Abstract
We reported an efficient one-pot two-step synthesis of 3-(tert-butyl)-N-(4-methoxybenzyl)-1-methyl-1H-pyrazol-5-amine 3 in good yield by a solvent-free condensation/reduction reaction sequence starting from 3-(tert-butyl)-1-methyl-1H-pyrazol-5-amine 1 and p-methoxybenzaldehyde 2. The one-pot reductive amination proceeded by the formation in situ of the N-(5-pyrazolyl)imine 4 as key synthetic intermediate of other valuable pyrazole derivatives. This methodology is distinguished by its operational easiness, short reaction time, isolation and purification of the aldimine intermediate is not required. The structure of the synthesized N-heterocyclic amine 3 was fully characterized by FTIR-ATR, 1D and 2D NMR experiments, EIMS, and elemental analysis.
1. Introduction
Amines are one of the most important functional groups in organic chemistry [1]. In particular, N-heterocyclic amines are valuable building blocks in drug discovery and modern organic synthesis because they are key precursors in the preparation of active pharmaceutical ingredients, bioactive molecules, natural occurring products, and agrochemicals [1,2,3,4]. Therefore, the ongoing need for new and functionalized N-heterocyclic amines to probe novel reactivity has driven the development of innovative synthetic methods for their preparation in high yields, easy workup procedure, and mild reaction conditions [4,5,6,7]. Until now, the reductive amination is the most commonly used approach in the pharmaceutical industry for C–N bond construction due to its operational simplicity and a wide toolbox of protocols [2].
Pyrazole is a five-membered heterocycle containing two nitrogen atoms in adjacent positions. Nowadays, pyrazole derivatives have attracted more attention due to their wide range of physiological and pharmacological activities [8,9,10,11,12,13], proving to be a promising scaffold for the discovery of novel active pharmaceutical ingredients. For instance, the antipsychotic agent CDPPB [11], the nonsteroidal anti-inflammatory drugs Lonazolac and Epirizole [12], the histamine H2-receptor agonist Betazole [13], among others marketed drugs have this structural motif of pyrazole, as depicted in Figure 1. The interesting structural features of pyrazoles as well as their diverse applications in medicinal chemistry, drug discovery, and materials science have stimulated chemists to develop novel and efficient synthetic protocols for obtaining structurally diverse pyrazoles [14,15,16,17,18,19].
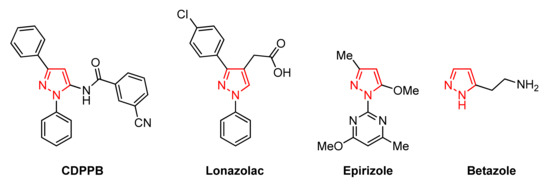
Figure 1.
Marketed drugs containing pyrazole moiety.
Due to the powerful physiological activities of pyrazole derivatives and secondary amines as building blocks for the synthesis of potential druglike compound libraries and important pharmaceutical intermediates, we described the synthesis of a novel N-pyrazolyl amine 3 as a potential bioactive N-heterocycle through a simple and efficient one-pot reductive amination.
2. Results and Discussion
In connection with the ongoing development of novel protocols for the construction of C–N bonds [20,21,22], and our current studies on the synthetic utility of N-(5-pyrazolyl)imine derivatives for the preparation of N-heterocycles of biological interest [23,24,25], we reported a one-pot two-step synthesis of 3-(tert-butyl)-N-(4-methoxybenzyl)-1-methyl-1H-pyrazol-5-amine 3 through a solvent-free condensation reaction between equimolar amounts of 3-(tert-butyl)-1-methyl-1H-pyrazol-5-amine 1 and p-methoxybenzaldehyde 2 to form the N-(5-pyrazolyl)imine 4, followed by reduction with sodium borohydride in methanol at ambient temperature, as depicted in Scheme 1. After performing a liquid–liquid extraction and removing the excess of solvent under reduced pressure, the resulting crude product was purified by flash chromatography on silica gel using a mixture of DCM/MeOH (30:1, v/v) as eluent to give the N-heterocyclic amine 3 in 88% yield. Although the intermediate 4 has been described in our previous work [24], the synthesis and characterization of the target N-pyrazolyl amine 3 has not been reported on Reaxys database. For that reason, a complete spectroscopic and analytical characterization was carried out in this work, see Material and Methods. The IR, 1D NMR and MS spectra, and elemental analysis suggested that effectively the structure of the isolated yellow solid corresponded to the N-pyrazolyl amine 3. Moreover, 2D HSQC, HMBC, COSY, and NOESY experiments permitted us the assignment of all proton and carbon atoms, confirming the proposed structure for 3 without ambiguity, see Supplementary Materials.
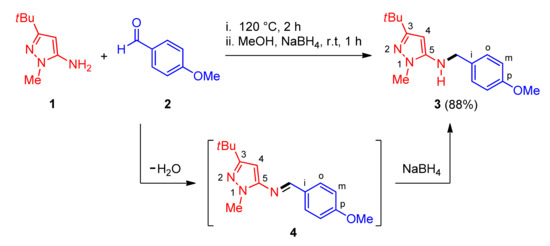
Scheme 1.
One-pot synthesis of the N-pyrazolyl amine via a reductive amination.
The presence of absorption bands at 3243 and 1240/1036 cm−1 assigned to the stretching vibrations of N–H and C–O–C functionalities, respectively, are the most relevant features of the IR spectrum. The 1H-NMR spectrum recorded in deuterated chloroform using TMS as internal standard showed a broad singlet at 3.34 ppm assigned to the NH proton, a doublet at 4.16 (J = 5.2 Hz, 2H) ppm associated with the methylene protons, and the absence of a singlet at 8.50 ppm of the azomethine proton (CH=N), which indisputably confirmed the reduction of the aldimine intermediate 4 generated in situ by a solvent-free condensation reaction of 3-(tert-butyl)-1-methyl-1H-pyrazol-5-amine 1 with p-methoxybenzaldehyde 2, as illustrated in Scheme 1. The presence of three different types of methyl carbons at 30.6, 34.2, and 55.4 ppm, one methylene carbon at 50.1 ppm, three aromatic carbons at 85.2, 114.2, and 129.4 ppm, respectively, and five quaternary carbons, are the most relevant features of the 13C{1H} NMR spectrum. These results evidenced the high electron density (δ = 85.2 ppm) of the carbon atom at position 4 of the π-excedent pyrazole ring. Once again, the absence of the azomethinic carbon at 158.3 ppm of the aldimine intermediate 4 into the DEPT-135 spectrum, confirmed the formation of the N-pyrazolyl amine 3 in an excellent yield via a one-pot reductive amination. In particular, NOESY correlation was observed between methylene protons and the aromatic proton H-4, indicating the closeness of the NCH2 fragment to the pyrazole ring, which is in good agreement with the structure drawn, see Supplementary Materials, Figure S11. Ultimately, a molecular ion with m/z 273 and a base peak with m/z 121 corresponding to the (4-methoxyphenyl)methylium ion, in the mass spectrum, is also consistent with the structure 3.
In summary, this one-pot reductive amination is distinguished by its operational easiness, short reaction time, isolation and purification of the aldimine intermediate is not required; thus, the experimental procedure is simple and efficient in terms of energy, waste, and human resource economy. Remarkably, amine and pyrazole moieties could be susceptible for further functionalization reactions for obtaining novel pyrazole derivatives with potential applications in medicinal chemistry and drug discovery.
3. Materials and Methods
3.1. General Information
All reagents were purchased from commercial sources and used without further purification. All starting materials were weighed and handled in air at ambient temperature. The IR spectrum was recorded on a Shimadzu FTIR 8400 spectrophotometer by ATR technique (Scientific Instruments Inc., Seattle, WA, USA). Spectrum is reported in wavenumber (cm−1) and only selected resonances are reported. 1H and 13C{1H} NMR spectra were recorded on a Bruker Avance 400 spectrophotometer (Bruker BioSpin GmbH, Rheinstetten, Germany), operating at 400 MHz and 101 MHz, respectively, while using tetramethylsilane (0 ppm) as the internal reference. NMR spectroscopic data were recorded in CDCl3 using as internal standards the residual nondeuterated signal (δ = 7.26 ppm) for 1H-NMR and the deuterated solvent signal (δ = 77.16 ppm) for 13C{1H} NMR spectroscopy [26]. DEPT spectra were used for the assignment of carbon signals. Chemical shifts (δ) are given in ppm and coupling constants (J) are given in Hz. The following abbreviations are used for multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, and m = multiplet. Mass spectrum was run on a SHIMADZU-GCMS 2010-DI-2010 spectrometer (Scientific Instruments Inc., Columbia, WA, USA) (equipped with a direct inlet probe) operating at 70 eV. Microanalysis was performed on an Agilent CHNS elemental analyzer (Thermo Fischer Scientific Inc., Madison, WI, USA) and the values are within ±0.4% of the theoretical values. Melting point was determined on a Büchi melting point B-450 apparatus (Instrumart, South Burlington, VT, USA). The 3-(tert-butyl)-1-methyl-1H-pyrazol-5-amine 1 was prepared by a known procedure [27].
3.2. Synthesis of 3-(tert-Butyl)-N-(4-methoxybenzyl)-1-methyl-1H-pyrazol-5-amine (3)
A 10.0 mL open-topped tube was charged with 3-(tert-butyl)-1-methyl-1H-pyrazol-5-amine 1 (153 mg, 1.0 mmol) and p-methoxybenzaldehyde 2 (136 mg, 1.0 mmol, CAS 123-11-5), and the resulting mixture was heated in a sand bath at 120 °C for 2 h under solvent-free conditions. Then, the water vapor condensed on the walls of the open-topped tube was removed with a small piece of cotton attached to a spatula. After complete disappearance of starting materials, as monitored by thin-layer chromatography (TLC), the mixture was allowed to cool to ambient temperature. The resulting N-(5-pyrazolyl)imine 4 was dissolved in methanol (3.0 mL, CAS 67-56-1) and solid NaBH4 (75 mg, 2.0 mmol, CAS 16940-66-2) was added portionwise with stirring over a period of 5 min. The stirring was continued at ambient temperature for 1 h. After the reaction was complete (monitored by TLC), the volume of the reaction mixture was reduced to 1.0 mL under reduced pressure, and distilled water (5.0 mL) was added. The aqueous solution was extracted with ethyl acetate (2 × 5.0 mL, CAS 141-78-6), and the combined organic extracts were dried with anhydrous sodium sulfate (CAS 7757-82-6). After removal of the solvent, the resulting crude product was purified by flash chromatography on silica gel using a mixture of DCM/MeOH (30:1, v/v) as eluent to give the N-heterocyclic amine 3 as a yellow solid (241 mg, 88% yield). Rf (CH2Cl2/MeOH: 30/1) = 0.37. M.p 76 °C. FTIR–ATR: ν = 3243 (v N–H), 2958, 2863, 1611 (v C=Npyrazole), 1558 (v C=C), 1506 (v C=C), 1449, 1356, (1240 and 1036 for va and vs. C–O–C, respectively), 832, 753, 717 cm−1. 1H-NMR (400 MHz, CDCl3): δ = 1.28 (s, 9H, tBu), 3.34 (br s, 1H, NH), 3.57 (s, 3H, NCH3), 3.81 (s, 3H, OCH3), 4.16 (d, J = 5.2 Hz, 2H, NCH2), 5.41 (s, 1H, H-4), 6.90 (d, J = 8.4 Hz, 2H, Hm), 7.30 (d, J = 8.4 Hz, 2H, Ho) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ = 30.6 (CH3, tBu), 32.3 (Cq, tBu), 34.2 (NCH3), 50.1 (NCH2), 55.4 (OCH3), 85.2 (CH, C-4), 114.2 (2CH, Cm), 129.4 (2CH, Co), 130.9 (Cq, Ci), 148.1 (Cq, C-5), 159.3 (Cq, Cp), 160.7 (Cq, C-3) ppm. Anal. calcd. for C16H23N3O (273.37): C, 70.30; H, 8.48; N, 15.37. Found: C, 70.07; H, 8.36; N, 15.51. MS (EI, 70 eV) m/z (%): 273 (47) [M+], 121 (100), 91 (19), 77 (25), 67 (15).
3.3. Synthesis of (E)-3-(tert-butyl)-N-(4-methoxybenzylidene)-1-methyl-1H-pyrazol-5-amine (4)
A 10.0 mL open-topped tube was charged with 3-(tert-butyl)-1-methyl-1H-pyrazol-5-amine 1 (153 mg, 1.0 mmol) and p-methoxybenzaldehyde 2 (136 mg, 1.0 mmol, CAS 123-11-5), and the resulting mixture was heated in a sand bath at 120 °C for 2 h under solvent-free conditions. Then, the water vapor condensed on the walls of the open-topped tube was removed with a small piece of cotton attached to a spatula. After complete disappearance of starting materials, as monitored by thin-layer chromatography (TLC), the mixture was allowed to cool to ambient temperature. The resulting crude product was purified by flash chromatography on silica gel using a mixture of DCM/MeOH (30:1, v/v) as eluent to give the N-(5-pyrazolyl)imine 4 as a yellow solid (247 mg, 91% yield). Rf (CH2Cl2/MeOH: 30/1) = 0.65. M.p 138 °C [24]. FTIR–ATR: ν = 2955, 2862, 1619 (v C=Npyrazole), 1596 (v C=Nimine), 1573, 1518 (v C=C), 1461, 1362, (1251 and 1025 for va and vs. C–O–C, respectively), 837, 760, 729 cm−1. 1H-NMR (400 MHz, CDCl3): δ = 1.33 (s, 9H, tBu), 3.87 (s, 3H, OCH3), 3.91 (s, 3H, NCH3), 6.05 (s, 1H, H-4), 6.97 (d, J = 8.8 Hz, 2H, Hm), 7.84 (d, J = 8.8 Hz, 2H, Ho), 8.50 (s, 1H, CH=N) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ = 30.7 (CH3, tBu), 32.4 (Cq, tBu), 34.7 (NCH3), 55.6 (OCH3), 87.5 (CH, C-4), 114.4 (2CH, Cm), 129.2 (Cq, Ci), 130.6 (2CH, Co), 150.1 (Cq, C-5), 158.3 (CH=N), 161.1 (Cq, C-3), 162.7 (Cq, Cp) ppm. Anal. calcd. for C16H21N3O (271.36): C, 70.82; H, 7.80; N, 15.49. Found: C, 70.98; H, 8.01; N, 15.32. MS (EI, 70 eV) m/z (%): 271 (86) [M+], 256 (100), 229 (46), 128 (22), 91 (13), 77 (11).
Supplementary Materials
The following are available online. Figure S1: MS spectrum of the compound 3 (EI technique); Figure S2: IR spectrum of the compound 3 (ATR technique); Figure S3: 1H-NMR spectrum of the compound 3; Figure S4: Expansion 1H-NMR spectrum of the compound 3; Figure S5: 13C{1H} NMR and DEPT-135 spectra of the compound 3; Figure S6: Expansion 13C{1H} NMR and DEPT-135 spectra of the compound 3; Figure S7: HSQC 2D C–H correlation spectrum of the compound 3; Figure S8: HMBC 2D C–H correlation spectrum of the compound 3; Figure S9: Expansion HMBC 2D C–H correlation spectrum of the compound 3; Figure S10: COSY 2D H–H correlation spectrum of the compound 3; Figure S11: NOESY 2D H–H correlation spectrum of the compound 3; Figure S12: MS spectrum of the compound 4 (EI technique); Figure S13: IR spectrum of the compound 4 (ATR technique); Figure S14: 1H-NMR spectrum of the compound 4; Figure S15: 13C{1H} NMR and DEPT-135 spectra of the compound 4; Figure S16: HSQC 2D C–H correlation spectrum of the compound 4; Figure S17: HMBC 2D C–H correlation spectrum of the compound 4; Figure S18: COSY 2D H–H correlation spectrum of the compound 4; Figure S19: NOESY 2D H–H correlation spectrum of the compound 4.
Author Contributions
Investigation, data curation, writing—original draft preparation, D.B.; resources, writing—review and editing, H.R.; conceptualization, data curation, writing—original draft preparation, J.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
APC was sponsored by MDPI.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
Authors thank to the Dirección de Investigaciones at the Universidad Pedagógica y Tecnológica de Colombia for financial support (project number SGI-2829). We also acknowledge Luis Hurtado and Carlos Rodríguez at the Universidad del Valle for acquiring 1D and 2D NMR experiments, FT–IR, EI–MS, and elemental analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Roughley, S.D.; Jordan, A.M. The medicinal chemist’s toolbox: An analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Kuchuk, E.; Usanov, D.L.; Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2020, 40, 709–752. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, A.; Walton, S.M.; Gaunt, M.J. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 2020, 120, 2613–2692. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Orrego-Hernández, J.; Portilla, J. Cs2CO3-Promoted direct N-alkylation: Highly chemoselective synthesis of N-alkylated benzylamines and anilines. Eur. J. Org. Chem. 2016, 2016, 3824–3835. [Google Scholar] [CrossRef]
- Hahn, G.; Kunnas, P.; de Jonge, N.; Kempe, R. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst. Nat. Catal. 2019, 2, 71–77. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Adam, R.; Papa, V.; Beller, M. Homogeneous and heterogeneous catalytic reduction of amides and related compounds using molecular hydrogen. Nat. Commun. 2020, 11, 3893. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.M.; Sharma, K.S. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Vélez, I.D.; Upegui, Y.; Muñoz, J.A.; et al. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo [3,4-b][1,4]diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2015, 93, 401–413. [Google Scholar] [CrossRef]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef] [PubMed]
- Kleizienė, N.; Arbačiauskienė, E.; Holzer, W.; Šačkus, A. (2E)-3-(3-Methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal. Molbank 2009, 2009, M644. [Google Scholar] [CrossRef]
- Kleizienė, N.; Arbačiauskienė, E.; Holzer, W.; Šačkus, A. 4-Bromo-3-methoxy-1-phenyl-1H-pyrazole. Molbank 2009, 2009, M639. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, C.; Tang, Y.; Ma, S. Copper-mediated pyrazole synthesis from 2,3-allenoates or 2-alkynoates, amines and nitriles. Chem. Commun. 2014, 50, 7677–7679. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, D.; Castillo, J.; Becerra, D.; Rojas, H.; Abonia, R. Synthesis of biologically active molecules through multicomponent reactions. Molecules 2020, 25, 505. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar] [CrossRef]
- Pearce, A.J.; Harkins, R.P.; Reiner, B.R.; Wotal, A.C.; Dunscomb, R.J.; Tonks, I.A. Multicomponent pyrazole synthesis from alkynes, nitriles, and titanium imido complexes via oxidatively induced N–N bond coupling. J. Am. Chem. Soc. 2020, 142, 4390–4399. [Google Scholar] [CrossRef]
- Acosta, P.; Becerra, D.; Goudedranche, S.; Quiroga, J.; Constantieux, T.; Bonne, D.; Rodriguez, R. Exploiting the reactivity of 1,2-ketoamides: Enantioselective synthesis of functionalized pyrrolidines and pyrrolo-1,4-benzodiazepine-2,5-diones. Synlett 2015, 26, 1591–1595. [Google Scholar] [CrossRef]
- Bianchini, G.; Ribelles, P.; Becerra, D.; Ramos, M.T.; Menéndez, J.C. Efficient synthesis of 2-acylquinolines based on an aza-vinylogous Povarov reaction. Org. Chem. Front. 2016, 3, 412–422. [Google Scholar] [CrossRef]
- Moreno-Fuquen, R.; Arango-Daraviña, K.; Becerra, D.; Castillo, J.-C.; Kennedy, A.R.; Macías, M.A. Catalyst- and solvent-free synthesis of 2-fluoro-N-(3-methylsulfanyl-1H-1,2,4-triazol-5-yl)benzamide through a microwave-assisted Fries rearrangement: X-ray structural and theoretical studies. Acta Cryst. 2019, C75, 359–371. [Google Scholar] [CrossRef]
- Abonia, R.; Castillo, J.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J. An efficient synthesis of 7-(arylmethyl)-3-tert-butyl-1-phenyl-6,7-dihydro-1H,4H-pyrazolo [3,4-d][1,3]oxazines. Eur. J. Org. Chem. 2010, 2010, 6454–6463. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Quiroga, J.; Abonia, R.; Rodriguez, J.; Coquerel, Y. The aryne aza-Diels–Alder reaction: Flexible syntheses of isoquinolines. Org. Lett. 2015, 17, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.-C.; Agudelo, B.C.; Gálvez, J.; Carissan, Y.; Rodriguez, J.; Coquerel, Y. Periselectivity in the aza-Diels–Alder cycloaddition between α-oxoketenes and N-(5-pyrazolyl)imines: A combined experimental and theoretical study. J. Org. Chem. 2020, 85, 7368–7377. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Bagley, M.; Davis, T.; Dix, M.; Widdowson, C.; Kipling, D. Microwave-assisted synthesis of N-pyrazole ureas and the p38α inhibitor BIRB 796 for study into accelerated cell ageing. Org. Biomol. Chem. 2006, 4, 4158–4164. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).