2-(2-(Fluorosulfonyloxy)phenyl)benzoxazole
Abstract
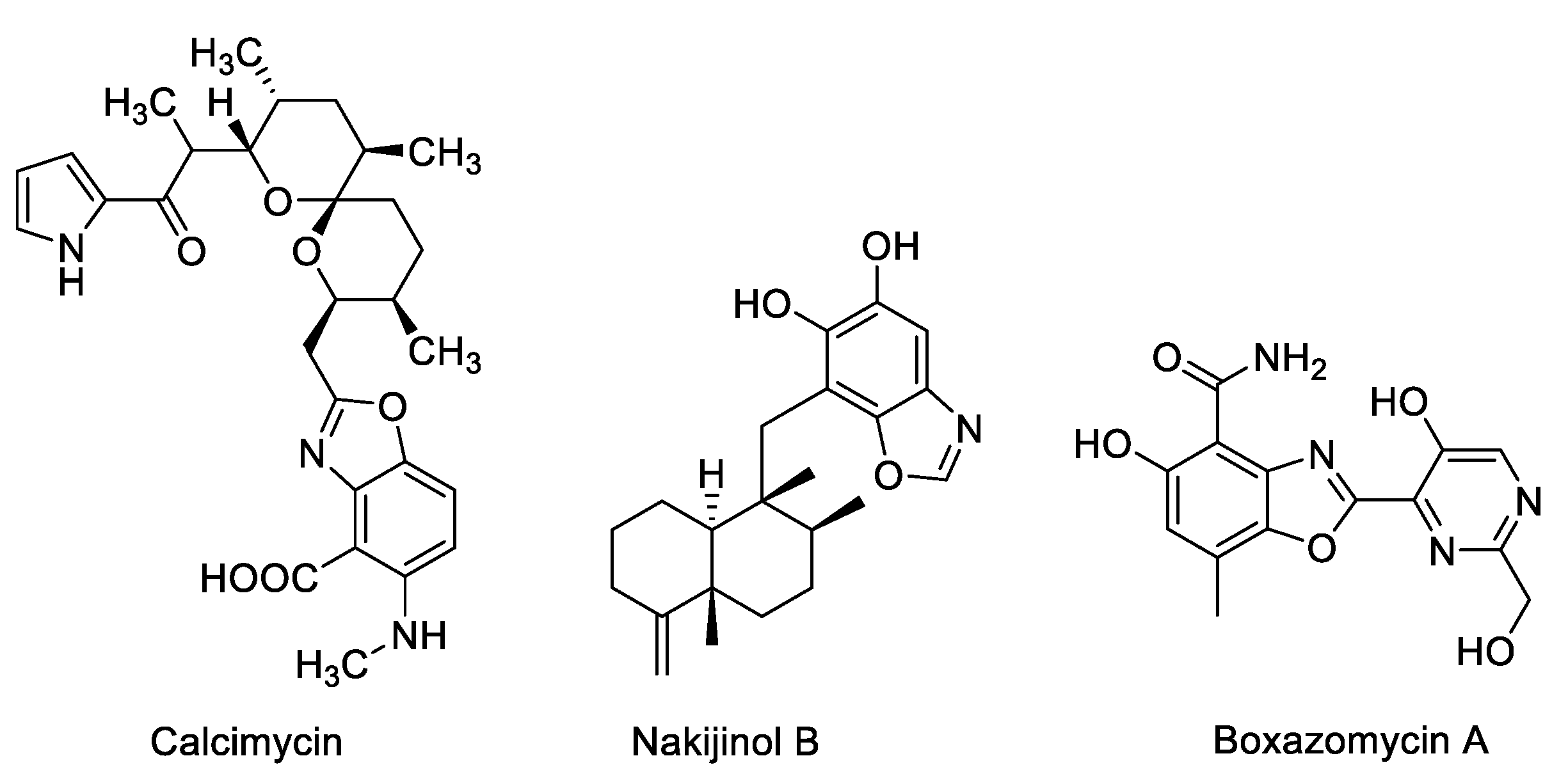
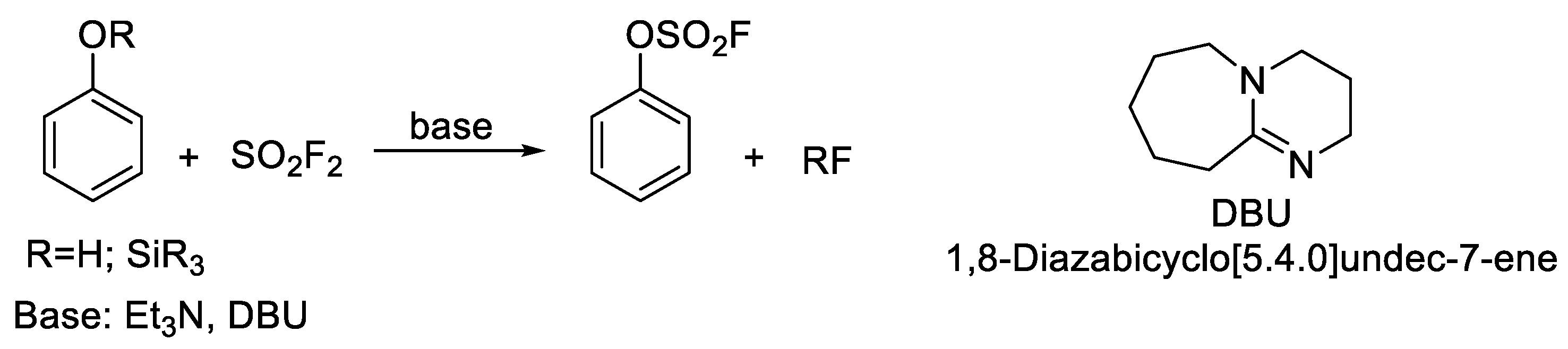
:1. Introduction
2. Results and Discussion
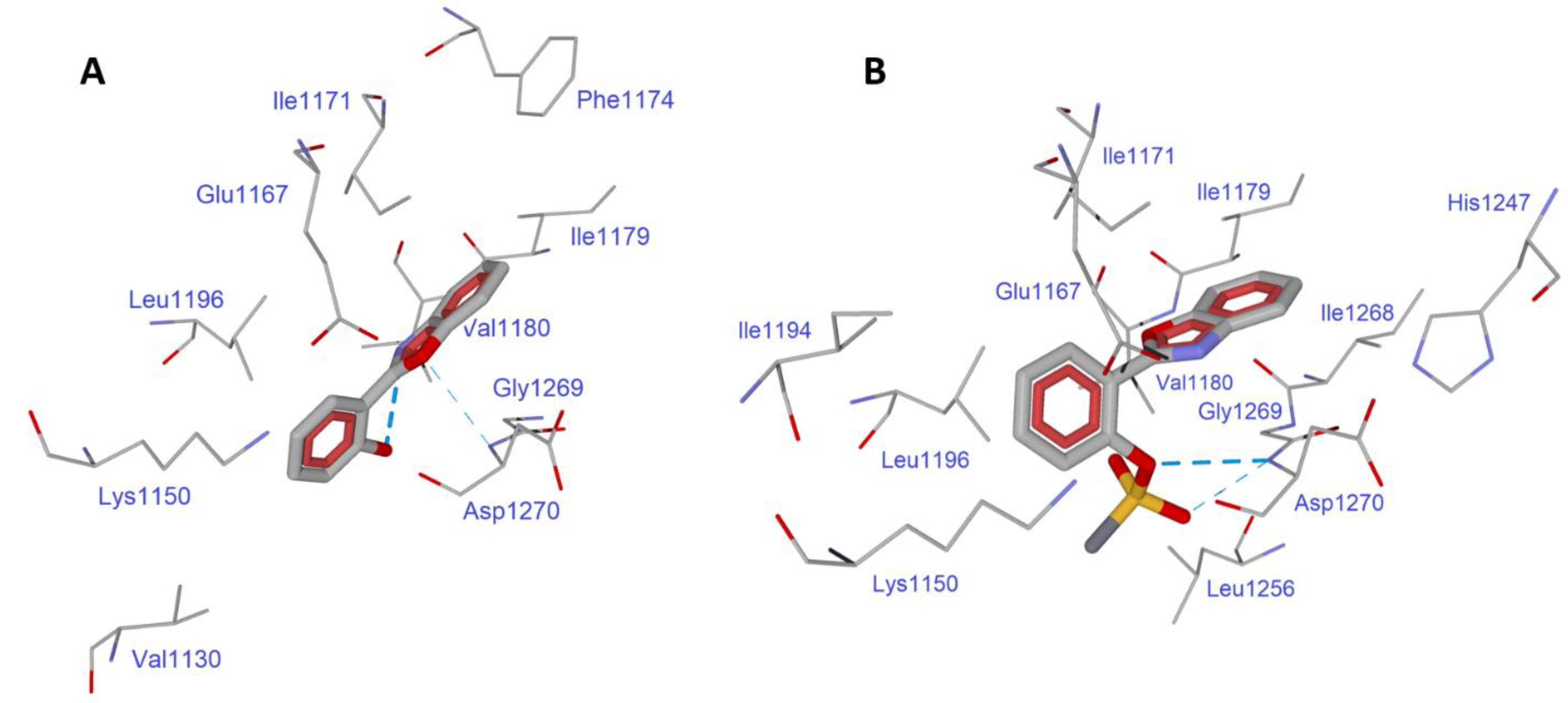
Molecular Docking
3. Materials and Methods
3.1. General Information and Compound 2 Synthesis
3.2. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Veeraswamy, G.; Bhattarai, D.; Goo, J.-I.; Lee, K.; Choi, Y. Recent Advances in the Development of Pharmacologically Active Compounds that Contain a Benzoxazole Scaffold. Asian J. Org. Chem. 2015, 4, 1338–1361. [Google Scholar] [CrossRef]
- Demmer, C.S.; Bunch, L. Benzoxazoles and oxazolopyridines in medicinal chemistry studies. Eur. J. Med. Chem. 2015, 97, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Rida, S.M.; Ashour, F.A.; El-Hawash, S.A.M.; ElSemary, M.M.; Badr, M.H.; Shalaby, M.A. Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Q.; Chen, J.; Takaki, K.; Johnson, G.; Iben, L.; Mahle, C.D.; Ryan, E.; Xu, C. Design and synthesis of benzoxazole derivatives as novel melatoninergic ligands. Bioorg. Med. Chem. Lett. 2004, 14, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, T.; Patel, T.M. Anticancer activity of benzoxazole derivative (2015 onwards): A review. Futur. J. Pharm. Sci. 2020, 6, 94. [Google Scholar] [CrossRef]
- Jin, X.; Ma, X.; Zhong, W.; Cao, Y.; Zhao, H.; Leng, X.; Yang, J.; Zhou, H.; She, M. Fluorescent sensing film decorated with ratiometric probe for visual and recyclable monitoring of Cu2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119217. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. Engl. 2014, 53, 9430–9448. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.S.; Smedley, C.J.; Zheng, Q.; Li, S.; Dong, J.; Moses, J.E. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; McDonald, J.J.; Kolodziej, S.A.; Kurumbail, R.G.; Williams, J.M.; Warren, C.J.; O’Neal, J.M.; Skepner, J.E.; Roberds, S.L. Discovery of Potent Inhibitors of Soluble Epoxide Hydrolase by Combinatorial Library Design and Structure-Based Virtual Screening. J. Med. Chem. 2011, 54, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.F.; Chen, H.; Emkey, R.; Whittington, D.A. The R1275Q Neuroblastoma Mutant and Certain ATP-competitive Inhibitors Stabilize Alternative Activation Loop Conformations of Anaplastic Lymphoma Kinase. J. Biol. Chem. 2012, 287, 37447–37457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
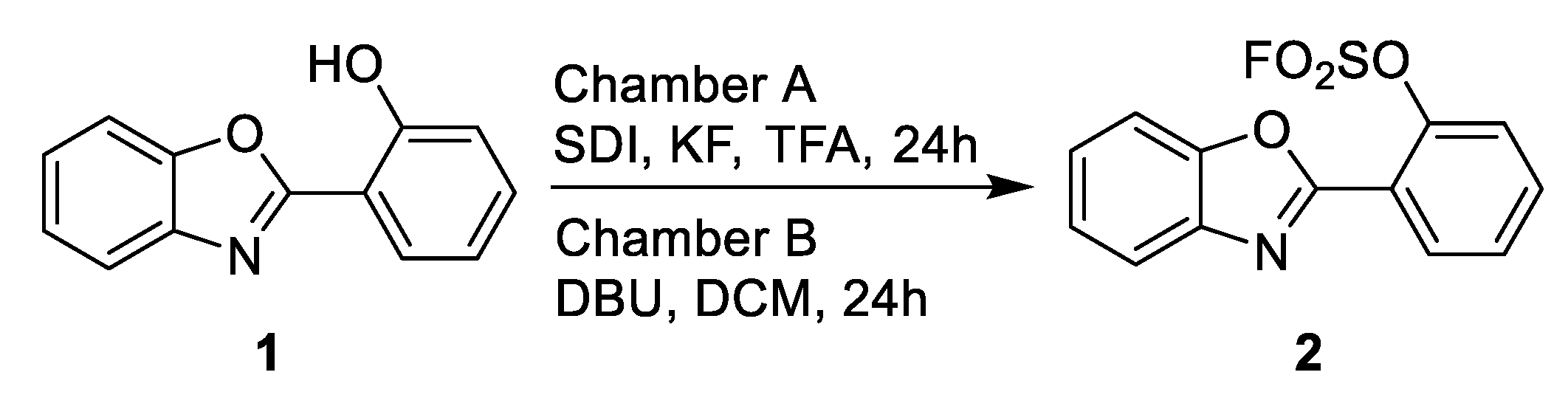
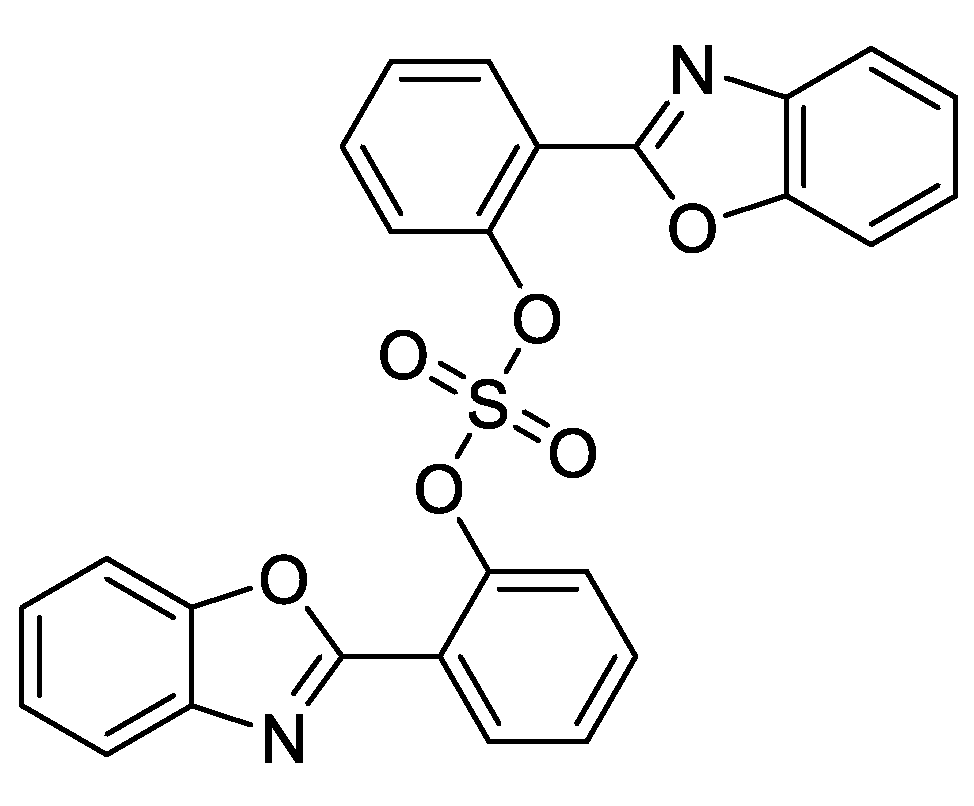
| No. * | Chamber A | Target Product Yield, g (%) ** | By-Product Yield, g (%) ** | ||
|---|---|---|---|---|---|
| SDI, mmol | KF, mmol | TFA, mL | |||
| 1 | 1.5 | 4 | 1 | 0.0498 (34) | 0.0695 (57) |
| 2 | 2.5 | 6.5 | 1.6 | 0.1095 (75) | 0.0285 (24) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilenko, N.V.; Shmalyuk, V.I.; Khlebnikov, A.I. 2-(2-(Fluorosulfonyloxy)phenyl)benzoxazole. Molbank 2021, 2021, M1242. https://doi.org/10.3390/M1242
Danilenko NV, Shmalyuk VI, Khlebnikov AI. 2-(2-(Fluorosulfonyloxy)phenyl)benzoxazole. Molbank. 2021; 2021(3):M1242. https://doi.org/10.3390/M1242
Chicago/Turabian StyleDanilenko, Nadezhda V., Vladimir I. Shmalyuk, and Andrei I. Khlebnikov. 2021. "2-(2-(Fluorosulfonyloxy)phenyl)benzoxazole" Molbank 2021, no. 3: M1242. https://doi.org/10.3390/M1242
APA StyleDanilenko, N. V., Shmalyuk, V. I., & Khlebnikov, A. I. (2021). 2-(2-(Fluorosulfonyloxy)phenyl)benzoxazole. Molbank, 2021(3), M1242. https://doi.org/10.3390/M1242







