5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-1H-pyrazole
Abstract
1. Introduction
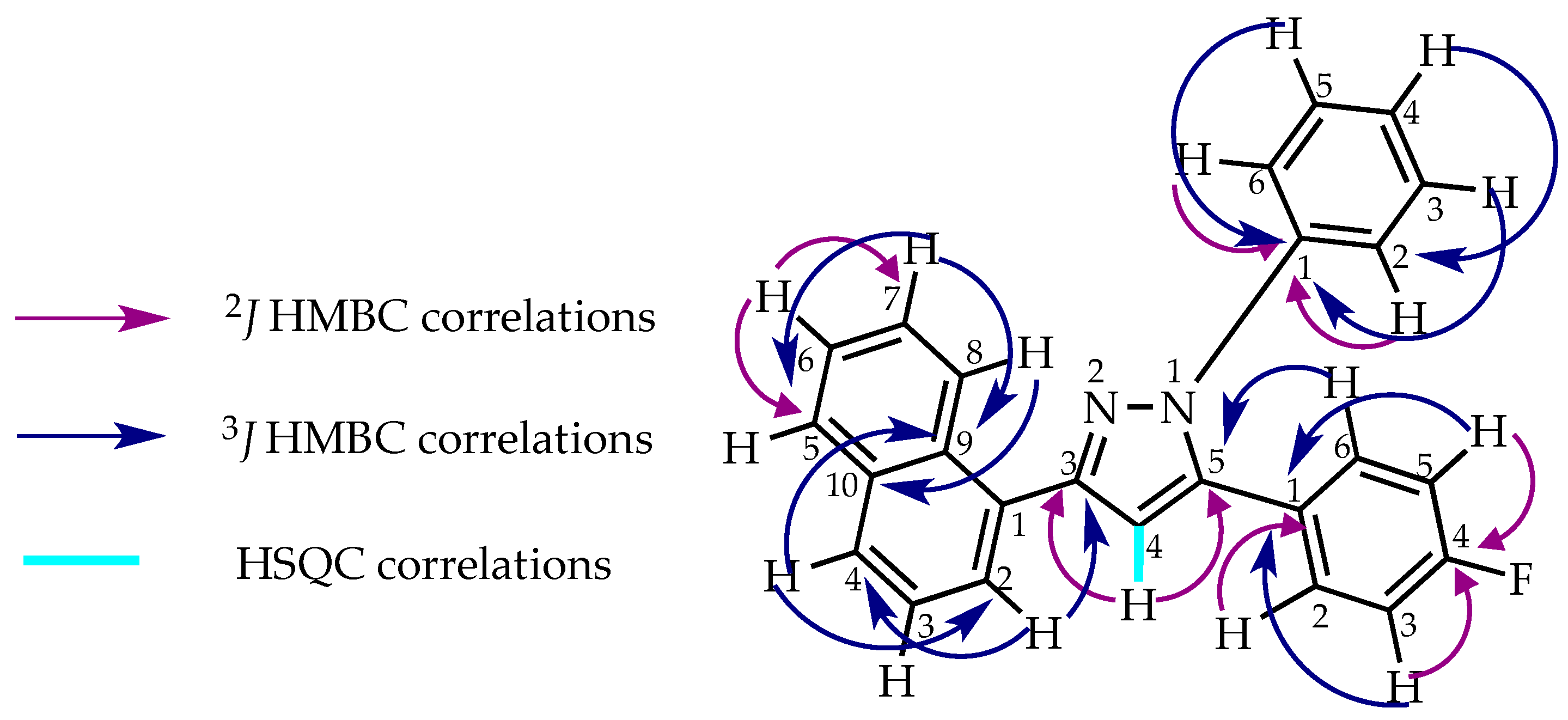
2. Results and Discussion
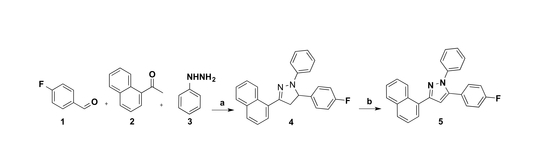
2.1. Synthesis
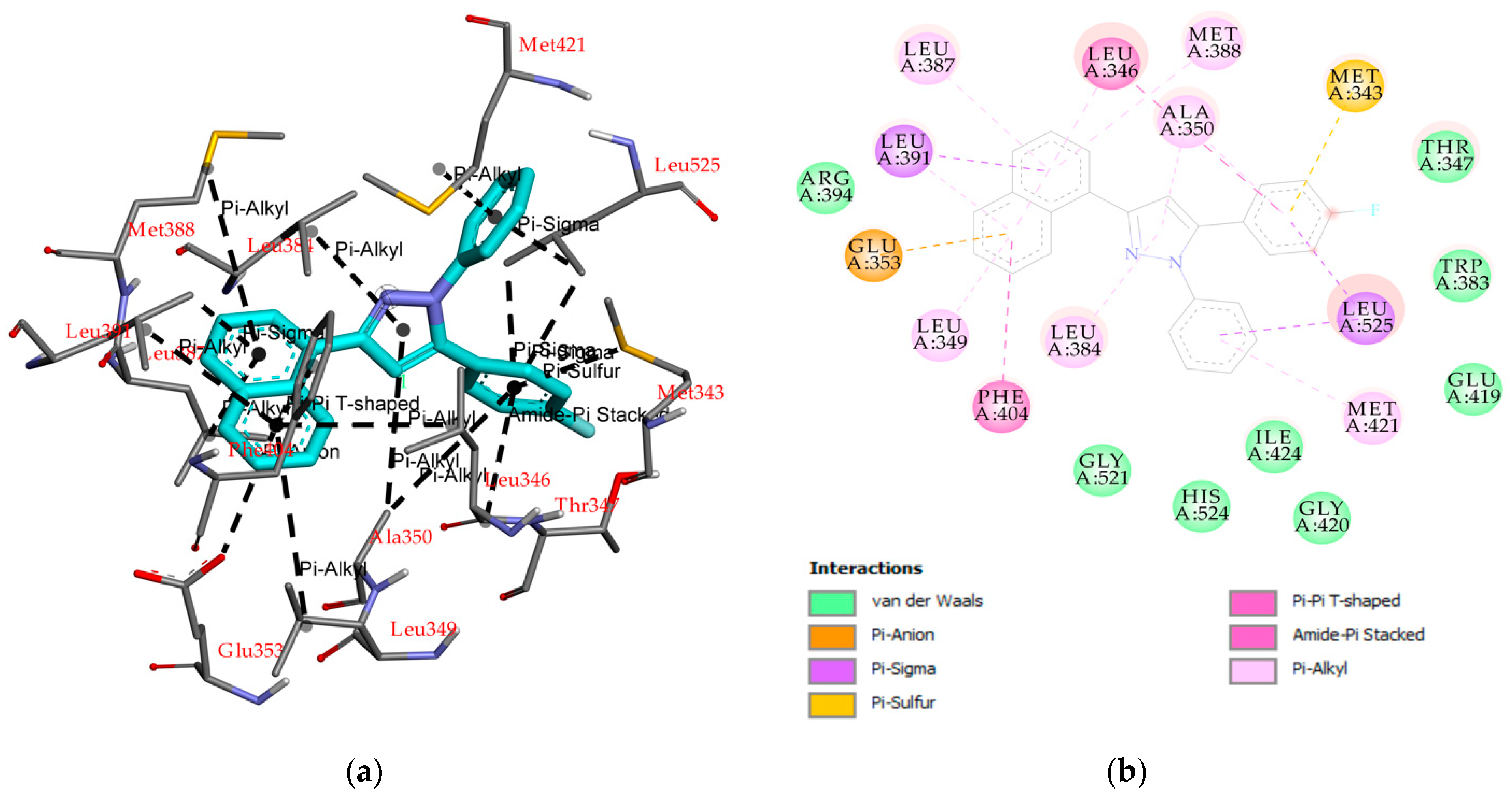
2.2. Molecular Docking
3. Materials and Methods
3.1. Materials
3.2. Synthesis of 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole 4
3.3. Synthesis of 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-1H-pyrazole 5
3.4. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thumar, N.J.; Patel, M.P. Synthesis, characterization, and antimicrobial evaluation of carbostyril derivatives of 1H-pyrazole. Saudi Pharm. J. 2011, 19, 75–83. [Google Scholar] [CrossRef]
- Harikrishna, N.; Isloor, A.M.; Ananda, K.; Obaid, A.; Fun, H.-K. Synthesis, and antitubercular and antimicrobial activity of 1′-(4-chlorophenyl)pyrazole containing 3,5-disubstituted pyrazoline derivatives. New J. Chem. 2016, 40, 73–76. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Gupta, A.K.; Kumar, S. Developments in synthesis of the anti-inflammatory drug, celecoxib: A review. Recent Pat. Inflamm. Allergy. Drug Discov. 2013, 7, 124–134. [Google Scholar] [CrossRef]
- Jasril, J.; Teruna, H.Y.; Aisyah, A.; Nurlaili, N.; Hendra, R. Microwave assisted synthesis and evaluation of toxicity and antioxidant activity of pyrazoline derivatives. Indones. J. Chem. 2019, 19, 583–591. [Google Scholar] [CrossRef]
- Zaninetti, R.; Cortese, S.V.; Aprile, S.; Massarotti, A.; Canonico, P.L.; Sorba, G.; Grosa, G.; Genazzani, A.A.; Pirali, T. A concise synthesis of pyrazole analogues of combretastatin A1 as potent anti-tubulin agents. Chem. Med. Chem. 2013, 8, 633–643. [Google Scholar] [CrossRef]
- Jasril, J.; Teruna, H.Y.; Ikhtiarudin, I.; Frimayanti, N. Search for new potential breast cancer inhibitors (MCF7) based on molecular docking and biological assay of pyrazoline analogue compounds. ASTESJ 2020, 5, 122–126. [Google Scholar] [CrossRef]
- Padmini, T.; Kamal, B.R. Synthesis, anti-inflammatory, analgesic and antipyretic activity of novel 1,3,5-trisubstituted pyrazole derivatives. Asian J. Chem. 2019, 31, 1225–1229. [Google Scholar] [CrossRef]
- Jasril, J.; Ikhtiarudin, I.; Zamri, A.; Teruna, H.Y.; Frimayanti, N. New fluorinated chalcone and pyrazoline analogs: Synthesis, docking, and molecular dynamic studies as anticancer agents. TJPS 2017, 41, 93–98. [Google Scholar]
- Palleapati, K.; Kancharlapalli, V.R.; Shaik, A.B. Synthesis, characterization and antitubercular evaluation of some new isoxazole appended 1-carboxamido-4,5-dihydro-1H-pyrazoles. J. Res. Pharm. 2019, 23, 156–163. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kawashita, Y.; Hayashi, M. Oxidative aromatization of 1,3,5-trisubstituted pyrazolines and hantzsch 1,4-dihydropyridines by Pd/C in acetic acid. Org. Lett. 2002, 4, 3955–3957. [Google Scholar] [CrossRef]
- Liu, D.C.; Gao, M.J.; Huo, Q.; Ma, T.; Wang, Y.; Wu, C.Z. Design, synthesis, and apoptosis-promoting effect evaluation of novel pyrazole with benzo[d]thiazole derivatives containing aminoguanidine units. J. Enzym. Inhib. Med. Chem. 2019, 34, 829–837. [Google Scholar] [CrossRef]
- Shaik, A.B.; Rao, G.K.; Kumar, G.B.; Patel, N.; Reddy, V.S.; Khan, I.; Routhu, S.R.; Kumar, C.G.; Veena, I.; Shekar, K.C.; et al. Design, synthesis and biological evaluation of novel pyrazolochalcones as potential modulators of PI3K/Akt/mTOR pathway and inducers of apoptosis in breast cancer cells. Eur. J. Med. Chem. 2017, 139, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, N.M.H.; Al-Omar, M.A. Substituted Pyrazole Derivatives. US Patent 010039749B1, 7 August 2018. [Google Scholar]
- Gao, D.; Hlinak, A.J.; Mazhary, A.M.; Truelove, J.E.; Vaughn, M.B.W. Celecoxib Compositions. WIPO (PCT) 2000032189A1, 8 June 2000. [Google Scholar]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef]
- Jasril, J.; Zamri, A.; Ikhtiarudin, I.; Teruna, H.Y. 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole. Molbank 2016, 2016, M891. [Google Scholar] [CrossRef]
- Zamri, A.; Teruna, H.Y.; Wulansari, S.; Herfindo, N.; Frimayanti, N.; Ikhtiarudin, I. 3-(3,4-Dimethoxyphenyl)-5-(2-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole. Molbank 2019, 4, M1088. [Google Scholar] [CrossRef]
- Hawaiz, F.E.; Hussein, A.J.; Samad, M.K. One-pot three-component synthesis of some new azo-pyrazoline derivatives. Eur. J. C 2014, 5, 233–236. [Google Scholar] [CrossRef][Green Version]
- Movassagh, B.; Bijanzadeh, H.R. A mild and highly efficient one-pot synthesis of 1,3,5-triaryl-2-pyrazolines. Chem. Heterocycl. Compd. 2013, 48, 1719–1721. [Google Scholar] [CrossRef]
- Guo, W.; Wong, T.C. Study of 13C-19F and 1H-19F couplings in some fluorinated aromatic compounds using two-dimensional 13C-1H chemical shift correlation spectroscopy with proton homonuclear decoupling. Magn. Res. Chem. 1986, 24, 75–79. [Google Scholar] [CrossRef]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of This Interaction by Tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Smyth, M.S.; Martin, J.H. X ray crystallography. Mol. Pathol. 2000, 53, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Plewczynski, D.; Łaz’niewski, M.; Augustyniak, R.; Ginalski, K. Can we trust docking results? Evaluation of seven commonly used programs on PDBbind database. J. Comput. Chem. 2010, 32, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Mardianingrum, R.; Yusuf, M.; Hariono, M.; Gazzali, A.M.; Muchtaridi, M. Mangostin and its derivatives against estrogen receptor alpha. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
| Compounds | ∆G (Kcal/mol) | Ki (nM) | Interaction Compound—Erα | ||
|---|---|---|---|---|---|
| H-Bond | Hydrophobic | Van der Waals | |||
| 5 | −10.61 | 16.71 | - | Leu391, Leu525, Met343, Glu353, Leu384, Leu349, Ala350, Met388, Met421, Leu387, Phe404, Leu346 | Thr347, Trp383, Glu419, Ile424, His524, Gly420, Gly521, Arg394 |
| 4-OHT a | −11.04 | 3.76 | Arg394, Glu353 | Leu391, Leu387, Ala350, Leu346, Ile424, Met388, Leu428, Leu525, Met421 | Leu384, Phe404 Met343, Gly420, His524, Gly521, Leu349, Thr347, Asp351, Leu354, Trp383 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasril, J.; Frimayanti, N.; Nurulita, Y.; Zamri, A.; Ikhtiarudin, I.; Guntur, G. 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-1H-pyrazole. Molbank 2021, 2021, M1197. https://doi.org/10.3390/M1197
Jasril J, Frimayanti N, Nurulita Y, Zamri A, Ikhtiarudin I, Guntur G. 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-1H-pyrazole. Molbank. 2021; 2021(1):M1197. https://doi.org/10.3390/M1197
Chicago/Turabian StyleJasril, Jasril, Neni Frimayanti, Yuana Nurulita, Adel Zamri, Ihsan Ikhtiarudin, and Guntur Guntur. 2021. "5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-1H-pyrazole" Molbank 2021, no. 1: M1197. https://doi.org/10.3390/M1197
APA StyleJasril, J., Frimayanti, N., Nurulita, Y., Zamri, A., Ikhtiarudin, I., & Guntur, G. (2021). 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-1H-pyrazole. Molbank, 2021(1), M1197. https://doi.org/10.3390/M1197