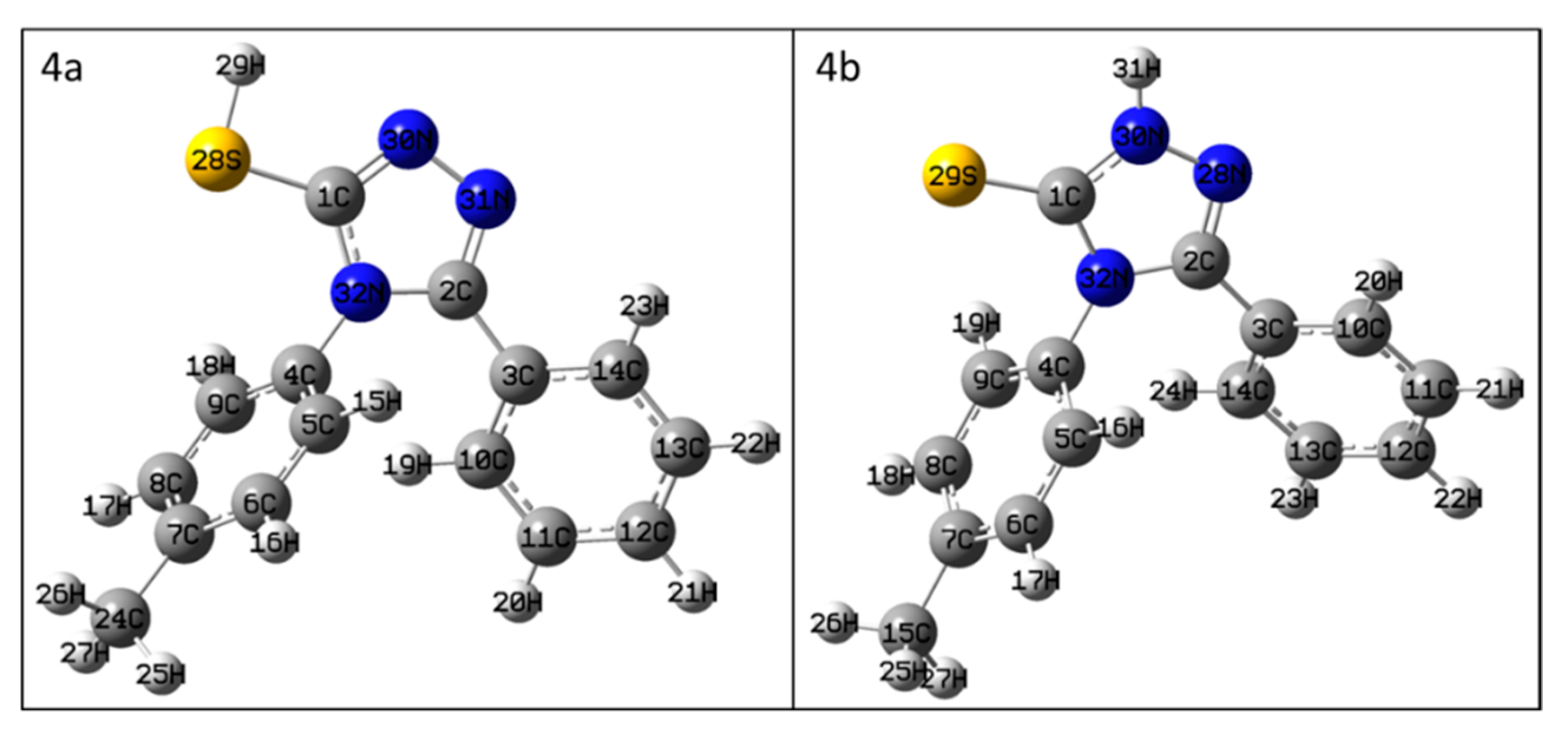
The geometry optimization and frequency calculations showed that all structures were global minima with no imaginary frequencies. The optimized structures are presented in Figure 1 and were used for the NMR chemical shift calculations.
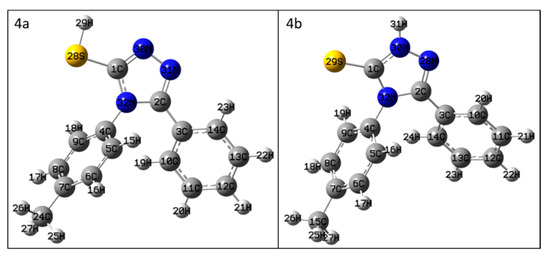
Figure 1.
The geometry-optimized structures of compounds 4a and 4b.
Table 1 shows the calculated NMR chemical shifts for compounds 4a and 4b and experimentally determined for compound 4.

Table 1.
Calculated NMR chemical shifts in ppm with TMS (for H and C atoms) and NH3 standards (for N atoms) by the GIAO method at the B3LYP/6-31+G (d, p) level of theory and experimentally determined.
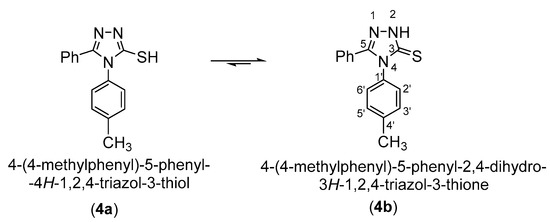
Scheme 3.
Tautomeric equilibrium of the 5-phenyl-4-(4-methylphenyl)-4H-1,2,4-triazol-3-thiol (4).
It can be seen from Table 1 that the value of 159.79 ppm of the chemical shift calculated for carbon atom 1C (experimentally numbered 3-C) for structure 4b (thione form) is the closest to the value of 168.6 ppm determined experimentally. It is also observed that the difference between the calculated chemical shift values for 4a and 4b for carbon atoms 1C (experimentally numbered 3-C) of 17.81ppm (159.79 ppm − 141.98 ppm) is similar to that between carbon atoms 3-C in compound 4 and that in S-alkylated compound 5, 168.6 ppm − 152.1 ppm = 16.5 ppm. Moreover, the experimentally determined chemical shift value for the 4-N nitrogen atom from the 2D HMBC 1H-15N spectrum of 183.9 ppm is very close to the experimentally calculated 190.79 (32N).
From the 1H and 13C NMR spectra, it is confirmed that the tautomeric equilibrium is completely shifted to the tautomeric form (4b) in DMSO-d6. This shift of equilibrium is confirmed by the deshielded signal of the 2-N-H proton at 14.11 ppm, as well as by the deshielded signal of the 3-C carbon atom at 168.6 ppm, corresponding to a C=S thionic carbon atom.
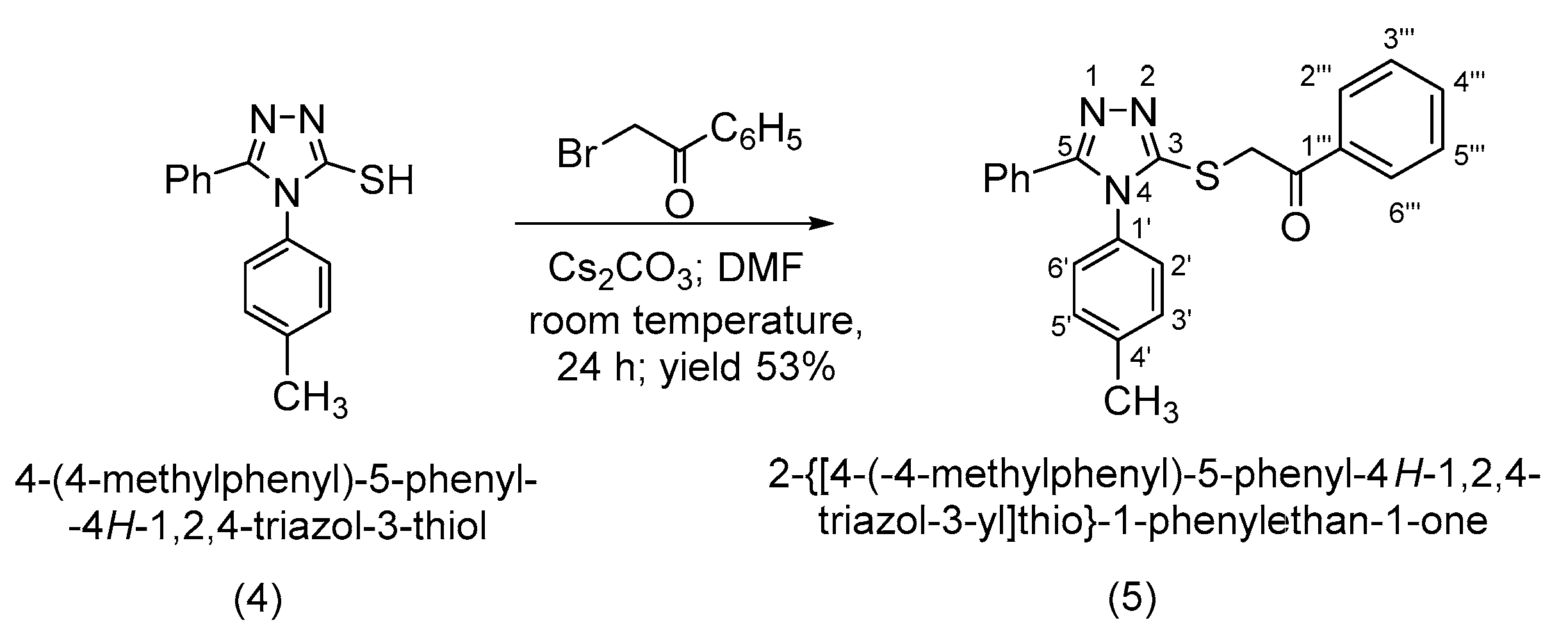
Following the alkylation using cesium carbonate as a base in N,N-dimethylformamide, it has been observed that the alkylation occurs exclusively at the thiol group as S-alkylation. This is observed from a 2D NMR spectroscopic analysis by analyzing the couplings over 2 or 3 bonds in the HMBC spectrum as well as by the shifting the signal of the triazolic carbon 3-C atom to a lower δ value, at 152.1 ppm, corresponding to a thiol type carbon atom (Scheme 4).
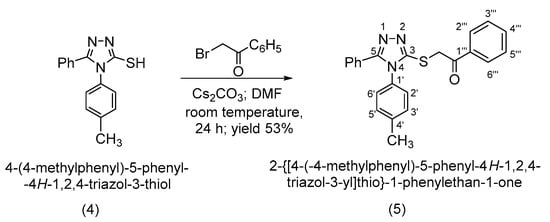
Scheme 4.
Synthetic route to 2-{[4-(4-methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5).
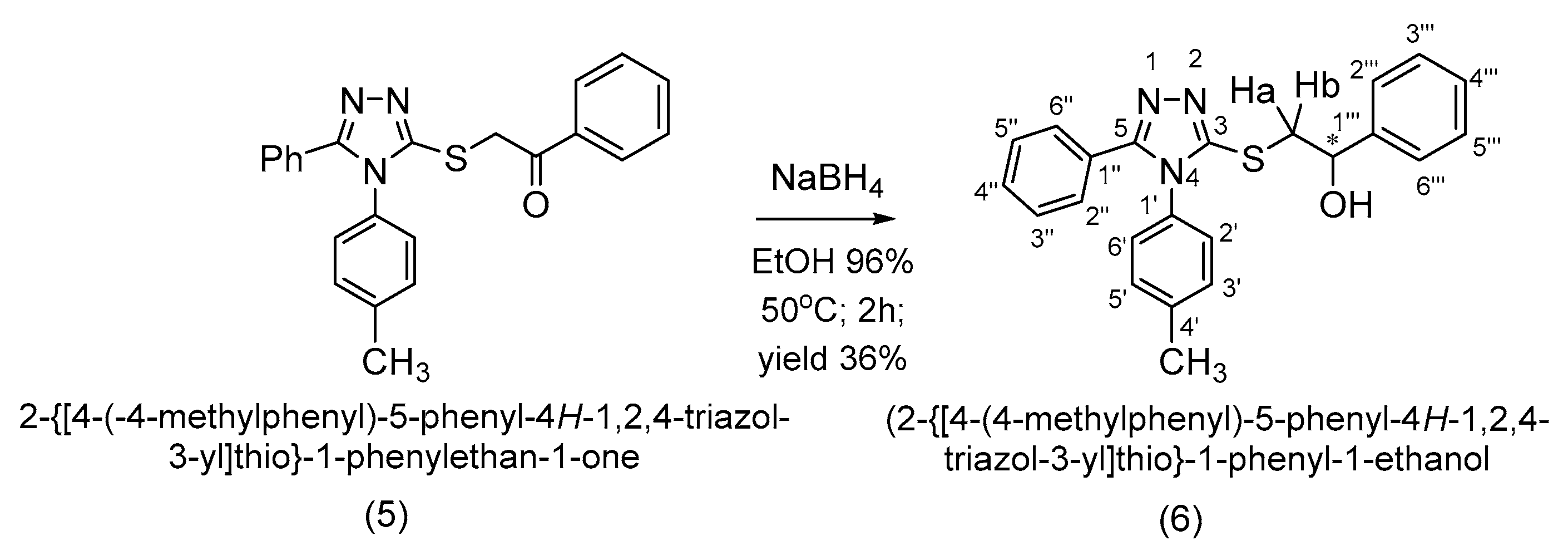
Th reduction of the carbonyl group to the secondary alcohol group was accomplished with sodium borohydride in ethanol. The secondary alcohol (6) was obtained in a yield of 36% after the recrystallization of ethanol (Scheme 5).
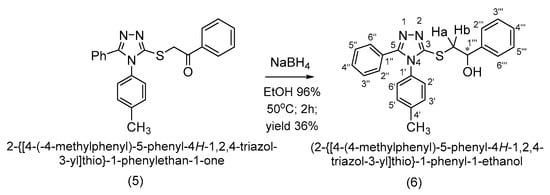
Scheme 5.
Synthetic route to 2-{[4-(4-methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (6).
From the correlative spectra 1H-15N HMBC, the signal for the 4-N nitrogen atom could be identified in all the synthesized compounds, by its coupling over 3 bonds with hydrogen atoms in the ortho positions of the phenyl ring attached to this atom. This long range coupling was very useful in the assignment of the corresponding 1H NMR signals for the ortho protons on the phenyl ring bound to the 4-N nitrogen atom.
The methylene protons from the obtained secondary alcohol (5) are diastereotopic and appear in the 1H NMR spectrum at different δ values as two distinct doublets of doublets. This is specific for a methylene group attached to an asymmetric carbon atom. From the 1H-13C HMBC spectrum, the long range coupling over 3 bonds of the methylene diastereotopic protons with the 3-C triazole carbon atom is observed, thus further confirming the S-alkylation.
3. Materials and Methods
The chemical reagents were purchased from commercial sources and used in syntheses with no further purification. Melting points were determined on a Böetius PHMK (Veb Analytik Dresden, Dresden, Germany melting point apparatus and are uncorrected. Infrared spectra (IR) were recorded as KBr disks on a Jasco FT/IR-410 spectrometer. NMR spectra were recorded on a Bruker AVANCE III 500 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany), in DMSO-d6 and CDCl3 using TMS as an internal standard for protons and carbons. Chemical shifts are reported in ppm units, and the coupling constants are given in Hz.
All computations were performed using the Gaussian 09, Revision B01 program package (Gaussian, Inc., Wallingford, CT, USA). [Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, Jr., J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian, Inc., Wallingford CT, 2016] and the density functional theory (DFT) methods at the B3LYP/6-31+G (d, p) level of theory [Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652]. All structures were subjected to geometry optimization and frequency calculations in solvent phase (dimethylsulfoxide) using the Polarizable Continuum Model (PCM) with the integral equation formalism variant (IEFPCM) [Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–29; Pascual-Ahuir, J.L.; Silla, E.; Tuñón, I. GEPOL: An improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J. Comp. Chem. 1994, 15, 1127–1138].
3.1. NMR Characterization of 4-(4-Methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-thione (4)
IR (KBr, cm−1): 3415 (), 3109 (), 2934 (), 1586 (), 1547; 1482 (), 1077, 1106, 1158 (), 819 (), 696 ().
1H-NMR (500 MHz, DMSO-d6) δ(ppm): 14.11 (s, 1H, -NH); 7.43–7.40 (m, 1H, 4″-H); 7.36–7.31 (m, 4H, 2″-H, 6″-H, 3″-H, 5″-H); 7.28 (d, 2H, J = 8.4 Hz, 3′-H, 5′-H); 7.22 (d, 2H, J = 8.4 Hz, 2′-H, 6′-H); 2.35 (s, 3H, -CH3);
13C-NMR (125 MHz, DMSO-d6) δ(ppm): 168.6 (3-C=S); 150.5 (5-C); 138.8 (4′-C); 131.8 (1′-C); 130.2 (4″-C); 129.7 (3′-C, 5′-C); 128.4 (2′-C, 6′-C); 128.3 (3″-C, 5″-C); 128.1 (2″-C, 6″-C); 125.8 (1″-C); 20.7 (-CH3);
15N-NMR (50 MHz, DMSO-d6) δ(ppm): 183.9 (4-N).
(All spectra are reported in Supplementary Materials).
3.2. Synthesis of 2-{[4-(4-Methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5)
4-(4-Methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-thiol (4) (0.004 moles, 1.00 g) was magnetically stirred with cesium carbonate (0.004 moles, 1.35 g) dissolved in N,N-dimethylformamide (0.310 moles, 24.00 mL), at room temperature. After the cesium salt was dissolved, a solution formed of 2-bromo-1-phenylethanone (0.005 moles, 1.005 g) in N,N-dimethylformamide (0.194 moles, 15.00 mL) was added to the reaction mixture. The resulting mixture was left to stir for 24 h and after that was precipitated in water. The solid formed was collected by filtration, washed with water, dried and recrystallized from ethanol. The ketone (5) was obtained in a yield of 53%.
m.p.: 185–186 °C
IR (KBr, cm−1): 3472 (), 3061 (), 2919 (), 1676 (), 1579; 1595 (), 1472 (), 1428 (), 1199 (), 825 (, 756 (.
1H-NMR (500 MHz, CDCl3) δ(ppm): 8.05 (dd, 2H, J = 8.0 Hz, J = 1.2 Hz, 2″′-H, 6″′-H); 7.60 (tt, 1H, J = 7.4 Hz, J = 1.2 Hz, 4″′-H); 7.50–7.47 (m, 2H, 3″′-H, 5″′-H); 7.44–7.42 (m, 2H, 2″-H, 6″-H); 7.35–7.31 (m, 1H, 4″-H); 7.29–7.25 (m, 4H, 3′-H, 5′-H, 3″-H, 5″-H), 7.14–7.12 (m, 2H, 2′-H, 6′-H) 4.97 (s, 2H, -CH2); 2.42 (s, 3H, -CH3).
13C-NMR (125 MHz, CDCl3) δ(ppm): 193.2 (-C=O); 155.1 (5-C); 152.1 (3-C); 140.3 (1′-C); 135.3 (1″′-C); 133.9 (4′-C); 131.13 (4″′-C); 130.7 (3′-C, 5′-C); 129.7 (4″-C); 128.8 (3″′-C, 5″′-C); 128.6 (2″′-C, 6″′-C); 128.5 (3″-C, 5″-C); 128.1 (2″-C, 6″-C); 127.0 (2′-C, 6′-C); 126.6 (1″-C); 41.3 (-CH2); 21.3 (-CH3).
15N-NMR (50 MHz, CDCl3) δ(ppm): 176.4 (1-N).
(All spectra are reported in Supplementary Materials)
Elemental analysis for C23H19N3OS Calcd. (%): C, 71.66; H, 4.97; N, 10.90; S, 8.32. Found (%): C, 71.58; H, 4.90; N, 10.78; S, 8.20.
3.3. Synthesis of 2-{[4-(4-Methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenyl-1-ethanol (6)
2-{[4-(-4-Methylphenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]thio}-1-phenylethan-1-one (5) (0.00126 moles, 0.486 g) was dissolved in ethanol (0.879 moles, 50.00 mL) and heated to 50 °C on a water bath until the complete dissolution of the ketone. Afterwards, sodium borohydride (0.00193 moles, 0.07 g) was added in five stages at 25 min time intervals. After the last portion, the conversion of the reaction was checked, and the resulting mixture was precipitated in water. The solid formed was collected by filtration, washed with water, dried and recrystallized from ethanol. The secondary racemic alcohol (6) was obtained in a yield of 36%.
m.p.: 173–174 °C;
IR (KBr, cm−1): 3356 (), 3059, 3033 (), 2945 (), 2917 (), 2862 (), 1665 (), 1602, 1581 (), 1511, 1474 (), 1425 (), 1270, 1234 (), 1091 (), 855, 822 (), 739 (, 696 ().
1H NMR (500 MHz, DMSO-d6) δ(ppm): 7.39–7.32 (m, 11H, 2″′-H, 6″′-H, 3′-H, 5′-H, 2″-H, 6″-H, 3″-H, 5″-H, 4″-H, 3″′-H, 5″′-H); 7.29–7.34 (m, 3H, 2′-H, 6′-H, 4″′-H); 5.80 (d, 1H, J = 4.7 Hz, -OH); 4.90–4.87 (m, 1H, CH-OH); 3.54 (dd, 1H, J = 12.9 Hz, J = 4.5 Hz, Ha); 3.37 (dd, 1H, J = 12.9 Hz, J = 8.1 Hz, Hb); 2.23 (s, 3H, -CH3).
13C NMR (125 MHz, DMSO-d6) δ(ppm): 154.2 (5-C); 152.4 (3-C); 143.7 (1″′-C); 139.6 (1′-C); 131.2 (4′-C); 130.3 (2′-C, 6′-C); 129.5 (3′-C, 5″-C); 128.4 (3″-C, 5″-C); 128.0 (3″′-C, 5″′-C); 127.7 (2″-C, 6″-C); 127.3 (4″′-C); 127.2 (4″-C); 126.7 (1″-C); 125.8 (2″′-C, 6″′-C); 70.9 (-CH); 40.6 (-CH2); 20.6 (-CH3).
15N-NMR (50 MHz, DMSO-d6) δ(ppm): 177.9 (1-N).
(All spectra are reported in Supplementary Materials)
Elemental analysis for C23H21N3OS Calcd. (%): C, 71.29; H, 5.46; N, 10.84; S, 8.27. Found (%): C, 71.20; H, 5.38; N, 10.70; S, 8.16.
Supplementary Materials
The following are available. Figure S1. FT-IR spectrum of compound 4; Figure S2. 1H-NMR spectrum of compound 4 in DMSO-d6; Figure S3. 13C-NMR spectrum of compound 4 in DMSO-d6; Figure S4. FT-IR spectrum of compound 5; Figure S5. 1H-NMR spectrum of compound 5 in CDCl3; Figure S6. 13C-NMR spectrum of compound 5 in CDCl3; Figure S7. COSY 1H-1H spectrum of compound 5 in CDCl3; Figure S8. 13C DEPT135 spectrum of compound 5 in CDCl3; Figure S9. HMBC 1H-13C spectrum of compound 5 in CDCl3 Figure S10. HMBC 1H-15N spectrum of compound 5 in CDCl3; Figure S11. HSQCCED 1H-13C spectrum of compound 5 in CDCl3; Figure S12. FT-IR spectrum of compound 6; Figure S13. 1H NMR spectrum of compound 6 in DMSO-d6; Figure S14. 13C-NMR spectrum of compound 6 in DMSO-d6; Figure S15. COSY 1H-1H spectrum of compound 6 in DMSO-d6; Figure S16. 13C DEPT135 spectrum of compound 6 in DMSO-d6; Figure S17. HMBC 1H-13C spectrum of compound 6 in DMSO-d6; Figure S18. HMBC 1H-15N spectrum of compound 6 in DMSO-d6; Figure S19. HSQCCED 1H-13C spectrum of compound 6 in DMSO-d6.
Author Contributions
Designed the experiments, V.B.; performed the experiments, V.-S.V.; analyzed the spectral data, V.B.; wrote the manuscript, V.B. and V.-S.V.; supervision and funding acquisitions, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI–UEFISCDI, project number PN-III-P2-2.1-PED-2019-3414, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dimri, A.K.; Parmar, S.S. Synthesis of 3-aryl-4-oxothiazolin-2-yl(4-ethoxy-3-methoxy)phenyl hydrazones as possible anticonvulsants. J. Heterocycl. Chem. 1978, 15, 335–336. [Google Scholar] [CrossRef]
- Radl, S. Preparation of some pyrazole derivatives by extrusion of elemental sulfur from 1,3,4-thiadiazines. Collect. Czech. Chem. Commun. 1992, 57, 656–659. [Google Scholar] [CrossRef]
- Nuțiu, M.; Bercean, V.; Birău, M. Synthesis of some 4-aryl-thiosemicarbazides. Ann. West Univ. Timiş. 1996, V, 7–10. [Google Scholar]
- Golovlyova, S.M.; Moskvichev, Y.A.; Alov, E.M.; Kobylinsky, D.B.; Ermolaeva, V.V. Synthesis of novel five-membered nitrogen-containing heterocyclic compounds from derivatives of arylsulfonyl and arylthioacetic and propionic acids. Chem. Heterocycl. Compd. 2001, 37, 1102–1106. [Google Scholar] [CrossRef]
- Ledeți, I.; Bercean, V.; Alexa, A.; Foica, C.; Șuta, L.-M.; Dehelean, C.; Trandafirescu, C.; Muntean, D.; Licker, M.; Fuliaș, A. Preparation and antibacterial properties of substituted 1,2,4-triazoles. J. Chem. 2015, 2015, 879343. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, R.N.; Smith, R.A.; Nischwitz, A.K.; Gavin, T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3–TBAI. Tetrahedron Lett. 2005, 46, 8931–8935. [Google Scholar] [CrossRef]
- Varala, R.; Rao, K.S. Cesium salts in organic synthesis: A Review. Curr. Org. Chem. 2015, 19, 1242–1274. [Google Scholar] [CrossRef]
- Gómez, A.B.; Ahlsten, N.; Platero-Prats, A.E.; Martín-Matute, B. Synthesis of 4,5-disubstituted 2-aminothiazoles from α,β-unsaturated ketones: Preparation of 5-benzyl-4methyl-2-aminothiazolium hydrochloride salt. Org. Synth. 2014, 91, 185–200. [Google Scholar] [CrossRef]
- Ahmed, F.F.; Abd El-Hafeez, A.A.; Abbas, S.H.; Abdelhamid, D.; Abdel-Aziz, M. New 1,2,4-triazole-chalcone hybrids induce caspase-3 dependent apoptosis in A549 human lung adenocarcinoma cells. Eur. J. Med. Chem. 2018, 151, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Godhani, D.R.; Sanja, D.B.; Sanghani, A.M. Synthesis and antimicrobial elucidation of [1,2,4]-triazole-3-thione derivatives. J. Chem. Pharm. Res. 2013, 5, 240–243. [Google Scholar]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Jingde, W.; Xinyong, L.; Xianchao, C.; Yuan, C.; Defeng, W.; Zhong, L.; Wenfang, X.; Christophe, P.; Myriam, W.; Erik, D.C. Synthesis of novel derivatives of 4-amino-3-(2-furyl)-5-mercapto-1,2,4-triazole as potential HIV-1 NNRTIs. Molecules 2007, 12, 2003–2016. [Google Scholar]
- Al-Aabdullah, E.S.; Asiri, H.H.; Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and antiInflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols. Drug. Des. Dev. Ther. 2014, 8, 505–517. [Google Scholar]
- Maxwell, J.R.; Wasdahl, D.A.; Wolfson, A.C.; Stenberg, V.I. Synthesis of 5-aryl-2H-tetrazoles, 5-aryl-2H-tetrazole-2-acetic acids, and [(4-phenyl-5-aryl-4H-1,2,4-triazol-3-yl)thio]acetic acids as possible superoxide scavengers and antiinflammatory agents. J. Med. Chem. 1984, 27, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).